
Contributed by Roy Curtiss III, November 25, 2020 (sent for review July 7, 2020; reviewed by Ferric Fang and M. Stephen Trent)
Author contributions: H.S. and Q.K. designed research; H.S., Q.L., X.B., and Q.K. performed research; S.W. and Q.K. contributed new reagents/analytic tools; H.S., S.W., R.C., and Q.K. analyzed data; and H.S., R.C., and Q.K. wrote the paper.
Reviewers: F.F., University of Washington; and M.S.T., University of Georgia.
1H.S. and Q.L. contributed equally to this work.
- Altmetric
Pneumococcal infection-caused diseases are responsible for substantial morbidity and mortality worldwide. Traditional pneumococcal vaccines are developed based on purified capsular polysaccharides (CPS) or CPS conjugated to a protein carrier. Production processes of the traditional vaccines are laborious, and thereby increase the vaccine cost and limit their use in developing nations. A cost-effective pneumococcal vaccine using the recombinant attenuated Salmonella vaccine (RASV) was developed in this study. We cloned and expressed genes for seven serotypes of CPSs in the RASV strain. The RASV-delivered CPSs induced robust humoral and cell-mediated responses and mediated efficient protection of mice against pneumococcal infection. Our work provides an innovative strategy for mass producing low-cost bioconjugated polysaccharide vaccines for needle-free mucosal delivery against pneumococcal infections.
Streptococcus pneumoniae capsular polysaccharides (CPSs) are major determinants of bacterial pathogenicity. CPSs of different serotypes form the main components of the pneumococcal vaccines Pneumovax, Prevnar7, and Prevnar13, which substantially reduced the S. pneumoniae disease burden in developed countries. However, the laborious production processes of traditional polysaccharide-based vaccines have raised the cost of the vaccines and limited their impact in developing countries. The aim of this study is to develop a kind of low-cost live vaccine based on using the recombinant attenuated Salmonella vaccine (RASV) system to protect against pneumococcal infections. We cloned genes for seven different serotypes of CPSs to be expressed by the RASV strain. Oral immunization of mice with the RASV-CPS strains elicited robust Th1 biased adaptive immune responses. All the CPS-specific antisera mediated opsonophagocytic killing of the corresponding serotype of S. pneumoniae in vitro. The RASV-CPS2 and RASV-CPS3 strains provided efficient protection of mice against challenge infections with either S. pneumoniae strain D39 or WU2. Synthesis and delivery of S. pneumoniae CPSs using the RASV strains provide an innovative strategy for low-cost pneumococcal vaccine development, production, and use.
The bacteria Streptococcus pneumoniae (pneumococcus) continues to be a leading cause of pneumonia, meningitis, and bacteremia in young children, elderly people, and immunocompromised populations (1), and therefore is responsible for millions of cases and deaths each year worldwide (2). S. pneumoniae synthesizes immunologically distinct capsular polysaccharides (CPSs), which cover the cell surface and define serotypes. At present, there are over 90 capsular serotypes identified (34–5). The CPSs prevent S. pneumoniae from complement-mediated opsonophagocytic killing, which is believed to be an important defense mechanism during S. pneumoniae infection (6, 7). Antibodies against CPSs mediate clearance of S. pneumoniae from the lung and protect the host against pneumococcal disease (89–10). Consequently, traditional pneumococcal vaccines are mainly developed based on purified CPS or CPS conjugated to a protein carrier, in order to induce the CPS-specific immune responses (11).
A 23-valent pneumococcal polysaccharide vaccine (PPV23; Pneumovax) was developed and recommended for people over 65 y or for children and adults with underlying medical conditions. The PPV23 contains 23 capsular types of purified CPSs: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. However, the purified CPSs are T cell-independent antigens that could not induce much IgM-to-IgG switching (12) and sustained memory immune responses (13). Therefore, the PPV23 is not effective in children younger than 2 y of age (14). The pneumococcal conjugate vaccine (PCV13; Prevnar13) developed by conjugating CPSs of 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19F, 19A, and 23F to diphtheria toxoid (CRM197) (15) provided effective memory responses and increased immunogenicity in children under 2 y of age (16). Although glycoconjugated vaccines have played a great role in controlling S. pneumoniae infections caused by vaccine serotypes (171819202122–23), there are also disadvantages to their use. PCV13 includes the most prevalent serotypes in the Western world and in developing countries (24, 25) and helps decrease the overall incidence of invasive pneumococcal disease. However, as the existing serotypes in PCV13 are not readily altered and the distribution of S. pneumoniae serotypes varies by geography, age, and time (26), the introduction of PCV13 resulted in variable immunogenicity among different populations (27) and the diseases caused by nonvaccine serotypes increased significantly in children younger than 5 y and in adults older than 45 y (28).
The production process of the PCV involves multiple stages and rounds of purification, which greatly increases their cost and limits the vaccine use in developing countries where the disease burden is heaviest. Although the vaccine price drops significantly because of the support of the Gavi Pneumococcal Advance Market Commitment, the global average coverage of pneumonia vaccine is currently only at 47%. More efforts are still needed to reduce the vaccine price and increase immunization coverage for lower-income countries. In addition, as each polysaccharide is structurally distinct and the chemical conjugation may change its conformation and epitopes, each reaction requires optimization and the produced glycoconjugate vaccines are often poorly characterized, heterogeneous, and variably immunogenic (11, 29, 30).
The protein glycan coupling technology was developed recently for biosynthesis of polysaccharide conjugate vaccine. This approach uses the oligosaccharyltransferases from Campylobacter jejuni (PglB) (3132–33) or Neisseria (PglL) (34, 35). This technology still needs the complicated process of isolation and purification of protein-CPS conjugates. Another limitation for both of the PPV and PCV is that the induced predominant serum immunoglobulin G (IgG) isotype is IgG2 in responses of people older than 2 y of age (36), and neither of the two vaccines induces significant mucosal IgA responses (37). Live attenuated strains of S. pneumoniae are used for pneumococcal vaccine development as well (37, 38). However, the potential reversion to virulence of attenuated vaccines remains a major concern, as the pneumococci are naturally competent for DNA uptake from circulating wild-type strains.
As an alternative approach, we focused on using recombinant attenuated Salmonella vaccine (RASV) strains as delivery platforms for pneumococcal polysaccharides. RASV strains presenting foreign antigens from unrelated pathogens are promising oral vaccine vectors, as they were developed to provide novel, needle-free, and low-cost strategies for preventing infectious diseases (39). There are gram-negative bacteria, such as Escherichia coli strains with a waaL gene deletion, which showed a capability to synthesize heterologous polysaccharides from gram-negative and gram-positive bacteria (40, 41). In our previous study (42), genes required for Salmonella O-antigen synthesis were also deleted for construction of RASV strains delivering heterologous polysaccharide antigens. Lipopolysaccharide (LPS)-associated O-antigen in gram-negative bacteria is covalently ligated to the lipid A-core in the outer membrane (OM) (43). Several lines of evidence suggest that OM proteins (OMPs), including matrix porin (OmpF) and siderophore transporter FhuA, form a tight complex with LPS (44). The OMP–LPS complexes offer high potentials for triggering T cell-dependent (TD) immune responses against O-antigen, which may help explain that antibodies to O-antigen could provide protective activity after natural infection of Salmonella Typhimurium (S. Typhimurium) (45). Attenuated Salmonella strains synthesizing Shigella O-antigens elicited strong anti-Shigella–LPS immune responses and conferred efficient protection against challenge with virulent Shigella strains in murine models (4647–48). Therefore, the RASV platform may provide a novel strategy for producing bioconjugated polysaccharide vaccines against pneumococcal infections. In this study, the gene clusters for synthesis of S. pneumoniae CPSs of 2, 3, 5, 6A, 9V, 14, and 18C were cloned into our balanced lethal vector and the resultant plasmids were introduced into the RASV strain to establish a platform for vaccine development against pneumococcal infection.
Methods
Bacterial Strains and Plasmids.
Bacteria strains and plasmids utilized in this study are described in Table 1. S. Typhimurium and E. coli strains containing Asd+ plasmids were grown at 37 °C in Luria-Bertani (LB) broth or on LB agar. For the growth of strains with asdA gene deletions, diaminopimelic acid (DAP; 50 μg/mL) was added to the growth medium. S. pneumoniae strains D39 (serotype 2), WU2 (serotype 3), STREP5 (serotype 5), EF6796 (serotype 6A), EMC9V (serotype 9V), STREP14 (serotype 14), and OREP18C (serotype 18C) were grown on Trypticase soy agar plates supplemented with 5% sheep blood or in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY medium) at 37 °C in an anaerobic jar system (GasPak system, BBL).

| Strains or plasmids | Description | Source |
| S. pneumoniae strains | ||
| D39 | Wild-type S. pneumoniae CPS2 strain | Laboratory collection |
| WU2 | Wild-type S. pneumoniae CPS3 strain | Lababoratory collection |
| STREP5 | Wild-type S. pneumoniae CPS5 strain | BEI Resources |
| EF6796 | Wild-type S. pneumoniae CPS6A strain | Laboratory collection |
| EMC9V | Wild-type S. pneumoniae CPS9V strain | BEI Resources |
| STREP14 | Wild-type S. pneumoniae CPS14 strain | BEI Resources |
| OREP18C | Wild-type S. pneumoniae CPS18C strain | BEI Resources |
| E. coli strain | ||
| χ6097 | ∆(lac-pro) rpsL ∆asdA4 ∆(zhf-2::Tn10) thi ϕ80dlacZ∆M15 | K-12/F− |
| Salmonella enterica serovar Typhimurium strains | ||
| χ3761 | Wild-type UK-1 | Laboratory collection |
| χ11316 | ∆pabA1516 ∆pabB232 ∆asdA16 ∆araBAD23 ∆relA198::araC PBAD lacI TT ∆wbaP45 | χ9241 (42) |
| Recombinant plasmids | ||
| pG8R184 | Asd+ vector, pSC101 ori, Kan+ | Laboratory stock |
| pG8R307 | pG8R184 - S. pneumoniae CPS2 operon (wchA-rmlD), pSC101 ori | Present study |
| pG8R308 | pG8R184 - S. pneumoniae CPS3 (ugd-pgm), pSC101 ori | Present study |
| pG8R309 | pG8R184 - S. pneumoniae CPS5 (wcil-fnlC), pSC101 ori | Present study |
| pG8R310 | pG8R184 - S. pneumoniae CPS6A (wchA-rmlD), pSC101 ori | Present study |
| pG8R311 | pG8R184 - S. pneumoniae CPS9V (wchA-wcjE), pSC101 ori | Present study |
| pG8R312 | pG8R184 - S. pneumoniae CPS14 (wchA-Irp), pSC101 ori | Present study |
| pG8R313 | pG8R184 - S. pneumoniae CPS18C (wchA-rmlD), pSC101 ori | Present study |
| Strains for immunization | ||
| RASV-CPS2 | χ11316 electroporated with pG8R307 | |
| RASV-CPS3 | χ11316 electroporated with pG8R308 | |
| RASV-CPS5 | χ11316 electroporated with pG8R309 | |
| RASV-CPS6A | χ11316 electroporated with pG8R310 | |
| RASV-CPS9V | χ11316 electroporated with pG8R311 | |
| RASV-CPS14 | χ11316 electroporated with pG8R312 | |
| RASV-CPS18C | χ11316 electroporated with pG8R313 | |
| RASV-184 | χ11316 electroporated with pG8R184 | |
Cloning of S. pneumoniae CPS Genes into pG8R184.
Genomic DNA from S. pneumoniae strains was isolated using the GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich). All capsule loci were amplified from the genomic DNA using the PrimeSTAR GXL DNA Polymerase (Takara Bio) with primers listed in SI Appendix, Table S1. For each capsule locus, multiple primers were designed to ligate fragments amplified from S. pneumoniae genomic DNA with pG8R184 to create plasmids pG8R307 (CPS2), pG8R308 (CPS3), pG8R309 (CPS5), pG8R310 (CPS6A), pG8R311 (CPS9V), pG8R312 (CPS14), and pG8R313 (CPS18C). The resulting amplicons from each capsular type were ligated to pG8R184 by gene assembly using NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs). The products were transformed into E. coli strain χ6097 by electroporation for propagation of plasmids. The plasmids were then confirmed by PCR, sequencing, and CPS production, before electroporated into S. Typhimurium strain χ11316. Both E. coli and S. Typhimurium transformants were detected on LB agar plates lacking DAP.
Detection of Capsule Expression by Western Blotting.
S. Typhimurium strains with or without recombinant plasmids, grown overnight at 37 °C, were diluted in fresh LB media to an OD600 of 0.03. All strains were OD600 matched (0.8 to 0.9). The LPS of the S. Typhimurium transformants and wild-type S. Typhimurium χ3761 were separated by SDS/PAGE (49). Samples were then transferred onto nitrocellulose membranes and immunologically detected with 1:1,000 dilutions of serotype-specific rabbit anti-CPS antibodies (Statens Serum Institute) for identification of CPS synthesis. Salmonella O Group B Antiserum (BD) was used for identification of S. Typhimurium native O-antigen at a 1:1,000 dilution.
Animal Immunization and Challenge.
The animal experiments were performed in accordance with the recommendations of the Animal Welfare Act and US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training. The protocols were approved by the University of Florida Institutional Animal Care and Use Committee and Southwest University Institutional Animal Care and Use Committee.
For immunization, groups of 7-wk-old female BALB/c mice (Charles River Laboratories) were orally vaccinated with strains of RASV-CPS2 (χ11316 with pG8R307), RASV-CPS3 (χ11316 with pG8R308), RASV-CPS5 (χ11316 with pG8R309), RASV-CPS6A (χ11316 with pG8R310), RASV-CPS9V (χ11316 with pG8R311), RASV-CPS14 (χ11316 with pG8R312), RASV-CPS18C (χ11316 with pG8R313), and RASV-184 (χ11316 with pG8R184) at a dose of 1 × 109 CFU in 20 μL of buffered saline with gelatin (BSG) on day 0. The same dose of the same strain was given 2 wk later (on day 14). Mice orally administrated with 20 μL of BSG were included as the negative control. For the positive control group, the PPV23 (Chengdu Institute of Biological Products) was diluted at 1:10 and 100 μL per mouse was intraperitoneally administered on day 0 and day 14. Three mice in each group were killed for the assay of cytokine expression on day 21. Blood was collected on day 28 (2 wk after secondary immunization) via mandibular vein puncture. Vaginal secretion samples were collected as described previously (47). For RASV-CPS2 (χ11316 with pG8R307) and RASV-CPS3 (χ11316 with pG8R308) immunized groups, mice (n = 10) were challenged on day 35 (3 wk after secondary immunization) intraperitoneally with 2 × 104 CFU (150 times the LD50) of D39 and 2 × 104 CFU (100 times the LD50) of WU2 in 100 μL BSG, respectively.
Antigen Preparation and ELISA.
Purified pneumococcal polysaccharide powders of types 2, 3, 5, 6A, 9V, 14, and 18C were purchased from the American Type Culture Collection (ATCC). S. Typhimurium LPS were extracted using the LPS extraction kit (Boca Scientific) from χ3761. Both of the pneumococcal polysaccharides and the S. Typhimurium LPS were coated on the 96-well ELISA plates (100 ng per well). Serum antibodies and vaginal secretion IgA (SIgA) against CPSs and S. Typhimurium LPS were measured using ELISA, as described previously (47).
Cell Stimulation and Flow Cytometry.
Single-cell preparations of spleen were obtained as previously described (47). Splenocytes were stimulated with each type of the CPSs or with the LPS of χ3761 (50 ng/2 × 106 cells) in the presence of Protein Transport Inhibitor Mixture (eBioscience). The cells were then incubated for 8 h at 37 °C with 5% CO2. Before staining, dead cells were excluded using Zombie Red (Biolegend) and mouse FcR were blocked with the rat anti-mouse CD16/CD32 monoclonal antibody (eBioscience). For surface staining, cells were incubated with fluorophore-conjugated antibodies on ice for 30 min. The eBioscience Intracellular Fixation and Permeabilization Buffer Set was used for intracellular staining. Cells were run on a BD LSRFortessa cell analyzer and analyzed with FlowJo software (TreeStar).
For detection of cytokine expression in CD4+ T cells at 21 d after primary immunization, the following antibodies purchased from Biolegend were used, including rat anti-mouse CD3-Alexa Fluor 700, CD4-FITC, IFN-γ–APC/Cy7, APC/Cy7-conjugated rat IgG1κ (isotype), IL-4–Alexa Fluor 647, Alexa Fluor 647-conjugated rat IgG1κ (isotype). Data are presented as described in the figure legends.
Complement Deposition Assays.
Serum samples collected from each group were heat-inactivated and pooled. Each strain of S. pneumoniae (107 CFU) was resuspended in 90 μL of PBS containing 1% bovine serum albumin (BSA) (Sigma-Aldrich) and mixed with 10 μL of inactivated pooled serum from the corresponding group for 40 min at 37 °C. Treated S. pneumoniae strain cells were then washed twice in PBS and incubated with 100 μL of PBS-1% BSA containing 20 μL of baby rabbit complement (BRC, Sigma-Aldrich) for 30 min at 37 °C. FITC conjugated goat anti-rabbit complement polyclonal antibody (1% final concentration) (MP Biomedical Cappel) was used for staining of the bacteria for 45 min on ice. The samples were fixed with 2% paraformaldehyde solution and analyzed by flow cytometry on the BD LSRFortessa cell analyzer.
Opsonophagocytic Assay.
The opsonophagocytic assay/activity (OPA) was performed mainly as previously described (50). Briefly, differentiation of HL-60 cells (CCL240, ATCC) into granulocytes were carried out in RPMI-1640 supplemented with 1% l-glutamine, 10% fetal calf serum (FCS), and 100 mM N,N- dimethylformamide (DMF) at a cell density of 2 × 105 cells/mL, in a 5% CO2 incubator at 37 °C. HL-60 cells from day 4, 6, or 7 postdifferentiation were used as the effector cells for OPA. Cell viability greater than 90% was assessed by Trypan blue exclusion. Inactivated pooled sera from each group were serially diluted in twofold steps in the Hanks’ buffer with Ca2+, Mg2+, and 0.1% gelatin. The diluted serum samples were then incubated with the corresponding S. pneumoniae strain (∼1,000 CFU per well) for 15 min at 37 °C in a 5% CO2 incubator. Following this incubation, BRC (final concentration: 12.5%; Sigma-Aldrich) and differentiated HL-60 cells (4 × 105 cells) were added. The reaction mixtures were incubated at 37 °C for 45 min with rotation at 220 rpm. After incubation, viable counts of S. pneumoniae strains were determined by dilution plating on blood agar plates. Reaction mixtures containing all the test reagents except serum samples were included as negative controls. The percentage of bacterial killing was calculated by the equation [1 − (number of colonies presented in tested sample/number of colonies presented in negative control)] × 100. All the samples were tested and plated in duplicate. The mean of four independent CFU counts for each sample was used to calculate the percentage of killing.
Statistical Analyses.
The GraphPad Prism 5 (Graph Software) was used to perform statistical analyses. One-way or two-way ANOVA followed by Bonferroni’s test was used for comparisons among groups, depending on the experimental design. All data except for animal survival were expressed as means with deviations. Animal survival analysis was performed using the Kaplan–Meier method followed by the log-rank (Mantel–Cox) test. The levels of significance were set at 0.05, 0.01, and 0.001.
Results
RASV Strains Were Established to Synthesize CPSs of S. pneumoniae.
The sequences of the cps locus have been reported for all 90 serotypes of S. pneumoniae (3). Most of the serotypes (except for types 3 and 37) utilize the wzy-dependent pathway for capsule assembly, which is similar to the mechanism of O-antigen synthesis in S. Typhimurium (7). In this study, we cloned and introduced seven serotypes of S. pneumoniae cps sequences encoding between two and six structural components into the RASV strain (Fig. 1A), to confirm if the CPSs from gram-positive S. pneumoniae could be expressed and ligated to Salmonella lipid A-core receptor. Sequences from the initial transferase gene to the last gene upstream of 3′ transposase gene in the cps locus of serotypes 2, 3, 5, 6A, 9V, 14, and 18C were cloned into the vector pG8R184, respectively (Fig. 1B). Each of the resulting constructs was then transformed into the RASV strain χ11316, which had been modified to facilitate the synthesis of heterologous polysaccharides by introduction of a ΔwbaP mutation (42).

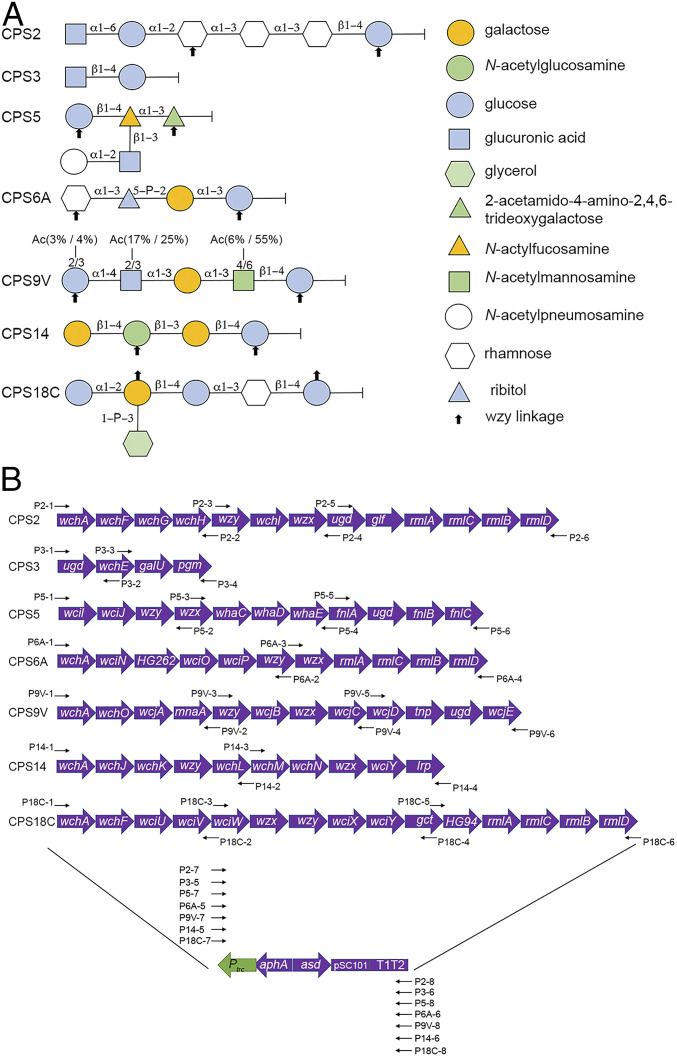
S. pneumoniae CPS repeat units and construction of plasmids for CPS biosynthesis. (A) The capsule repeat unit of each serotype is exhibited to be linked with the reducing end sugar on the right-hand side. Monosaccharides are depicted using different colored shapes according to the graphic symbols. (B) Plasmids designed and constructed for CPS synthesis. The vector backbone used for cloning CPS gene clusters is a balanced lethal vector pG8R184, which contains low copy ori of pSC101, Asd+, and Kan-resistance marker and Ptrc promoter with T1T2 terminator. Sequences of each pG8R184 derivative include the capsule coding genes from one of the seven serotypes, pSC101 ori, Asd+, Kan-resistance marker, and T1T2 terminator. The diagrams of CPS structures and CPS biosynthesis genes are modified from Bentley et al. (3). “P#” designations refer to primer names used to construct the plasmids, and the purposes of primers are listed in SI Appendix, Table S1.
To test the expression and polymerization of S. pneumoniae CPSs in the RASV system, polysaccharides from plasmid-bearing χ11316 derivatives RASV-CPS2 (pG8R307), RASV-CPS3 (pG8R308), RASV-CPS5 (pG8R309), RASV-CPS6A (pG8R310), RASV-CPS9V (pG8R311), RASV-CPS14 (pG8R312), RASV-CPS18C (pG8R313), along with empty plasmid control RASV-184 (pG8R184), were extracted and detected by Western blotting with CPS-specific antibodies. All the reconstituted polysaccharides could be detected by antisera against S. pneumoniae CPS, as indicated in Fig. 2 and SI Appendix, Fig. S2. RASV-CPS 2, RASV-CPS3, and RASV-CPS14 showed clearly ladder-like patterns, when detected with antibodies against CPS2, -3, and -14. RASV-CPS5 produced only CPS5-specific high molecular weight (HMW) polysaccharides, while RASV-CPS9V and RASV-CPS18C expressed CPSs with low molecular weight (LMW) (SI Appendix, Fig. S1). The recombinant CPSs synthesized by RASV-CPS6A contained both LMW and medium molecular weight polysaccharides. However, there were also LMW signals detected in the wild-type Salmonella strain χ3761 in the CPS6A detection assay, which indicated the cross-reaction of the antisera against S. pneumoniae CPS6A.

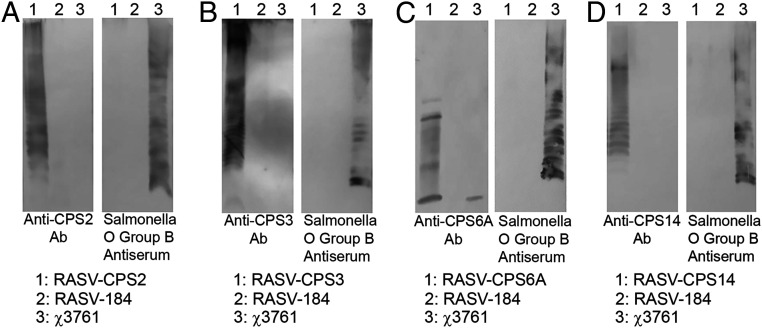
Immunoblot detection of S. pneumoniae CPS synthesis in RASV strains. Each plasmid carrying the capsule coding genes of one serotype was electroporated into the RASV strain χ11316. The transfected RASV strains were lysed and separated by 12% SDS/PAGE. The CPS profiles of serotype 2 (A), 3 (B), 6A (C), and 14 (D) were then detected using serotype-specific anti-CPS antibodies. The S. Typhimurium O-antigen was also identified by Western blot using the rabbit polyclonal Salmonella group B antisera. The χ11316 strain containing empty plasmid (RASV-CPS184) were included as the control sample. Blots of CPS profiles shown are representative of three independent experiments.
RASV-CPSs Strains Induced Significant CD4+ T Cell Immune Responses Against S. pneumoniae CPSs.
Flow cytometry assays were performed to investigate the CPS-specific CD4+ T-cell responses elicited by the RASV-CPS strains. Splenocytes were collected from mice at day 21 (7 d after secondary immunization) and stimulated with the type-specific CPSs for 8 h. After stimulation, cells were gated and analyzed as described in SI Appendix, Fig. S2. CD4+ T-cells from mice immunized with the corresponding RASV-CPS strains could stimulate significantly increased IFN-γ compared to those from PPV23-immunized mice (Fig. 3A and SI Appendix, Fig. S3A). In contrast, CD4+ T cells from PPV23-immunized mice induced more interleukin-4 (IL-4) than most of the RASV-CPS strains (Fig. 3B and SI Appendix, Fig. S3B). However, the IL-4 production levels showed no statistically difference between CD4+ T-cells from RASV-CPS18C–immunized mice and those from the PPV23 group when stimulated with the CPS18C.

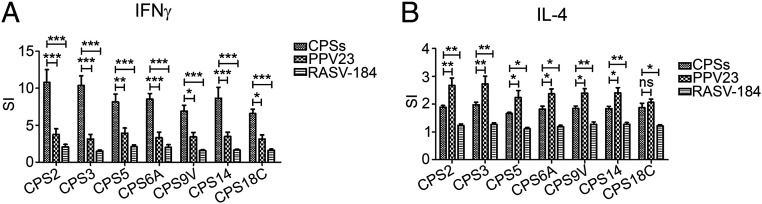
Cytokine expression in CD4+ T cells stimulated with S. pneumoniae CPSs ex vivo. Mice splenocytes were isolated on day 21 (7 d after secondary immunization) and stimulated with the corresponding serotype-specific CPS for 8 h. Fluorochrome-conjugated antibodies, including rat anti-mouse CD3-Alexa Fluor 700, CD4-FITC, IFN-γ–APC/Cy7, and IL-4–Alexa Fluor 647 were used for cell staining and analysis with flow cytometry. The cytokine stimulation index (SI) was expressed relative to the percentage of IFN-γ+ (A) or IL-4+ (B) cell subsets in the BSG-treated group. Data from two independent experiments were pooled and analyzed (n = 6). Mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant, two-way ANOVA and Bonferroni’s multiple comparison test.
RASV-CPS Strains Elicited CPS-Specific Humoral Immune Responses.
Groups of mice were vaccinated with the seven RASV-CPS strains using a two-dose schedule. The PPV23-, RASV-CPS184– (empty plasmid control), and BSG-treated mice were included as control groups. All the RASV-CPS strains stimulated similar or higher levels of serum anti-CPS IgG compared to the PPV23-immunized group (Fig. 4A). Moreover, the IgA levels in the vaginal secretions were significantly increased in the RASV-CPS–immunized groups (except for RASV-CPS9V and RASV-CPS18C) (Fig. 4B). The antibody response to RASV-CPS strains was dominated by IgG2a (Fig. 4C). In contrast, the PPV23 induced significantly higher levels of IgG1 production (Fig. 4D). The levels of type-specific anti-CPS antibodies varied among groups. Lower concentrations of serum type-specific IgG were induced by RASV-CPS5, RASV-CPS9V, and RASV-CPS18C compared to other RASV-CPSs.

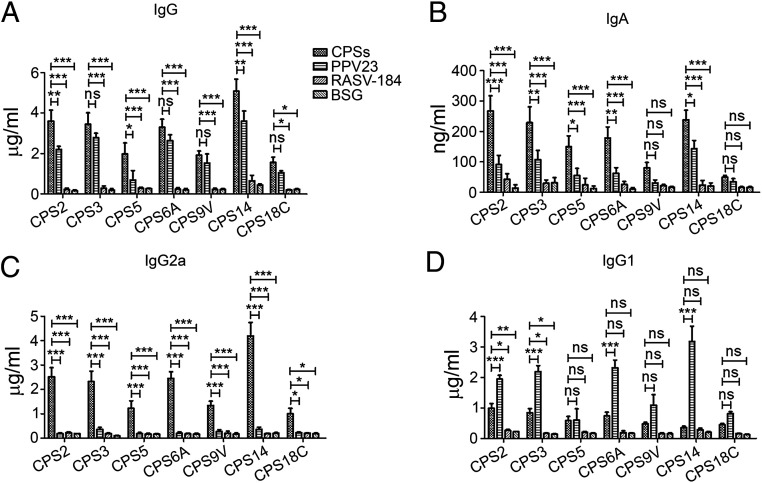
Humoral immune responses against S. pneumoniae CPSs. Serum (A, C, and D) and mucosal (B) antibody concentrations were measured by ELISA on day 28 (14 d after secondary immunization) (n = 12). The RASV-CPS strains stimulated comparable or higher levels of serum IgG, Ig2a, and mucosal IgA compared to the PPV23 group. Data were represented as mean ± SD *P < 0.05, **P < 0.01, ***P < 0.001, ns = not significant. Data were analyzed by using two-way ANOVA with Bonferroni’s multiple comparison test.
Serum Antibody Mediated Complement Deposition and OPA.
The complement system plays a vital role for host immunity to S. pneumoniae, especially in opsonophagocytosis of S. pneumoniae (5152–53). Serum antibodies mediated opsonization of S. pneumoniae activates the complement system, depositing C3 complement on the pneumococcal surface, which enables phagocytosis of the bacteria by phagocytic cells. Serum samples from mice immunized with RASV-CPS strains deposited similar or higher levels of C3 on the corresponding S. pneumoniae strains, compared to sera from PPV23-immunized mice (Fig. 5). Whereas the ability of sera to deposit C3 was reduced in RASV-184 (empty plasmid control) vaccinated mice, no C3 deposition was detected on bacteria for sera from BSG-treated group.

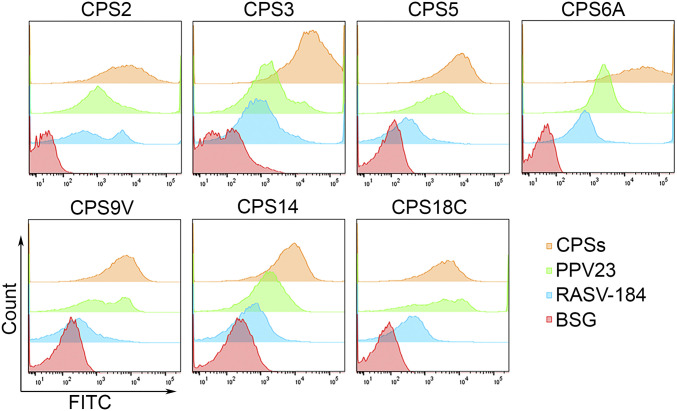
Complement deposition assays. Representative data of flow plots for antibody deposition on S. pneumoniae in 10% pooled antiserum from each group of RASV-CPSs immunized mice. PPV23, RASV-184 (empty plasmid control), and BSG vaccinated serum were included as controls.
OPA is suggested to be a good indicator of protection against pneumococcal infection (54, 55). All the RASV-CPSs elicited opsonizing antibodies that mediated the killing of pneumococci in a titer-dependent manner. Data in Fig. 6 indicated that sera from mice vaccinated with RASV-CPS2, RASV-CPS3, RASV-CPS5, RASV-CPS6A, and RASV-CPS14 showed increased or equivalent OPA compared to those from PPV23 treated mice. However, serum antibodies from RASV-CPS9V and RASV-CPS18C showed weaker responses than the PPV23 antibodies.

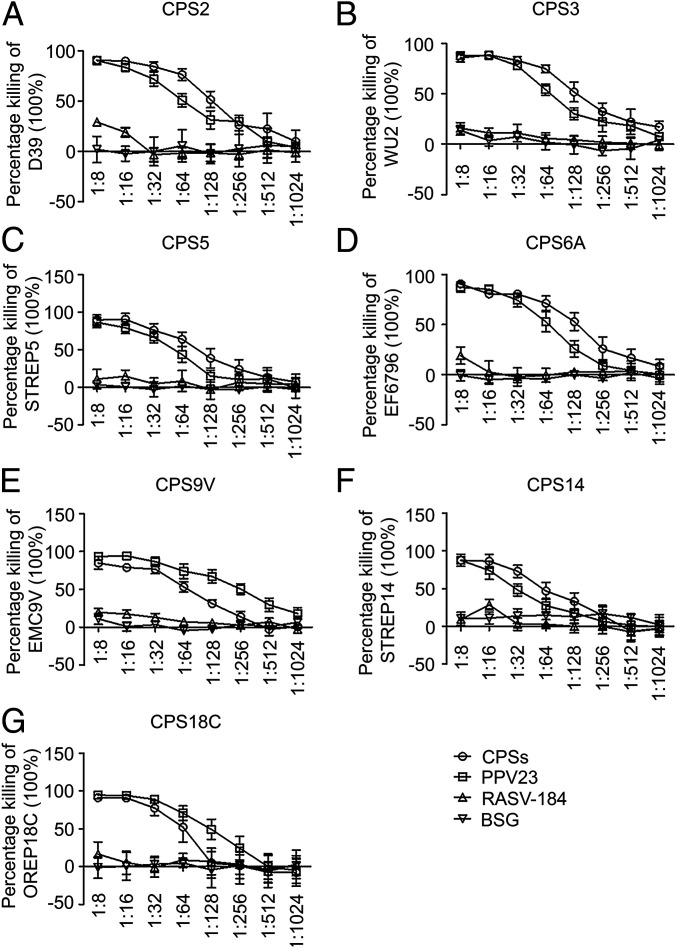
OPA in RASV-CPSs vaccinated mice. (A–G) OPAs were carried out using differentiated HL-60 cells and incubated with bacteria preopsonized RASV-CPS–specific antibodies or antibodies from control groups. Viable bacteria are counted after incubation at 37 °C for 45 min. Tested samples containing no serum (only bacteria, cells and complement) were included as negative control. The percentage of bacterial killing was determined relative to the negative control (contained only bacteria, cells and complement). Data shown are presented as mean ± SD.
Protection of Mice Vaccinated with RASV-CPS2 or RASV-CPS3 against Challenge of Wild-Type S. pneumoniae Strains.
To determine the protective efficacy of vaccinations with RASV-CPS2 and RASV-CPS3, survival experiments were performed using sepsis models of pneumococcal infection. The RASV-CPS2 and RASV-CPS3 vaccinated mice were challenged intraperitoneally with wild-type S. pneumoniae strains D39 (type 2) and WU (type 3), respectively. As shown in Fig. 7, immunization with either of these RASV-CPS strains resulted in significant protection against pneumococcal challenge compared to the RASV-CPS184– (empty plasmid control) and BSG-treated groups. However, there was no remarkable difference in protection between RASV-CPSs and PPV23-vaccinated mice.

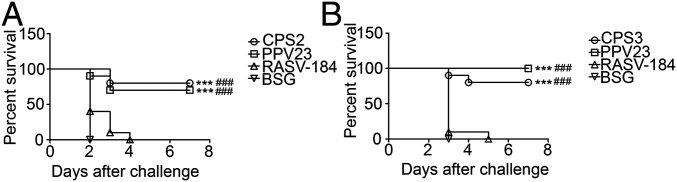
Survival of mice challenged with wild-type S. pneumoniae strains after immunization with RASV-CPSs. Female BALB/c mice were immunized orally twice at 2-wk intervals with 1 × 109 CFU of RASV-CPS2 (A) or RASV-CPS2 (B). Mice were challenged intraperitoneally with 2 × 104 CFU of D39 (A) or 2 × 104 CFU of WU2 (B) at 3 wk after the secondary immunization. Mice intraperitoneally immunized with PPV23 were included as positive control. The RASV-184–immunized or BSG-treated mice were used as negative control groups. Survival analysis was performed using the Kaplan–Meier method with the log-rank (Mantel–Cox) test. ***P < 0.001 compared to BSG, ###P < 0.001 compared to RASV-184.
Discussion
The RASV strains have been successfully tested for development of live vaccine vectors synthesizing heterologous polysaccharides from gram-negative bacteria, such as Shigella O-antigen (47). In this study, we used an RASV system to synthesize and deliver CPSs of seven serotypes from the gram-positive pathogen S. pneumoniae as live vaccines to immunize animals. CPSs biosynthesis in S. pneumoniae occurs through one of two methods, which includes the synthase-dependent pathway, whereby individual sugars are polymerized in a sequential, processive fashion, and the polymerase (Wzy)-dependent pathway whereby discrete repeat units are assembled in a polymerized fashion (7). Only CPS3 and CPS37 are synthesized through the synthase-dependent pathway. S. pneumoniae CPS 4, 8, 5, and 12F were successfully assembled in the gram-negative bacterium E. coli, which facilitated the development of bioconjugate vaccines using protein glycan-coupling technology (4, 56). Here, we further confirmed that S. pneumoniae CPS2, 3, 5, 6A, 9V, 14, and 18C could be successfully synthesized in the RASV system, which broadens the application of gram-negative bacteria for the synthesis of gram-positive CPSs. This study provided a strategy for pneumococcal vaccine development that uses RASV strains as live vaccine vectors to synthesize and deliver S. pneumoniae CPSs. In this way, the live vaccines serve as the factory for the manufacture of the protective antigen in the vaccinated animal host.
Polysaccharides attached to the OM of the RASV strains could elicit LPS-specific TD immune responses (47), which was deduced to be due to the presence of highly immunogenic proteins colocalized on the OM. Additionally, Salmonella exhibited excellent adjuvant characteristics and triggered robust antibody responses even in MyD88−/− and TRIF−/− mice (57). The immunization results from this study further confirmed that RASV delivered S. pneumoniae CPSs could successfully induce strong CPS-specific TD responses as well, since CD4+ T cells from RASV-CPS–immunized mice showed a remarkable increase in CPS-specific IFN-γ and IL-4 expression compared to the empty-plasmid control strain after ex vivo stimulation. Moreover, the RASV-CPS strains induced comparable or even higher levels of CPS-specific IgG than the PPV23 in mice. Meanwhile, the production of CPS-specific IFN-γ and IgG2a in the RASV-CPS group were superior to those in the PPV23 group, which is consistent with previous findings, in which the attenuated Salmonella strains induced Th1 biased immune responses (47, 58). The mice IgG2a corresponding to human IgG1, interacts with receptors for the Fc portion of immunoglobulins (FcR) on macrophages and NK cells, thereby mediates opsonophagocytosis and Ab-dependent cell-mediated cytotoxicity (ADCC) (59), providing protection against bacterial infections. Serum antibodies from the RASV-CPS–immunized mice exhibited stronger complement deposition and OPA (except for RASV-CPS9V and RASV-CPS18C) than those from the PPV23-treated mice, which further demonstrated that the RASV delivery system has great potential in inducing FcR-interacting antibodies against S. pneumoniae. The increased cytokine responses and complement deposition in the vector control group (RASV-184) compared to the BSG-treated group could be attribute to the adjuvant characteristics of Salmonella (57). Both wild-type and attenuated S. Typhimurium strains were able to cause self-limited infections in human (60, 61), so the RASV strains offer good prospect for human pneumococcal vaccine development to overcome the defect of traditional vaccines that induce mainly IgG2 (not the predominant FcR-interacting subtype) in humans.
As a live, attenuated vaccine vector, RASV is able to colonize mucosa-associated lymphoid tissues and even deeper lymphoid tissues, such as spleen, thereby eliciting strong immune responses in mucosal and systemic immune systems (62). Most of the orally administered RASV-CPS strains (except for RASV-CPS9V and RASV-CPS18C) induced remarkably increased levels of CPS-specific vaginal IgA. Another advantage of RASV strains delivering polysaccharide antigens is the capacity of live attenuated bacteria to stimulate global mucosal IgA responses (63). Thus, we observed that oral RASV strains delivering CPS of S. pneumoniae stimulated significant mucosal IgA responses against most of the CPS antigens, compared to relatively poor mucosal responses in mice injected with PPV23 (Fig. 5), which is essential for preventing S. pneumoniae infection on the first encountered nasal and lung mucosal surfaces.
Safety and immunogenicity are the two most important factors to be considered in developing live Salmonella-vectored vaccines for humans, especially in infants and young children. We have therefore taken multiple approaches to improve both immunogenicity and safety for future use (646566–67). There are strains proven to be totally safe and noninflammatory in newborn mice at the dose equal to 107 times the LD50 of the wild-type parent strain, which showed high levels of protection against S. pneumoniae challenge. The vaccine strains could maintain the ability to efficiently colonize lymphoid tissues, which may help the polysaccharide antigens to localize in the marginal zone and provide opportunities for induction of humoral immune responses. Therefore, the lack of CD21–C3d interaction in splenic marginal zone B cells in infants and young children could be overcome. However, although we showed that the strains were well tolerated in infant mice, the relevance of the mouse model to human infants is still questionable. Also of importance is the observation (64, 65) that maternal immunity to the RASV actually enhances the induction of protective immunity in neonatal and infant mice vaccinated with the same RASV. Thus, immune responses to the vector Salmonella might enhance rather than decrease vaccine efficacy when reused in the same host. Another concern for this study is that we immunized each group of mice with only one strain with a single capsule type, and compared the induced responses with the PPV23- (containing 23 capsule types) immunized group. The comparison method is not precise enough. Our future plan is to incorporate multiple serotypes of CPSs in one strain, which will facilitate the immunization of hosts with more types of CPSs and should be more reasonable when comparing with the PPV23 vaccine. It is also possible to administer mixtures of RASV strains delivering/displaying multiple protective antigens and this has been effective in inducing protective immunity (67).
The polysaccharide size exhibited a direct correlation with the immunogenicity of glycoconjugate vaccines (68, 69). In this study, we observed that the CPS9V and CPS18C delivered by RASV did not induce efficient CPS-specific vaginal IgA, which may be due to the pattern of CPS9V and CPS18C with LMW in the RASV strains (Fig. 4). The activities of complement deposition on S. pneumoniae and opsonophagocytosis driven by serum antibodies in the two groups appeared to be comparable or lower than those from the PPV23-treated groups. The results support a previous finding that the HMW polysaccharide provides more efficient protection against bacterial infection. One of the reasons for this was attributed to potent high-affinity antibodies induced by the HMW polysaccharide antigens (70). Wzz is a regulator, which can regulate length of polysaccharide during synthesizing polysaccharide with a Wzx/Wzy mixture (71). We are now evaluating various Wzz derived from different bacteria to generate longer CPS for the purpose of inducing high-level IgA and IgG antibodies against those CPS in which HMW forms are insufficiently synthesized. Other studies are under way to optimize the immunogenicity of RASV delivered CPS9V and CPS18C through genetic manipulation of CPS size or concentrated CPS by nanoparticles, such as OM vesicles.
In conclusion, we cloned gene clusters expressed to synthesize S. pneumoniae CPS2, 3, 5, 6A, 9V, 14, and 18C in the RASV strain. Using the RASV as a delivery system, the synthesized CPSs induced robust humoral and cell-mediated responses. The CPS-specific antisera could mediate efficient complement deposition on S. pneumoniae and opsonophagocytosis of the corresponding serotype of bacteria. Although mouse models for pneumococcal challenge with other serotypes were not successfully developed in our group, immunization with RASV-CPS2 or RASV-CPS3 could protect mice from fatal challenge by the wild-type S. pneumoniae strains D39 and WU2. In general, we report a vaccine development system that uses RASV strains for CPS synthesis and delivery to produce vaccines against S. pneumoniae infection. The application of using RASV strains for synthesis and delivery of polysaccharides could be expanded when further serotype CPSs of S. pneumoniae or other gram-positive bacteria are tractable for synthesis, and this system also paves a way to engineer and produce polysaccharide-based vaccines against other important pathogens. An economical advantage of this approach is that RASVs can be produced in fermenters and lyophilized in a thermo-stable form for reconstitution at time and place of needle-free mucosal administration.
Acknowledgements
We thank Dr. Kenneth L. Roland from Arizona State University for critical reading and editing. This work was supported by National Science Foundation of China Grant 31970874 and NIH Grant R01 AI112680.
Data Availability.
All study data are included in the article and supporting information.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
60
61
62
63
64
65
66
67
68
69
70
 Synthesis and delivery of Streptococcus pneumoniae capsular polysaccharides by recombinant attenuated Salmonella vaccines
Synthesis and delivery of Streptococcus pneumoniae capsular polysaccharides by recombinant attenuated Salmonella vaccines
