
These authors contributed equally to this work.
- Altmetric
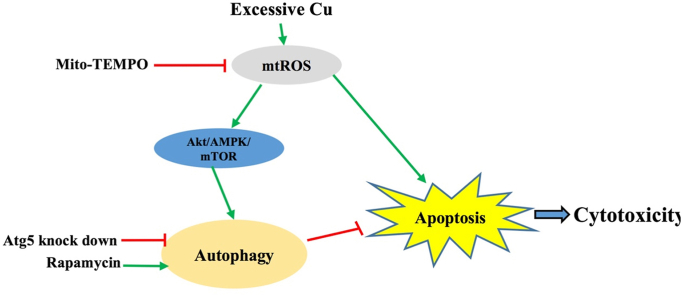
Copper (Cu) is a trace element necessary in animals as well as human beings. However, excessive Cu is toxic to immunocytes, but the precise mechanism is largely unclear so far. This work was conducted aiming to examine the Cu-mediated autophagy mechanism together with its role in Cu toxicology in RAW264.7 cells. Here, we demonstrated that CuSO4 reduced the cell viability depending on its dose. CuSO4 could obviously increase autophagy in RAW264.7 cells. According to the obtained results, CuSO4 induced autophagy through Akt/AMPK/mTOR pathway which characterized by down regulation of p-Akt (Ser473)/Akt, p-mTOR/mTOR, p-ULK1(Ser757)/ULK1 and subsequent up-regulation of p-AMPKα/AMPKα and p-ULK1(Ser555)/ULK1. Furthermore, CuSO4 significantly induced the production of mitochondrial reactive oxygen species (mtROS). In addition, CuSO4-mediated apoptosis and autophagy might be suppressed through suppressing mtROS generation by exposure to Mito-TEMPO. Intriguingly, autophagy promotion with rapamycin could decrease the apoptosis and the inhibition of autophagy with knock down Atg5 could enhance the apoptosis induced by CuSO4. Moreover, our results suggested that mtROS is the original cause in CuSO4-induced apoptosis and autophagy. Additionally, CuSO4 induced autophagy through mtROS-dependent Akt/AMPK/mTOR signalling pathwayin RAW264.7 cells. Moreover, autophagy activation might potentially generate a protection mechanism for improving CuSO4-induced RAW264.7 cell apoptosis.

•
mtROS is the original cause in CuSO4-induced apoptosis and autophagy.
•CuSO4 induced autophagy through mtROS-dependent Akt/AMPK/mTOR signalling pathway.
•Autophagy attenuates CuSO4-induced RAW264.7 cell apoptosis.
Introduction
Copper (Cu) has been identified as one of the essential metals in living body [1]. Cu acts as a critical cofactor of vital enzymes responsible for catalyzing electron transfer necessary for iron oxidation, cell respiration, neurotransmitter synthesis, pigment generation, antioxidant defense, peptide amidation, as well as connective tissue production [2]. Nonetheless, the disordered Cu homeostasis can be harmful, which may result in diseases like Wilson's disease (WD) and Menkes disease [2]. Recently, due to the widespread use of this metal, Cu is produced to the environment via industrial and agricultural activities and is recognized as the main polluting metal [3].
Cu accounts for an active transition metal for redox reaction, and oxidative stress (OS) is identified as a toxicity mechanism [4]. Patwa et al. [5] have demonstrated that treatment with 20 mg/kg copper sulfide (CuSO4) can increase reactivate oxygen species (ROS) production and decrease the levels of antioxidant enzymes in the liver of rats. In addition, CuSO4 induces down-regulation of antioxidant defense enzymes and up-regulation of caspase-3 in the brain of rats [6]. Our previous studies also have indicated that chronic exposure to excess Cu can lead to overproduction of ROS and decrease antioxidant functions, and ROS can promote apoptosis in livers of mice [7]. Results obtained by Wang et al. [8] have revealed that oxidative stress, cell cycle arrest and apoptosis induced by CuCl2 are the important toxicological mechanism in SGC-7901 cells.
Autophagy is recognized as the lysosomal decomposition-related pathway, which plays a vital role in cell development, differentiation, as well as homeostasis [9]. In the presence of stressful conditions, like OS, malignancy and serum deprivation, autophagy will be triggered for the sake of adapting to structural remodeling induced by the stresses, which is achieved through the synthesis of greater amounts of energy and nutrients, removal of misfolded and long-lived proteins in cells, and the elimination of redundant or injured organelles together with the invasive microorganisms [10]. It has shown that Cu can induce autophagy [[11], [12], [13]]. Fang et al. [14] have reported that CuSO4 promotes autophagy occurrence and ROS exert an important role in autophagy induced by CuSO4 in duck renal tubular epithelial cells. Besides, Liao et al. [15] have found that increased Cu consumption possibly induced autophagy of broiler chicken kidney via the AMPK-mTOR signal transduction pathway. Recently, investigation into the roles of autophagy has increased. Cell death as a result of various stressful conditions represents a complicated process under the joint control by apoptosis and autophagy; in some cases, the cross-talk of these two processes is quite intricate [16,17]. Many articles demonstrate the effect of Cu on inducing in vitro and in vivo [7,[18], [19], [20]].
Previous studies have demonstrated that over-exposure Cu can induce immunotoxicity including significant pathological damage, oxidative stress, inflammation and apoptosis in immune organs [[1], [2], [3], [4]]. Macrophages are the main immune cells in the immune system. In this study, cytotoxicity of CuSO4 in the RAW264.7 mouse monocytes was examined. Dysregulation or dysfunction of autophagy has been implicated in immune-related diseases. However, despite extensive studies on the roles of autophagy in Cu toxicity, little is known regarding its involvement in Cd-induced immunotoxic effects. Therefore, the present work aimed to examine the Cu toxicology mechanism in RAW264.7 cells including oxidative stress, autophagy, and apoptosis. Meanwhile, the relationship between autophagy and apoptosis in Cu toxicology is still unclear. The role (promotion or inhibition) of autophagy in CuSO4-induced apoptosis in RAW264.7 mouse monocytes is also be investigated.
Materials and methods
Cell culture and chemicals
The RAW264.7 monocytes were cultured within the DMEM (Gibco BRL, Grand Island, NY) that contained the 10% fetal bovine serum (FBS, heat-inactivated) and penicillin-streptomycin antibiotics (both 50 μg/ml), followed by incubation within the incubator under 37 °C and 5% CO2 conditions. The antioxidant Mito-TEMPO specific to mitochondria (CAS 1569257-94-8) was provided by Santa Cruz Biotechnology. CuSO4 (C1297) was provided by Sigma Aldrich Corporation. Bafilomycin A1 (B1793), 3-MA (M9281) and rapamycin (V900930) were bought from Sigma-Aldrich. MitoSOX (M36008) was provided by Invitrogen.
Antibodies
The antibodies such as rabbit anti-LC3B (2775S), rabbit anti-p62 (5114S), rabbit anti-AMPKα (5832), rabbit anti-p-AMPKα (2535), rabbit anti-Akt (9272), rabbit anti-p-Akt (4060), rabbit anti-mTOR (2972), rabbit anti-p-mTOR (2971), rabbit anti-ULK1 (8054), rabbit anti-p-ULK1(Ser757) (6888), rabbit anti-p-ULK1(Ser555) (5869), rabbit anti-Atg16 (8089T), rabbit anti-Atg7 (8558), rabbit anti-Atg3 (3415), rabbit anti-Beclin1 (3495), rabbit anti-Beclin1 (12,994), rabbit anti-cleaved-caspase-3 (9664), rabbit anti-cleaved-caspase-8 (8592), rabbit anti-cleaved-caspase-9 (9509) and rabbit anti-PARP (9548) were purchased from Cell Signaling Technology.
Cytotoxicity assessment
The MTT assay was conducted to assess the RAW264.7 cell viability. In brief, the complete culture medium was used to suspend RAW264.7 cells at 1 × 106 cells/ml, then each well of the 48-well plates was added with 150 μL cell suspension to incubate for 24 h. Afterwards, CuSO4 was added to treat the cells, later, 0.5 mg/ml MTT solution was used to incubate cells for 4 h. Thereafter, we used dimethyl sulfoxide to dissolve the resultant formazan crystals, and the microplate reader (PerkinElmer) was used to measure the absorbance (OD) value at 540 nm. All results were presented in the manner of mean ± SD from 3 independent assays.
Western blotting
Cells were treated with CuSO4 and lysed using the pre-chilled RIPA buffer. Then the cell lysate was subjected to 15 min centrifugation at 15,000g and 4 °C, and 12% SDS-PAGE was performed to separate the proteins, followed by transfer to the PVDF membranes. Later, 5% non-fat milk dissolved within TBST was used to block the membranes, followed by incubation with primary antibodies and HRP-labeled secondary antibodies in succession. Cells were then visualized by using the ECL detection kit (GE Healthcare, Piscataway, NJ, USA). Besides, the Bio-Rad ChemiDoc XRS + System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was utilized to visualize protein bands. The ImageJ2x software was utilized to determine the significant difference in protein expression.
Mitochondrial ROS detection
The ROS contents in mitochondria were measured using MitoSOX (M36008, Invitrogen). Briefly, CuSO4 was used to treat RAW264.7 cells for 24 h. After washing by PBS twice, cells were subjected to 10 min incubation using the 5 μM MitoSOX. Then, cells were rinsed by PBS twice, and the Synergy 2 multimode plate reader (BioTek Instruments) was used to determine fluorescence intensity. All results were standardized based on PBS controls.
Apoptosis analysis by flow cytometry
Cells were treated with CuSO4, and washed by the pre-chilled PBS (pH 7.2–7.4), followed by suspension within PBS to 1 × 106 cells/ml. Briefly, the 5 ml tube was added with 100 μL cell suspension, followed by. 15 min Annexin V- FITC (5 μL, Cat: 51-65874X, BD, USA) and PI (5 μL, Cat: 51-66211E, BD, USA) staining under 25 °C in dark. After the addition of 1 × binding buffer (400 μl), the flow cytometry (BD FACS Calibur) was used to assess stained cells in 40 min after they were prepared. In addition, the ModFit LT v3.0 software was employed to analyze the flow cytometric data.
Statistical analysis
Data were represented as mean ± SD. Significant differences between control and experimental groups were compared through one-way ANOVA by using SPSS17.0. A difference of P < 0.05 indicated statistical significance.
Results
CuSO4 induces cytotoxicity in RAW264.7 cells
To evaluate the RAW264.7 cytotoxicity of CuSO4, RAW264.7 were treated with CuSO4 (0, 10, 20, 50, 100, 200 and 500 μM) for 24 h. As presented in Fig. 1, CuSO4 inhibited cell viability, cell viability significantly (p < 0.05 or p < 0.01) decreased when the CuSO4 concentration excessed 50 μM.

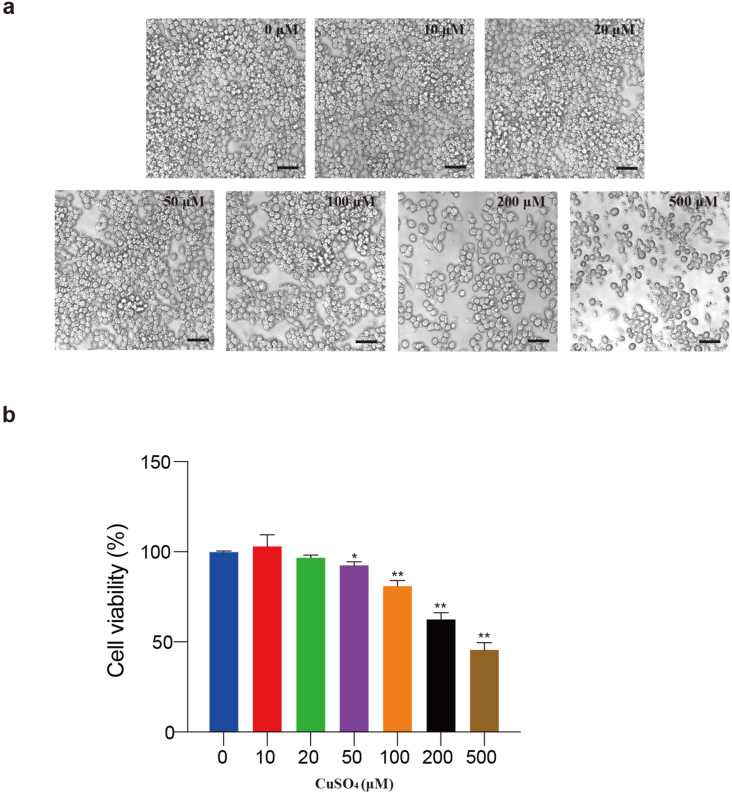
Cytotoxicity of CuSO4 in RAW264.7 cells. (a) Cells were treated with CuSO4 (0, 10, 20, 50, 100, 200 and 500 μM) for 24 h, and changes of cell numbers were observed by microscopy. Scale bar 50 μm. (b) Cell viability was analyzed by MTT assay. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group.
CuSO4 induces autophagy in RAW264.7 cells
Thus, the response of RAW264.7 cells to Cu-induced autophagy was analyzed. Firstly, we test the LC3 conversion and p62 degradation. In Fig. 2 a and b, LC3-II/LC3-I ratio markedly (p < 0.01) elevated in CuSO4-exposed groups relative to control. In addition, the p62 protein expression significantly (p < 0.01) decreased in CuSO4-treated cells.

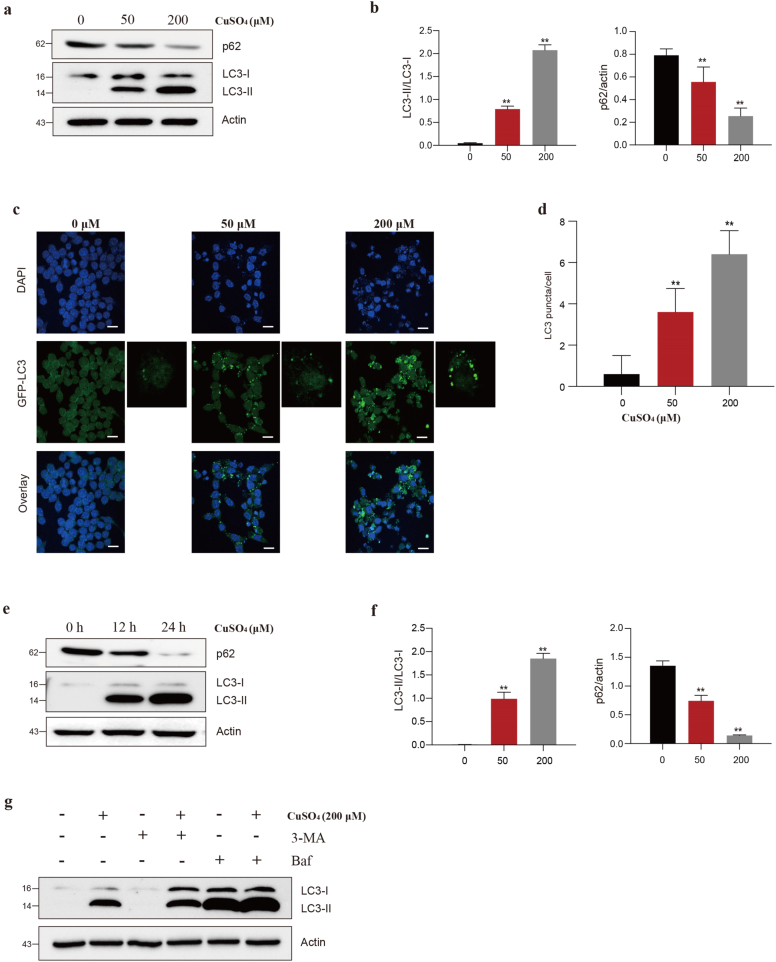
CuSO4 induces autophagy in RAW264.7 cells. (a and b) Cells were treated with CuSO4 (0, 50 and 200 μM) for 24 h, and immunoblotted for the whole cell lysis LC3 and p62 protein expression. (c and d) Cells were transiently transfected with GFP-LC3B and then treated with CuSO4 for 24 h. Fluorescence microscopy images show diffuse green staining in control cells, but GFP-LC3 B fluorescence puncta after CuSO4 exposure was observed. (e and f) Cells were treated with 200 μM CuSO4 for 0 h, 12 h and 24 h, and immunoblotted for the whole cell lysis LC3 and p62 protein expression. (g) Cells were pretreated with BaF1 (100 nM) 6 h and 3-MA (5 mM) for 3 h, and followed by CuSO4 (200 μM) treatment. After 12 h, the LC3 protein expression were detected. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In this regard, the green fluorescent protein (GFP)-microtubule-associated protein 1 light chain 3 B (LC3B) plasmid DNA was transfected into RAW264.7 cells. Even GFP fluorescence diffusion was seen in control groups. However, CuSO4 exposure caused GFP punctae, which suggested that LC3-II, the marker of autophagy, was recruited into autophagosomes (Fig. 3c and d). The autophagy inhibitors preventing autophagosomes from fusing with lysosomes, such as 3-MA and bafilomycin A1 (BaF1) involved in the autophagy flux, were used, which further verified the above results. BaF1 treatment prior to CuSO4 treatment led to the massively accumulated Cu-mediated LC3-II protein, but it made little difference to 3-MA, suggesting that RAW264.7 cells induced autophagy when they were challenged by CuSO4 (Fig. 2g).

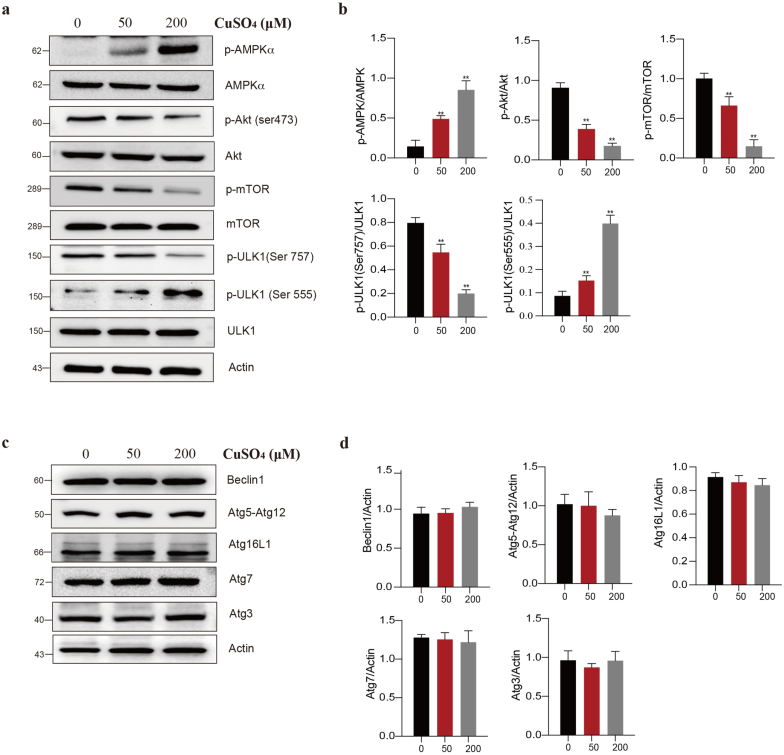
CuSO4 induces autophagy via Akt/AMPK/mTOR pathway in RAW264.7 cells. (a and b) Cells were treated with CuSO4 (0, 50 and 200 μM) for 24 h, and immunoblotted for the protein expression of Akt/AMPK/mTOR pathway. (c and d) Cells were treated with CuSO4 (0, 50 and 200 μM) for 24 h, and immunoblotted for the protein expression of autophagy-related protein. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group.
CuSO4 induces autophagy through the Akt/AMPK/mTOR signaling
In the presence of external stimuli, the Akt/AMPK/mTOR signal transduction pathway exerts a vital part in cell autophagy. For exploring the effect of Akt/AMPK/mTOR/ULK1 on the CuSO4-mediated RAW264.7 cells autophagy, this study performed Western blotting to measure the critical proteins related to the Akt/AMPK/mTOR signal transduction pathway. As demonstrated in Fig. 3a and b, CuSO4 exposure markedly up-regulated the protein levels of p-ULK1 (Ser555) and p-AMPKα within RAW264.7 cells, but down-regulated those of p-Akt (Ser473), p-ULK1 (Ser757) and p-mTOR (P < 0.01). Meanwhile, proteins involved in the autophagy flux were also detected. The results showed that the Beclin1, Atg5-Atg12, Atg7 Atg3 and Atg16L1 were not changed in CuSO4-treated RAW264.7 cells (Fig. 3c and d).
CuSO4 induces apoptosis in RAW264.7 cells
According to Fig. 4, CuSO4 treatments at 50 and 200 μM significantly elevated the apoptosis rates (P < 0.01). In addition, we also measured the expression of apoptotic proteins. The protein levels of cleaved-caspase-3/-8/-9 and cleaved-PARP significantly (P < 0.01) increased in CuSO4-treated RAW264.7 cells.

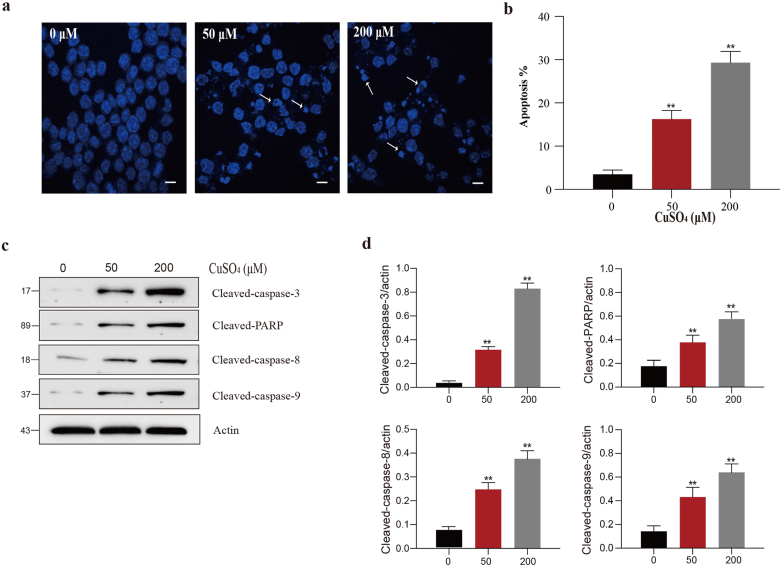
CuSO4 induces apoptosis in RAW264.7 cells. (a) RAW264.7 cells are treated with CuSO4 (0, 50 and 200 μM) for 24 h, and morphological changes (stain with DAPI) were observed by fluorescence microscopy. Arrows indicate apoptotic cells. Scale bar 50 μm. (b) Changes of apoptosis percentages in RAW264.7 cells after treated with CuSO4 for 24 h. (c and d) Immunoblot analysis of cleaved-caspase-8, cleaved-caspase-9, cleaved-caspase-3 and cleaved-PARP in lysates of the CuSO4-treated RAW264.7 cells. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group.
CuSO4 induces autophagy and apoptosis through mtROS in RAW264.7 cells
We detected the fluorescent intensity related to the CuSO4-induced mtROS level (Fig. 5a). Following CuSO4 treatment, mtROS level markedly elevated (P < 0.01) (Fig. 5b).

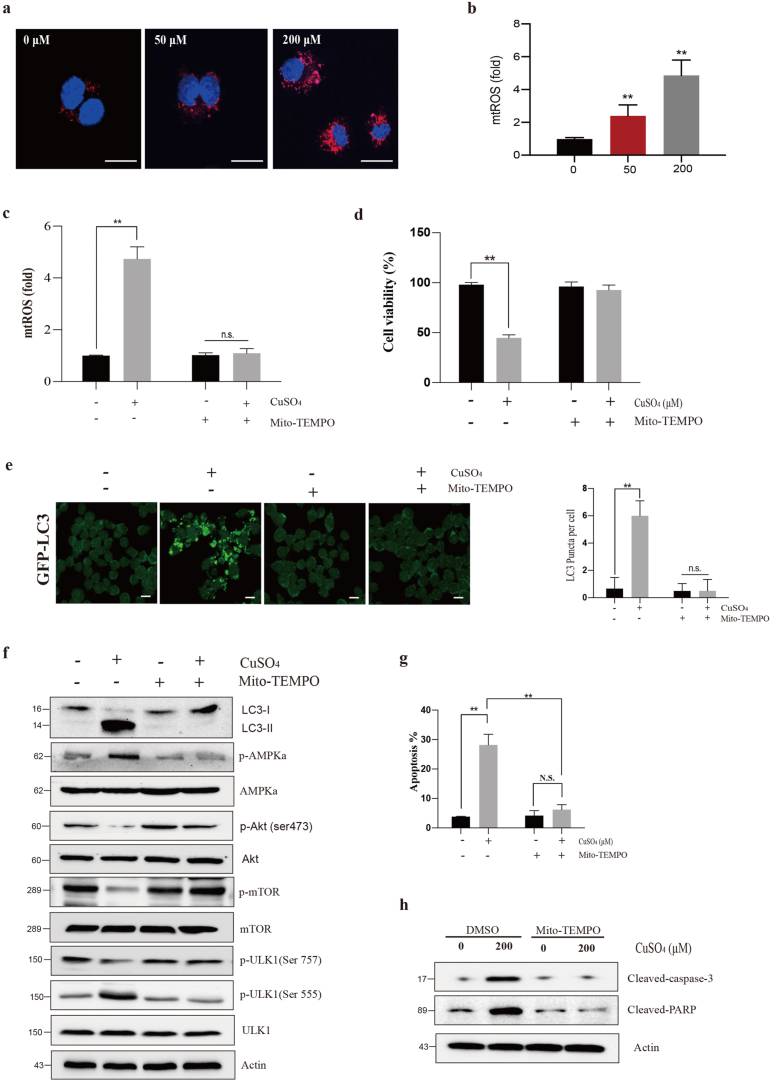
CuSO4 induces autophagy and apoptosis through mtROS. (a and b) Relative mtROS amounts determined by MitoSOX-red staining of CuSO4-primed RAW264.7 cells. Scale bar 50 μm. (c) mtROS changes in CuSO4-treated (200 μM, 24 h) RAW264.7 cells in the presence/absence of Mito-TEMPO (500 μM, 1 h) pre-treatment. Changes of cell viability (d), GFP-LC3 fluorescence puncta (e), autophagy proteins (f), apoptosis (g) and apoptotic proteins (h) in CuSO4-treated (200 μM, 24 h) RAW264.7 cells in the presence/absence of Mito-TEMPO (500 μM, 1 h) pre-treatment. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To determine the role of mtROS in CuSO4-induced autophagy and apoptosis, we used Mito-TEMPO to suppress mtTOS generation. It was illustrated from Fig. 5c that, pretreatment with Mito-TEMPO suppressed the CuSO4-mediated mtROS generation (Fig. 6c), while suppressing ROS enhanced the CuSO4-mediated cell viability. Mito-TEMPO attenuated autophagy induced by CuSO4 (Figure e and f). Mito-TEMPO also can suppress apoptosis induced by CuSO4 (Figure g and h). The related protein of autophagy and apoptosis including LC3-II/LC3-I, p-AMPKα, p-Akt (Ser473), p-mTOR, p-ULK1 (Ser757), p-ULK1 (Ser555), cleaved-caspase-3 and cleaved-PARP was dramatically reversed by Mito-TEMPO. According to these findings, CuSO4-induced autophagy and apoptosis within RAW264.7 cells may be induced by mtROS.

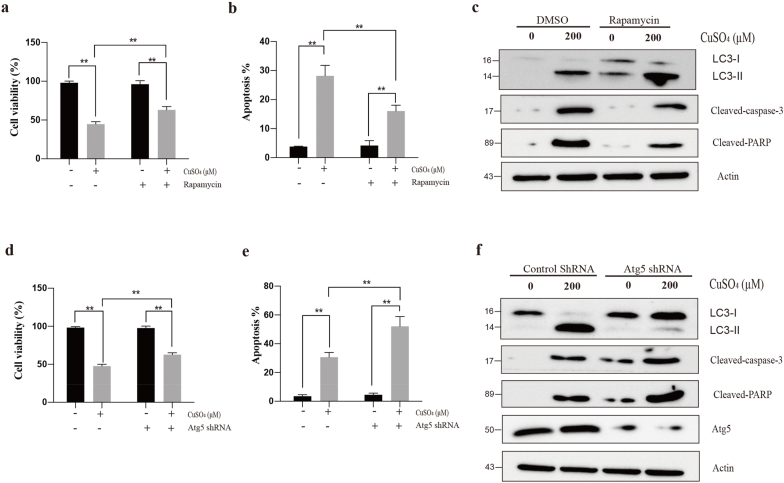
Autophagy inhibits apoptosis in Raw264.7 cells. Cells were pretreated with rapamycin (100 nM) for 2 h and followed by CuSO4 (200 μM) treatment for 24 h, changes of cell viability (a), apoptosis (b) and autophagy and apoptotic proteins expression (c) were detected. After transfection with Atg5 shRNA cultured for 24 h, and transfection efficiency was evaluated by immunoblotting for Atg5 protein. Cells transfected with control shRNA and ATG5 shRNA exposed with CuSO4 (200 μM) for 24 h, changes of cell viability (d), apoptosis (e) and autophagy and apoptotic protein expression (f) were detected. Data are presented with the means ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group.
Autophagy inhibits apoptosis in Raw264.7 cells
More and more studies have indicated the important roles of autophagy, including cytoprotection and cytotoxicity. For investigating the relationship of CuSO4-induced autophagy with the apoptosis of RAW264.7 cells, we used an autophagy promotor (rapamycin) to increase autophagy induced by CuSO4. As a result, combining CuSO4 with rapamycin markedly increase cell viability and inhibit apoptosis (Fig. 6a and b). Meanwhile, LC3-II/LC3-I, the autophagic protein, was up-regulated, and apoptosis-related protein cleaved-caspase-3 and cleaved-PARP were decreased in comparison with those in CuSO4-treated RAW264.7 cells (Fig. 6c). On the contrary, down-regulation of cell viability and up-regulation of apoptosis were observed in the combination of CuSO4 and autophagy inhibition (Atg5 knock down) group (Fig. 6d and e). In comparison with CuSO4 treatment group, CuSO4 treatment combined with autophagy suppression (Atg5 deletion) markedly suppressed the expression of autophagic protein LC3-II/LC3-I while increasing that of apoptotic proteins cleaved-PARP and cleaved-caspase-3, as revealed by Western blotting analysis (Fig. 6f).
Discussion
More attention should be paid to macrophages in terms of toxicology because they have diverse activities. Results in this work suggested that CuSO4 showed high toxicity to macrophages (RAW264.7 cells) in vitro. Our data are in consistence with the findings of Triboulet et al. [21], who reported that copper nanoparticles can alter macrophage function and toxic to macrophage. Then, the molecular mechanism of Cu-induced RAW264.7 cells toxicology is explored, including oxidative stress, autophagy, and apoptosis.
Autophagy, a form of cell decomposition, is related to material transfer from cytoplasm to lysosomes [17]. It has been reported that Cu is a novel stimulator of autophagy [11,22]. However, it is still unknown about the involvement of autophagy in the Cu-induced toxicity to RAW264.7 cells. In the case of autophagy, LC3 will be increasingly cleaved into LC3-I and LC3-II, while LC3-II relates to the amount of autophagosomes formed. The findings showed that CuSO4 overexposure can increase the LC3 puncta and ratio of LC3-II/LC3–I as well as decrease p62 protein expression, which indicated that autophagy level is the up-regulation in RAW264.7 cells. In consistence with our results, Cu compounds can increase autophagy in male germ cells [19] and in the kidney as well as brain of broiler chickens [15,23]. Furthermore, our results demonstrated that down regulation of p-Akt (Ser473)/Akt, p-mTOR/mTOR p-ULK1 (Ser757)/ULK1 and subsequent up-regulation of p-AMPKα/AMPK and p-ULK1 (Ser555)/ULK1 were observed in CuSO4-treated RAW264.7 cells. The canonical PI3K/Akt-mTOR signal transduction pathway has been identified to be the critical factor to adversely regulate autophagosome formation [24]. The PI3K/Akt signal transduction pathway controls mTOR activity [25]. Moreover, the activation of AMPK is recently suggested to result in autophagy by negatively modulating mTOR [26]. Besides, AMPK can directly phosphorylate ULK1 to induce autophagy [26]. These results demonstrate that CuSO4 induces autophagy through Akt/AMPK/mTOR/ULK1 signaling. The above results conform to findings from Liao et al. [15], in which CuSO4 induces autophagy in kidney of broiler chickens through activation of AMPK-mTOR pathway. Meanwhile, proteins involved in the autophagy flux also were detected. According to the results, Beclin1, Atg5-Atg12, Atg7 Atg3 and Atg16L1 were not changed in CuSO4-treated RAW264.7 cells. In contrary, the result of Fang et al. [14] showed that CuSO4 treatment can increase Beclin-1, ATG7, ATG5, ATG3 expression in duck renal tubular epithelial cells. The inconsistency of the findings is possibly a result of cell type. Autophagy usually occurs concurrently with apoptosis, and it represents the well-aligned and autonomous cell death process, which mainly functions to maintain the balance of certain cell populations within the tissues. Findings in the present work revealed that CuSO4 treatment promoted RAW264.7 cell apoptosis while up-regulating cleaved-caspase-3/-8/-9 and cleaved-PARP.
It has confirmed oxidative stress in the basic mechanism of Cu toxicology [4]. Here, we find that CuSO4 treatment increased the mtROS production. Many studies indicate that ROS synthesis induces autophagy and apoptosis [17]. However, how mtROS affects the CuSO4-induced autophagy and apoptosis of RAW264.7 cells has not been reported. Afterwards, this study examined whether mtROS production was an upstream event in the CuSO4-mediated autophagy and apoptosis. Mito-TEMPO (mtROS scavenger) treatment evidently mitigated apoptosis, autophagy and mtROS formation induced by CuSO4 in RAW264.7 cells. Besides, Mito-TEMPO pretreatment abolished the effects of CuSO4 treatment on the Akt/AMPK/mTOR/ULK1 signal transduction pathway. Consistently, Fang and colleagues [14] discovered that Cu activated the ROS/HO-1/NQO1 signal transduction pathway to cause autophagy. Collectively, findings in this work indicated the vital part of mtROS in the CuSO4-mediated autophagy and apoptosis. Additionally, the mtROS-induced interference with the Akt/AMPK/mTOR/ulk1 signal transduction pathway was related to the CuSO4-mediated RAW264.7 cell apoptosis.
Autophagy plays a role of a double-edged sword, which can regulate cell death and survival. The autophagy level is low under physiological condition, and this contributes to cell survival. In the presence of some chemicals, autophagy can be significantly activated, resulting in cell death. It has been widely indicated that apoptosis and autophagy are both critical for cell death. The association of autophagy with apoptosis is generally categorized as three types, namely, interdependence, mutual transformation and mutual antagonism. Besides, autophagy may interact with apoptosis in diverse manners. Autophagy acts as an antagonist to resist apoptosis and facilitate cell apoptosis at diverse conditions. For investigating how autophagy affected RAW264.7 apoptosis induced by CuSO4, autophagy promotion (rapamycin) and inhibition (Atg5 knock down) were used. Noteworthily, findings in this work indicated that, rapamycin pretreatment suppressed the CuSO4-mediated RAW264.7 cell apoptosis; by contrast, Atg5 deletion had the opposite effect. These observations suggested the possible cytoprotective effect of autophagy on CuSO4-mediated RAW264.7 cell apoptosis.
Conclusions
To conclude, our results suggested that mtROS is the original cause in CuSO4-induced apoptosis and autophagy. In addition, CuSO4 induced autophagy through mtROS-dependent Akt/AMPK/mTOR signalling pathwayin RAW264.7 cells. Moreover, autophagy activation may protect RAW264.7 cells from CuSO4-induced apoptosis.
Author contributions
Hongrui Guo, Qin Luo and Yuzhen Song designed the experiments. Qin Luo, Yuzhen Song, Jingjing Kang, Yuchen Wu and Fengsun Wu carried out the experiments. Qin Luo, Yuzhen Song, Yueqin Li, Qing Dong, Jun Wang and Chao Song analyzed and interpreted the data. Hongrui Guo, Qin Luo and Yuzhen Song wrote and revised the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
References
1
2
3
4
5
6
7
8
10
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
Acknowledgments
The study was supported by Sichuan Science and Technology Program (2020YJ0113), Doctoral Science Foundation of Henan University of Animal Husbandry and Economy (24030105; 24030044) and Scientific Research and Innovation Fund of Henan University of Animal Husbandry and Economy (M4030110).
 mtROS-mediated Akt/AMPK/mTOR pathway was involved in Copper-induced autophagy and it attenuates Copper-induced apoptosis in RAW264.7 mouse monocytes
mtROS-mediated Akt/AMPK/mTOR pathway was involved in Copper-induced autophagy and it attenuates Copper-induced apoptosis in RAW264.7 mouse monocytes