
Edited by Paul E. Turner, Yale University, New Haven, CT, and approved February 17, 2021 (received for review July 3, 2020)
Author contributions: L.W.A., R.B.-S., L.C.K., G.K., A.B., E.H., and M.B. designed research; L.W.A., R.B.-S., and M.B. performed research; L.W.A. and R.B.-S. analyzed data; and L.W.A., R.B.-S., L.C.K., E.H., and M.B. wrote the paper.
- Altmetric
In many important human diseases, a large proportion of infections do not lead to symptoms, but we typically have little understanding of the cause, impact, and dynamics of such inapparent infections. Our data from the Nicaragua Pediatric Cohort show that the dynamics of both symptomatic and inapparent infections are complex, such that in some years there are many symptomatic cases, but in others the cases are predominantly inapparent. Using epidemiologic models, we show that boosting the immune system is a parsimonious mechanism that can explain these patterns. This is important because it suggests that boosting is an important to the epidemiology of dengue and moreover that immune mechanisms can lead to complex inapparent infection dynamics.
Dengue is the most prevalent arboviral disease worldwide, and the four dengue virus (DENV) serotypes circulate endemically in many tropical and subtropical regions. Numerous studies have shown that the majority of DENV infections are inapparent, and that the ratio of inapparent to symptomatic infections (I/S) fluctuates substantially year-to-year. For example, in the ongoing Pediatric Dengue Cohort Study (PDCS) in Nicaragua, which was established in 2004, the I/S ratio has varied from 16.5:1 in 2006–2007 to 1.2:1 in 2009–2010. However, the mechanisms explaining these large fluctuations are not well understood. We hypothesized that in dengue-endemic areas, frequent boosting (i.e., exposures to DENV that do not lead to extensive viremia and result in a less than fourfold rise in antibody titers) of the immune response can be protective against symptomatic disease, and this can explain fluctuating I/S ratios. We formulate mechanistic epidemiologic models to examine the epidemiologic effects of protective homologous and heterologous boosting of the antibody response in preventing subsequent symptomatic DENV infection. We show that models that include frequent boosts that protect against symptomatic disease can recover the fluctuations in the I/S ratio that we observe, whereas a classic model without boosting cannot. Furthermore, we show that a boosting model can recover the inverse relationship between the number of symptomatic cases and the I/S ratio observed in the PDCS. These results highlight the importance of robust dengue control efforts, as intermediate dengue control may have the potential to decrease the protective effects of boosting.
Dengue virus (DENV) is the most prevalent vector-borne viral disease of humans, with recent estimates of around 105 million individuals infected annually (1). It comprises four antigenically distinct serotypes, DENV-1 to -4 (2), and is transmitted to humans by Aedes aegypti and, less frequently, Aedes albopictus mosquitoes (34–5). While most studies have focused on symptomatic infections, epidemiologic studies have shown that for dengue, the majority of infections are inapparent (3, 5), that is, infections that do not cause detected disease but result in a fourfold or greater rise in antibody titers. However, large fluctuations in annual dengue inapparent:symptomatic (I/S) ratios have been documented worldwide (5). For example, cohort studies able to detect inapparent DENV infections in Nicaragua (678–9), Peru (10), and Thailand (11) have shown that the I/S ratio of DENV infections ranges widely year to year. In the Pediatric Dengue Cohort Study (PDCS) in Nicaragua, the longest running dengue cohort study, the I/S ratio has varied widely, from 16.5:1 in 2006–2007 (7) to 1.2:1 in 2009–2010 (9). We currently do not understand the drivers of these fluctuations; however, we do know that potential extrinsic drivers, such as differences in replication rates of the predominating serotype, cannot explain them (5). Gaining a mechanistic understanding of these fluctuations in the I/S ratio is likely to be critical for understanding potential drivers of epidemic potential and severe dengue disease and for enacting effective control policies.
Extensive research has been conducted into the causes of DENV infection and disease, and there is now some evidence to suggest that immune interactions among viruses and strains may be responsible for fluctuating patterns (1213–14). In particular, this extensive body of work has shown that severe disease occurs due to immunopathology (4, 15, 16). The most important risk factor for severe dengue disease is secondary heterologous infections (4), due in part to a phenomenon called antibody-dependent enhancement (ADE), in which antibodies from a first infection cross-react with virus from a secondary infection, leading to incomplete neutralization. The resulting partially neutralized immune complexes enhance infection into Fc receptor-bearing cells (17). Low to intermediate titers of cross-reactive anti-DENV antibodies have been shown to enhance subsequent dengue disease severity in human populations (15, 18, 19). However, neutralizing antibody titers are thought to be protective against dengue disease, and a recent study showed that higher preinfection neutralizing antibody titers correlated with lower probability of symptomatic infection in children in the PDCS (20). Importantly, individuals with inapparent heterologous secondary infections had significantly higher preinfection titers than individuals with symptomatic heterologous secondary infections (2021–22), providing direct evidence that preinfection neutralizing antibody titer is an important determinant of disease outcome. Therefore, it is plausible that the variability in preinfection antibody titer could explain fluctuations in I/S ratios.
Recent work has suggested that frequent exposure to DENV may boost the immune response and result in modest increases in neutralizing antibody titer (20), which in turn may protect individuals against symptomatic infection. Evidence for boosting comes from analysis of neutralizing antibodies following primary infection. Here we have defined boosting as exposures to DENV that do not lead to extensive viremia and that result in a less than fourfold rise in antibody titers. Traditionally, the temporary period of cross-protection against heterotypic serotypes following a primary infection is explained by waning cross-reactive antibodies, resulting in a decrease in neutralizing antibody titers (23). However, an analysis of neutralizing antibody titers from the PDCS showed that neutralizing antibody titers did not decrease in the time between primary and secondary DENV infection, but in fact increased marginally (20). A comparable trend was seen in Thailand (24) and in a long-term hospital-based study in Nicaragua (25, 26). The increase in neutralizing antibody titer may be due to immune boosts (20), suggesting that children may be regularly exposed to DENV without experiencing symptoms or meeting the criteria for inapparent infection. There is also evidence of a phenomenon similar to boosting in a human vaccine study (27) and in a study in nonhuman primates (28), where in both cases there was initial exposure that resulted in viremia and seroconversion and a second challenge that did not result in viremia but did result in increased antibody titers. Clearly, in years with a high incidence of dengue, we would expect boosting to occur more frequently, and thus in the years immediately following high dengue incidence, we would expect fewer symptomatic infections, as individuals would be protected against symptomatic infection due to boosts (5).
Here we used mathematical models to determine which mechanisms can recover the fluctuations in the I/S ratio in DENV infections. Since our aim was to gain a conceptual qualitative understanding of the role of the impact of a range of mechanisms, we took the classic simplifying approach of not explicitly modeling the mosquito population dynamics. All models are adapted from existing dengue epidemiologic models (12, 29) and include immunity against homologous reinfection, a period of cross-protection following infection, and seasonality. For simplicity, we model the whole population but also present results from a model of the pediatric cohort from which our data are taken. With only these factors, a year-to-year variation in case number is seen, but not a variation in I/S ratio. This model was first modified to include the basic assumption that antibody titer decreases with time since infection and is predictive of infection outcome (20), to evaluate whether I/S fluctuations can be recovered by shorter periods of cross-protection between primary infections and secondary heterotypic infections for inapparent secondary infections than for symptomatic secondary infections, as previously suggested (6, 23).
We then explored whether I/S ratio differences can be explained by protection against symptomatic disease due to boosting of the immune response. We define boosts as exposures to homotypic or heterotypic DENV serotypes that “boost” the immune response and result in a modest rise in antibody titers (less than fourfold rise, below the threshold of classification as an inapparent infection), possibly due to limited viremia. It is important to note that with boosting, the antibody titer that we measure might not fall. Although it was previously thought that homologous DENV infection confers lifelong immunity against the infecting serotype (30), recent work has shown that homologous DENV reinfections do occur (31). We hypothesize that a boost in antibody titer can protect an individual during subsequent infections, resulting in the development of inapparent infection instead of symptomatic infection. We show that a boosting model can recover the fluctuations in the I/S ratio, recover the inverse relationship between the number of symptomatic cases and the I/S ratio in the PDCS, and recover a positive relationship between the I/S ratio in a given year and the number of cases in the previous year, as has been previously noted (5, 11). These models suggest that boosts may be occurring frequently in endemic areas and need to be considered when constructing effective dengue control policies.
Results
Large Fluctuations in I/S Ratio in the PDCS.
Fig. 1 shows the data from the PDCS study, including the annual number of symptomatic cases (Fig. 1A) and the annual number of inapparent cases. The I/S ratio from 2004 to 2015 is shown in Fig. 1B, illustrating the wide fluctuations in inapparent to symptomatic infections, from 16.5:1 in 2006–2007 to 1.2:1 in 2009–2010. An inverse relationship is observed between the number of symptomatic cases in a given year and the I/S ratio of that year (Fig. 1D). The number of detected symptomatic infections is similar for primary and secondary infections; however, more secondary inapparent infections were detected compared to primary inapparent infections (Fig. 1B). Interestingly, data from the PDCS show that in years with high numbers of symptomatic cases, the I/S ratio was low (Fig. 1D). This relationship is more pronounced for secondary infections compared to primary infections. For the purpose of this study, we only examined dengue dynamics before the 2016 Zika outbreak, which may have had a major impact on dengue transmission and will be explored elsewhere.

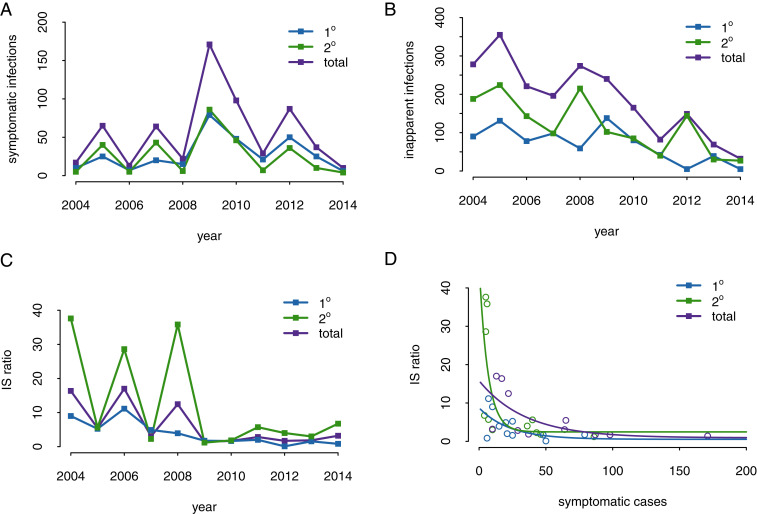
Symptomatic and inapparent DENV infections in the Nicaraguan PDCS, 2004 to 2015. (A) Annual number of symptomatic infections showing primary 1°, secondary 2°, and total infections. (B) Annual number of inapparent infections. (C) Annual fluctuations in I/S ratio. (D) Inverse relationship between annual number of symptomatic infections and I/S ratio with the slope of the nonlinear least squares fits all negative (−0.04369 primary, −0.155 secondary, and −0.02881 total).
Models without Boosting Cannot Recover the Large Fluctuations Observed in the I/S Ratio.
We first consider a null model, referred to as model 1 (SI Appendix), which was adapted from an existing dengue epidemiologic model (29). In this model, we assume that immunity against homologous reinfection is permanent, but that protection against a heterologous infection is temporary (we assume 2.2 y, but shorter periods give consistent results). We assume that a fraction, p, of infections is symptomatic, and the remaining infections are inapparent. We incorporate seasonality stochastically by allowing the amplitude of the seasonality to vary between peaks, using a similar approach as described by Adams et al. (12). All model equations and parameter values can be found in SI Appendix. It is clear that this model cannot recover any fluctuations in the I/S ratio (Fig. 2 C and E), which makes sense because there is no mechanism for the fraction, p, of symptomatic infections to change. As such, the baseline model demonstrates why this variation in I/S ratios is an interesting epidemiologic phenomenon that needs to be explained.

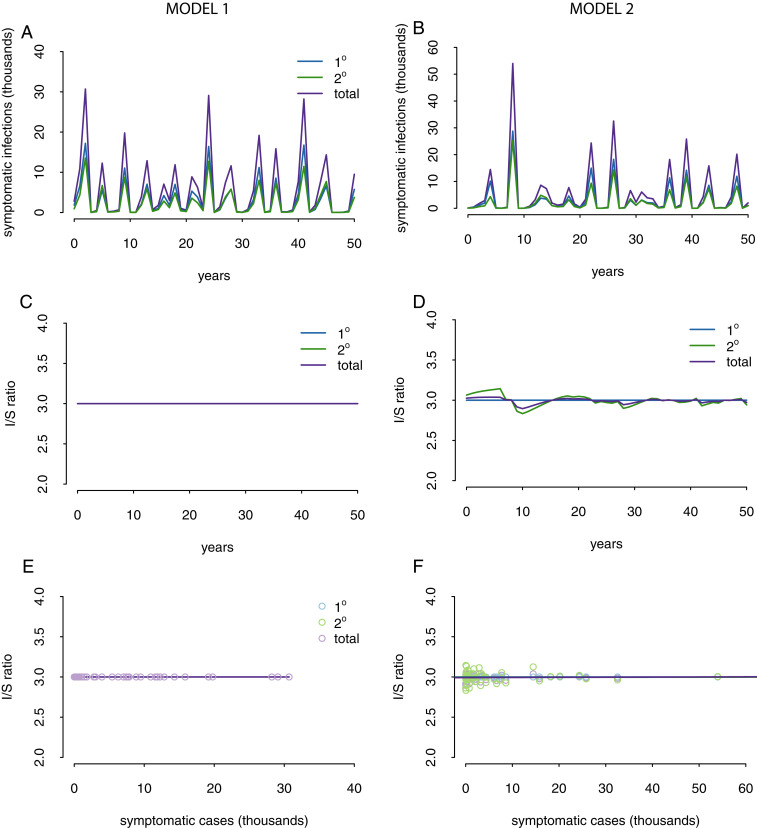
Null models cannot recover large fluctuations in the I/S ratio. (A, C, and E) Model 1. (B, D, and F) Model 2. (A and B) Simulated symptomatic infections. (C and D) Simulated I/S ratios. (E and F) Simulated relationship between simulated annual symptomatic infections and simulated I/S ratios.
We next modified model 1 to allow for differences in the period of cross-protection between primary and secondary infections. An analysis of the PDCS showed that the interval between consecutive infections was shorter if the second infection was inapparent rather than symptomatic (6). These results were insensitive to whether the first infection was inapparent or symptomatic (6). We incorporated into model 1 a shorter period of cross-protection prior to inapparent infections than symptomatic infections (SI Appendix). This model was able to recover small fluctuations in the I/S ratio in secondary infections, although not the general trend of higher I/S ratios in years with low numbers of symptomatic infections (Fig. 2 B, D, and F). The magnitude of the I/S fluctuations was small and could not recover the ranges in I/S ratios seen in the PDCS data (Fig. 1). These results are robust to different values for periods of cross-protection (from 6 mo to 3 y).
Boosting Can Explain Patterns in I/S Ratios.
We hypothesize that homotypic or heterotypic boosts occur frequently, resulting in modest rises in antibody titer. Such an increase in antibody titer can move an individual from a preinfection titer that results in a symptomatic infection to a preinfection titer that results in an inapparent infection. We make the baseline assumption that antibody titers decay following a primary infection due to waning of cross-reactive antibodies (23), and, following Katzelnick et al. (20), we assume that a higher preantibody titer is associated with an inapparent secondary infection rather than a symptomatic infection.
Fig. 1 shows that modest fluctuations in primary I/S ratios also occur. A recent study has shown that for some virologically confirmed but atypical dengue cases that resulted in a lower viral load, the antibody dynamics following a second infection resembled a primary infection (32). This suggests that a priming of the immune response due to modest DENV exposure can occur before a true detected DENV infection. In that study, antibody dynamics were not analyzed based on symptomatic or inapparent infection outcome. We hypothesize that this modest DENV exposure can prime the immune response, and that a subsequent “primary” infection is more likely to result in an inapparent rather than a symptomatic infection.
Model 3 combines two key assumptions: 1) there are differences in periods of cross-protection (model 2) and 2) there is the possibility of boosting/priming. Simulations are shown in Fig. 3 , and model equations are provided in SI Appendix. This model can recover I/S fluctuations in both primary and secondary infections, with the larger fluctuations in secondary infection I/S ratios relative to primary infection I/S ratios (Fig. 3C). Furthermore, this model can recover the inverse relationship between annual number of symptomatic infections and primary, secondary, and total I/S ratios. This suggests that in years with few symptomatic DENV infections, we would expect a large number of inapparent infections, whereas in years with large numbers of symptomatic infections, we would expect fewer numbers of inapparent infections.

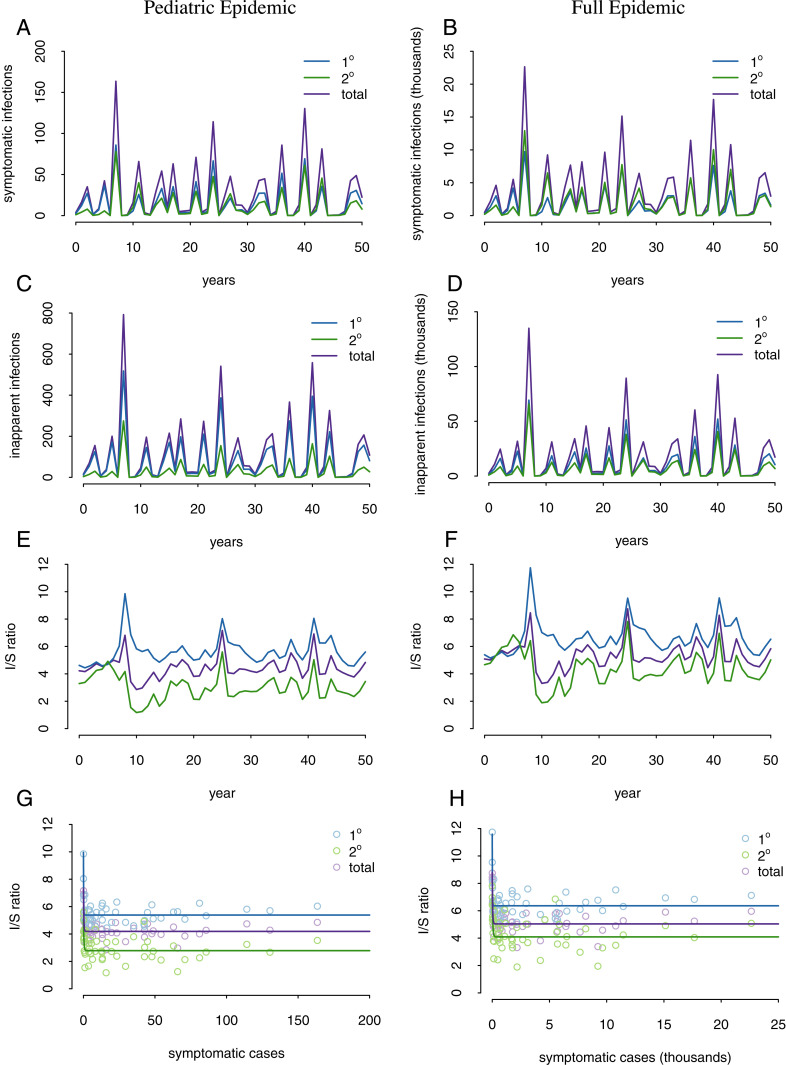
The boosting model can recover patterns in I/S ratios. Shown are results for both the full model (Right) and a pediatric model (Left) that samples the epidemic in the pediatric cohort. In the pediatric boosting model, the output of the full model 3 is used to determine the force of infection, but the population (i.e., cohort size) is 2,500, which represents the younger individuals in the pediatric cohort. (A and B) Annual number of symptomatic infections. (C and D) Annual number of inapparent infections. (E and F) Annual fluctuations in I/S ratio. (G and H) Inverse relationship between annual number of symptomatic infections and I/S ratio. Solid lines show exponential fits to the data.
Model 3 can recover these dynamics because in years with a large number of infections (both symptomatic and inapparent infections), a large number of boosts occur. In the following year, fewer infections occur because there are fewer susceptible individuals; however, the protective boosts result in more inapparent infections relative to symptomatic infections among the susceptible population. This results in a negative relationship between I/S ratio and symptomatic cases seen in the PDCS and elsewhere, as well as a positive relationship between cases in one year and the I/S ratio in the following year, as previously noted (5) and shown in Fig. 4C. Neither model 1 nor model 2 is able to reproduce this pattern (Fig. 4 A and B). As shown in Fig. 3, model 3 (with boosting) is able to recover the large fluctuations in I/S ratio found in the PDCS. Results are shown for a two-strain simulation, but three- and four-strain simulations (SI Appendix) produce qualitatively similar results, as shown in Fig. 5.

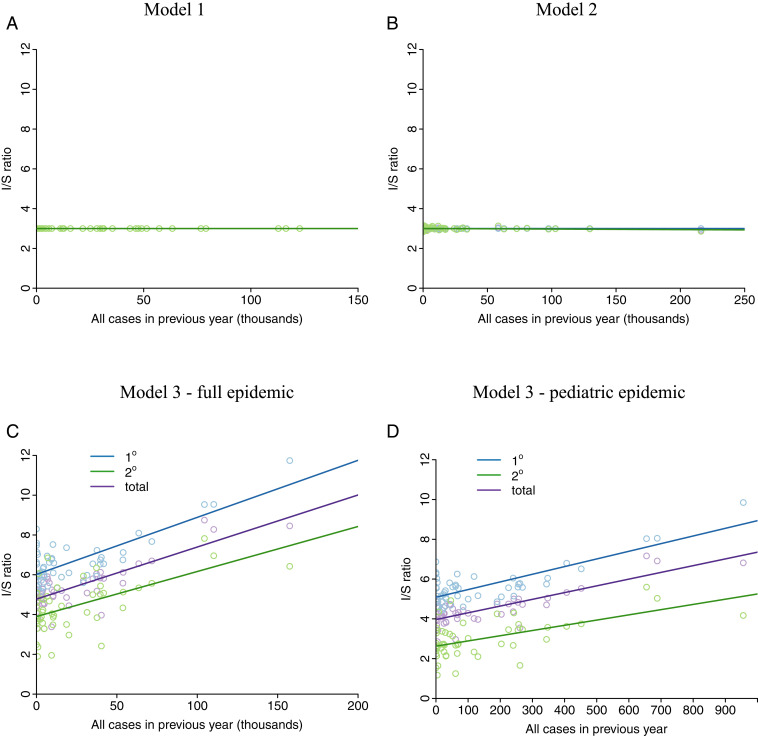
The boosting model can recover positive relationship between previous year cases and I/S ratios. (A) Results from model 1. (B) Results from model 2. (C and D) Results from model 3 (boosting model) of the full epidemic and for the pediatric cohort.

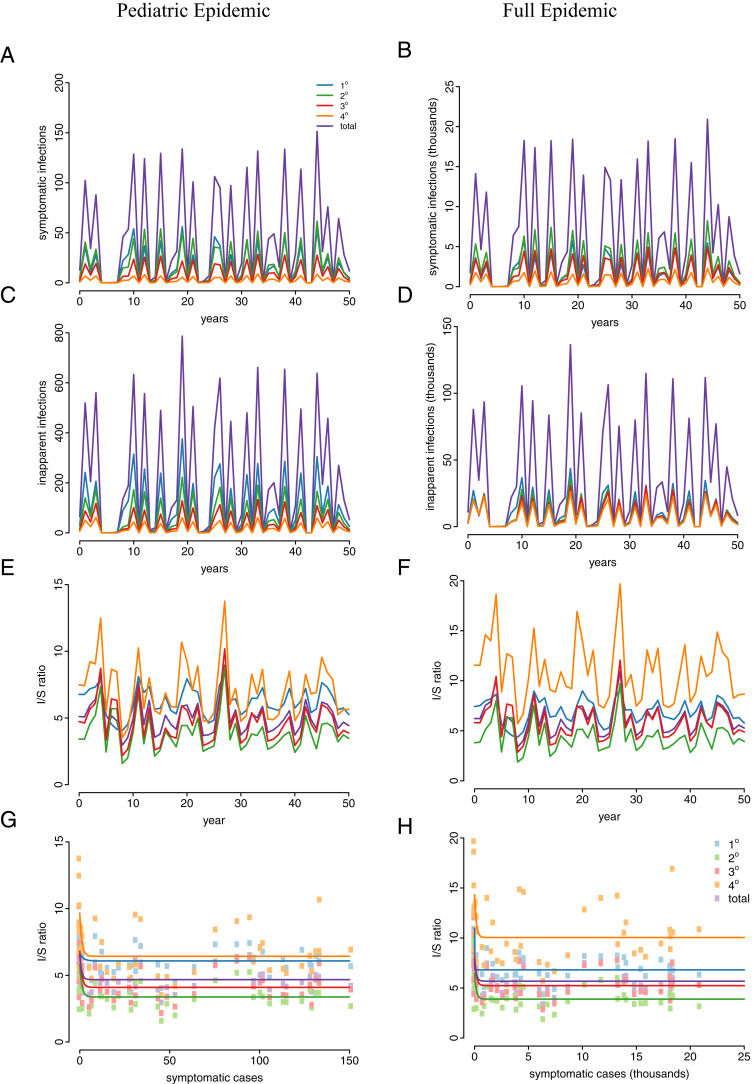
The boosting model with four strains can recover patterns in I/S ratios. Results are shown for both the full model (Right) and the pediatric cohort (Left). (A and B) Annual number of symptomatic infections. (C and D) Annual number of inapparent infections. (E and F) Annual fluctuations in I/S ratio. (G and H) Inverse relationship between annual number of symptomatic infections and I/S ratio. Solid lines show exponential fits to the data.
Discussion
Here we formulated mathematical models to understand potential mechanisms that can explain the large fluctuating patterns in I/S ratio seen in the PDCS in Nicaragua and more broadly. We show that accounting for shorter periods of cross-protection prior to inapparent infections relative to symptomatic infections cannot recover the large fluctuations in I/S ratio. Our key result is that by assuming that frequent boosting can increase the likelihood of a subsequent inapparent infection, we can recover the large fluctuations in these patterns. Furthermore, this phenomenon can recover the inverse relationship between annual numbers of symptomatic infections, the severity of an epidemic, and the I/S ratio. This pattern occurs because in years with high numbers of infections, boosts occur more frequently, decreasing the severity of infections in the following season.
Several mathematical models of dengue population dynamics have investigated dengue dynamics in hyperendemic settings (1213–14, 29, 32). These models have been useful in determining the immune mechanisms necessary to explain the typical out-of-phase oscillations of DENV serotypes, as well as in providing recommendations for potential control strategies. The models have shown that ADE (13, 14) or a temporary period of cross-protection (12, 29, 32) is needed to recover patterns of oscillating DENV serotypes. However, none of the models distinguish between inapparent and symptomatic infections. By extending these models to include inapparent and symptomatic infections, we show that another immune mechanism—boosting of the immune response—is needed to recover fluctuating patterns in inapparent and symptomatic infections.
Detecting boosts is difficult without detailed temporal data on antibody dynamics over time. Studies that already detect inapparent infections that can be extended to multiple annual samples may be useful for detecting boosts. Furthermore, studies that detect unusual primary DENV infections, such as that of Waggoner et al. (33), are needed to determine whether primary exposures that result in a secondary infection with antibody dynamics resembling a primary infection can be protective against symptomatic infection, as we assumed here. In addition, the level of preexisting antibody titers impacts the risk of severe disease in addition to protection (15), an effect that we did not consider in our models.
Other mechanisms besides boosting have been hypothesized to explain fluctuations in patterns of I/S ratio. Previous studies have observed a strong positive correlation between dengue incidence and the inapparent infection rate in the following year (5, 11) and have suggested that temporary cross-protection can explain the subsequent increase in the fraction of inapparent infections. However, we show here that shorter periods of cross-protection before inapparent infection compared to symptomatic infection cannot explain the wide fluctuations in I/S ratio. Another possible hypothesis that may explain these fluctuations in I/S ratio is differences in viral inoculum. In years with high numbers of DENV infections and more overall dengue transmission, it is possible (although speculative) that the average viral inoculum may be higher. A higher viral inoculum dose can increase viral growth and peak viral load (34), an important determinant of severe dengue disease (3536–37). Therefore, years with higher average viral inoculums may result in a higher proportion of symptomatic infections. Finally, viral evolution may result in the emergence of novel DENV strains that are associated with greater disease severity or epidemic force, as in, for example, the emergence of the more severe Asian DENV-2 strain in Latin America and Asia (2). However, in Nicaragua, the emergence of a novel DENV-2 clade could not explain the increase in disease severity across epidemic seasons (38).
The potential protective effects of boosting have very important implications for dengue control. The only currently registered dengue vaccine is a live-attenuated recombinant tetravalent vaccine (CYD-TDV) (39). In Phase 2b and 3 trials, vaccine efficacy was significantly higher in individuals who were seropositive compared to those who were seronegative prior to vaccination, and the risk of hospitalization and severe disease was increased in DENV-naïve vaccine recipients compared to recipients of placebo (40, 41). Given the vast differences in vaccine efficacy and vaccine safety based on serostatus, the vaccine was originally licensed for individuals age 9 to 45 y in endemic settings and is now restricted to DENV-seropositive individuals. A modeling consortium has also shown that the vaccine is most effective in high-transmission settings (42). In high-transmission settings, we would expect boosting to occur frequently. If boosts are protective, a vaccine or vector control policy that reduces but does not eliminate dengue transmission may result in more disease, as the protective effects of boosting will be minimized, although it is possible that a vaccine could potentially “step in” relative to these boosts. These results are consistent with another dengue epidemiologic model showing that incomplete control strategies may result in more severe cases if cross-protection is assumed to prevent disease, but seroconversion due to heterologous exposure can occur (29). Boosting is not a replacement for effective, multivalent dengue vaccines that induce strong type-specific immunity to each serotype simultaneously (43). In particular, boosting is not expected to provide sufficient protection against severe secondary dengue, because even in the highest DENV transmission settings, there will not be enough boosting to protect all individuals.
Models, such as the boosting model we present here, are useful in determining whether mechanisms that are difficult to study empirically can explain unusual patterns in the data. In particular, they produce qualitative general predictions of the impact of different mechanisms of epidemic patterns, with more detailed models explicitly modeling such processes as vector population dynamics more appropriate for making quantitative predictions. Here we have shown here that protective boosts protecting against symptomatic disease can explain the unusually large fluctuations in inapparent to symptomatic ratios of DENV infections. These results should be considered when designing dengue control policies, as even modest control could increase the number of symptomatic cases by decreasing the protective effects of boosting.
Methods
Data.
The PDCS follows a group of children from age 2 to 14 y through enhanced passive surveillance and collects annual healthy blood samples, which allows for detection of both symptomatic and inapparent DENV infections. The PDCS has been reviewed and approved by the Institutional Review Boards of the University of California, Berkeley (protocol 2010–09-2245), the University of Michigan (study ID HUM00091606), and the Nicaraguan Ministry of Health (protocol NIC-MINSA/CNDR CIRE-09/03/07–008). Parents or legal guardians of all subjects provide written informed consent, and subjects age ≥6 y provide assent. The PDCS is conducted by Sustainable Sciences Institute of Nicaragua and the University of California, Berkeley, at the Health Center Sócrates Flores Vivas (HCSFV) of the Municipal Health System of Managua, Nicaragua. The HCSFV is the primary health care facility serving the study area in District II of Managua. Its 17 neighborhoods are low- to mid-socioeconomic status and representative of Managua. In total, the cohort consists of ∼3,800 active children each year, with equal gender representation. To maintain age structure, each year during the period included in this study (2004 to 2016), ∼300 new 2-y-old children were enrolled as 15 y olds were withdrawn (the study was extended to 17 y olds in 2017), and ∼200 children age 3 to 11 y were enrolled to compensate for loss to follow-up (∼5% annually). The homes of all participants are identified with a GPS point.
Participants are followed closely for all illnesses, and children who present with fever are screened for signs and symptoms of dengue, chikungunya, or Zika. Acute and convalescent blood samples for serologic and virologic analysis are collected from children meeting the case definition of dengue, chikungunya, or Zika or presenting with undifferentiated fever. A healthy annual blood sample is collected from all participants each March/April (2010 to present) or July (2004 to 2009), before the start of the arbovirus transmission season (44). Dengue cases are laboratory-confirmed by 1) detection of DENV RNA by real-time or initially conventional RT-PCR (4546–47), 2) isolation of DENV in C6/36 cells (48, 49), 3) seroconversion as determined by a DENV-specific IgM capture ELISA using paired acute and convalescent sera (45, 48), and/or 4) a greater than fourfold rise in antibody titer between acute and convalescent sera as measured by the DENV inhibition ELISA (IE) (15, 44, 50, 51), with titer determined using the Reed–Muench method (15). Dengue cases are considered primary infections when convalescent samples have DENV IE titers <2,560 (52). Participants whose annual samples show seroconversion or a greater than fourfold rise in antibody titer during the year but who have not had an identifiable febrile episode associated with acute DENV infection are considered to have clinically inapparent infections (44).
Mathematical Models.
To model symptomatic and inapparent DENV infections, we extended an existing multistrain dengue model published by Nagao and Koelle (29). We included stochasticity in the amplitude of the seasonality term to allow for different epidemic sizes, similar to Adams et al. (12). The three models we considered are 1) a null model in which we assume that a constant proportion of infections are inapparent and the remaining infections are symptomatic, 2) a model in which the period of cross-protection preceding inapparent infections is shorter than that preceding secondary infections, and 3) a model that incorporates homologous and heterologous boosts, as well as priming of the immune response before a primary infection. All model equations and parameter values are provided in SI Appendix.
Acknowledgements
We thank our study team at the Centro de Salud Sócrates Flores Vivas and the Laboratorio Nacional de Virología at the Centro Nacional de Diagnóstico y Referencia, Nicaraguan Ministry of Health, as well as the Sustainable Sciences Institute in Nicaragua. We also thank the study participants and their families. This study was supported by Grants R01 GM122061-03 (to M.B.), P01 AI106695 (to E.H.), and U19 AI118610 (to E.H.) from the National Institute of Allergy and Infectious Diseases of the US NIH.
Data Availability
All study data are included in the main text and/or SI Appendix.
References
1
2
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
 Boosting can explain patterns of fluctuations of ratios of inapparent to symptomatic dengue virus infections
Boosting can explain patterns of fluctuations of ratios of inapparent to symptomatic dengue virus infections
