
- Altmetric
- 1 Introduction
- 2 Clinical aspects and pathogenesis of PE
- 3 Reactive oxygen species and oxidative stress in PE
- 4 Placental eNOS dysfunction and oxidative stress in PE pathogenesis
- 5 Inhibition of the NO/eNOS system in animal models for PE
- 6 Therapeutic perspectives targeting oxidative stress and NO/eNOS dysfunction
- 7 Conclusion
- Fundings
- Declaration of competing interest
Preeclampsia (PE) is a multifactorial pregnancy disease, characterized by new-onset gestational hypertension with (or without) proteinuria or end-organ failure, exclusively observed in humans. It is a leading cause of maternal morbidity affecting 3–7% of pregnant women worldwide. PE pathophysiology could result from abnormal placentation due to a defective trophoblastic invasion and an impaired remodeling of uterine spiral arteries, leading to a poor adaptation of utero-placental circulation. This would be associated with hypoxia/reoxygenation phenomena, oxygen gradient fluctuations, altered antioxidant capacity, oxidative stress, and reduced nitric oxide (NO) bioavailability. This results in part from the reaction of NO with the radical anion superoxide (O2•−), which produces peroxynitrite ONOO-, a powerful pro-oxidant and inflammatory agent. Another mechanism is the progressive inhibition of the placental endothelial nitric oxide synthase (eNOS) by oxidative stress, which results in eNOS uncoupling via several events such as a depletion of the eNOS substrate L-arginine due to increased arginase activity, an oxidation of the eNOS cofactor tetrahydrobiopterin (BH4), or eNOS post-translational modifications (for instance by S-glutathionylation). The uncoupling of eNOS triggers a switch of its activity from a NO-producing enzyme to a NADPH oxidase-like system generating O2•−, thereby potentiating ROS production and oxidative stress. Moreover, in PE placentas, eNOS could be post-translationally modified by lipid peroxidation-derived aldehydes such as 4-oxononenal (ONE) a highly bioreactive agent, able to inhibit eNOS activity and NO production. This review summarizes the dysfunction of placental eNOS evoked by oxidative stress and lipid peroxidation products, and the potential consequences on PE pathogenesis.

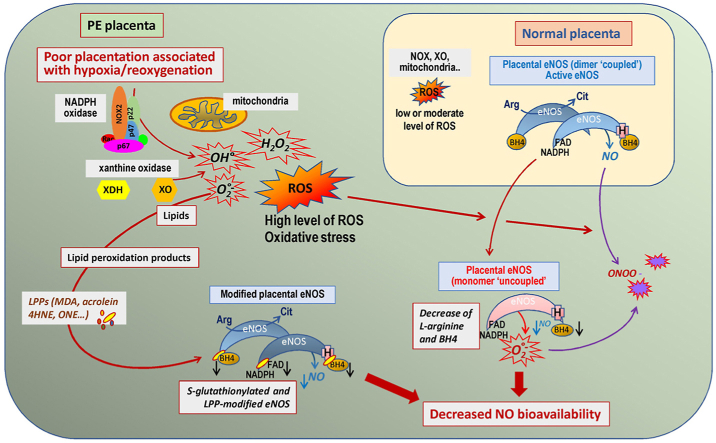
•
Physiological ROS production is enhanced during pregnancy.
•eNOS is one of the main target of oxidative stress in PE placenta.
•eNOS is S-glutathionylated in PE placentas.
•eNOS is modified by lipid oxidation products in PE placentas.
Introduction
Preeclampsia (PE) is a hypertensive disorder of pregnancy, characterized by a de novo high blood pressure development [[1], [2], [3], [4]], with (or without) proteinuria [5], and detected after 20 weeks of pregnancy. This disease affects around 2–8% of pregnancies worldwide, with an incidence depending on various geographical, nutritional, or ethnic factors, and an increased prevalence for patients affected with chronic hypertension, diabetes or obesity [6]. If untreated, PE can evolve to life-threatening complications such as eclampsia and HELLP syndrome (hemolysis, elevated liver enzymes and low platelet count), fetal growth restriction and fetal or perinatal death [7,8]. Only fetus delivery is efficient to halt the progression of the disease [9].
The mechanisms involved in the onset of PE are still unclear, but may involve an abnormal placentation, characterized by a disturbed trophoblastic invasion of spiral arteries, which enhances the production of reactive oxygen species (ROS), triggers oxidative stress, hypoxia, reduced placental perfusion and endothelial dysfunction [[1], [2], [3], [4], [5], [6]]. A main cause of abnormal placentation and endothelial dysfunction in PE, is the reduced bioavailability of nitric oxide (NO), a key-vasodilator and blood pressure regulator in placenta [10]. In endothelium and placenta, NO is biosynthesized by the endothelial nitric oxide synthase (eNOS), and confers to endothelium its vasorelaxing and anti-aggregant properties [[10], [11], [12]]. In addition, NO participates to placentation and the synthesis of the vascular endothelial growth factor (VEGF) [13]. Oxidative stress is thought to play a pivotal role in the decreased NO bioavailability in PE pathophysiology, via several mechanisms including an inhibition of eNOS (eNOS uncoupling) and subsequent defect of NO biosynthesis [10], or through the formation of peroxynitrite (ONOO-), via the reaction of NO with the radical anion superoxide O2•− [14].
In this review, we aimed at summarizing how oxidative stress evokes eNOS dysfunction in PE placentas, with focus on the role, and the mechanisms by which it may affect eNOS activity and NO production, thereby contributing to reduce NO bioavailability (Fig. 1).

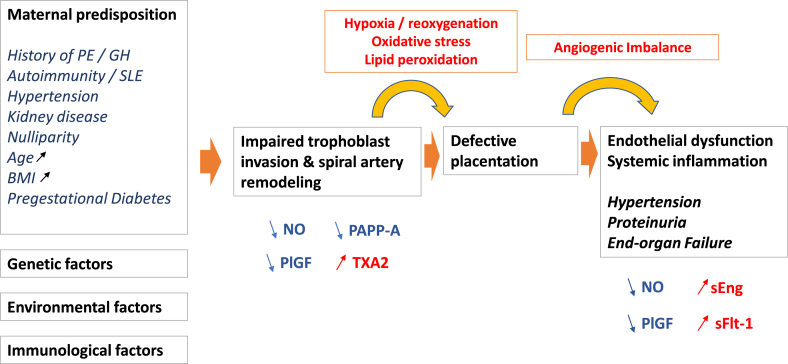
Scheme summarizing the mechanisms, risk factors, and outcomes of PE. PE, preclampsia, GH gestational hypertension, SLE systemic lupus erythematosus, NO nitric oxide, PlGF placental growth factor, sFLT-1 Soluble fms-like tyrosine kinase-1, TXA2 Thromboxane-A2, PAPP-A Pregnancy Associated Plasma Protein-A, sEng soluble endoglin.
Clinical aspects and pathogenesis of PE
Clinical aspects
Preeclampsia (PE) is a complex clinical syndrome specific to human pregnancy [1], and detected after 20 weeks of gestation, on the basis of de novo hypertension (≥140/90 mmHg) [[1], [2], [3], [4]], with (or without) proteinuria (>300 mg/24 h) [5] or end-organ. Other signs may include thrombocytopenia with a platelet count in the range of 100,000 per microliter, an impaired liver function evidenced by abnormally elevated liver enzymes, renal failure with elevated serum creatinine levels (>97.2 μmol/l), pulmonary oedema or new-onset cerebral or visual disturbances [[2], [3], [4], [5], [6]]. A high risk of PE would be defined by a combination of first trimester parameters (detected according to the Fetal Medicine Foundation [FMF] algorithm), including maternal factors (age, ethnicity, clinical risk factors, mean blood pressure, mean pulsatility indexes of both uterine arteries) and serum biomarker assays (PAPP-A or Pregnancy Associated Plasma Protein A and PlGF or Placental Growth Factor) [[1], [2], [3], [4], [5], [6]].
If untreated, PE can lead to serious complications such as eclampsia and the HELPP syndrome (hemolysis, elevated liver enzymes and low platelet count), that may rapidly become life-threatening in the absence of appropriate management [7,8].
PE is also responsible for one-third of severe preterm births, often associated with intrauterine growth restriction (IUGR) [15]. Women with PE history present an increased risk of recurrence for their other pregnancies, and a long-term vascular risk for chronic hypertension, coronary heart disease, stroke, chronic renal failure, and cardiovascular/neurovascular mortality [16,17].
The etiology of PE is difficult to define, in view of the heterogeneity of the clinical forms. Several risk factors are well characterized, including nulliparity vs multiparity, immunological risk factors including conflicts between the mother's system and antigens of fetal origin, exposure to sperm, obstetrical factors including multiple pregnancies, previous PE episodes, maternal factors (women aged over 40 years, high body mass index, chronic hypertension), as well as genetic (family history of PE) and environmental factors (high altitude, stress) [[18], [19], [20], [21], [22], [23], [24]]. There is currently no curative treatment for PE other than pregnancy termination and fetus delivery. Many prevention strategies have been reported, with poor convincing results. To date, several meta-analyses support an efficacy of low doses of acetylsalicylic acid (aspirin) (60–150 mg/24 h) in preventing PE and IUGR [[25], [26], [27], [28], [29], [30]].
Pathophysiology of PE
The pathophysiology of PE is mainly related to placental insufficiency, resulting from an impaired uteroplacental circulation, and a disruption of normal deciduo-trophoblastic interactions that affect placental development from the first trimester of pregnancy [31]. The reduced bioavailability of NO and oxidative stress are thought to play a key role in the maternal-placental circulation and in poor placentation (Fig. 1) [10,[31], [32], [33], [34], [35]].
Several successive stages are described, including an early defective placentation leading to placental hypoxia and ischemia-reperfusion of the placenta, and finally oxidative stress, maternal endothelial dysfunction and inflammation, which are strongly linked in PE pathophysiology [[2], [3], [4], [5], [6],31,33,36].
Defective placentation
During PE, the incomplete transformation of spiral arteries involves the persistence of smooth muscle cells (SMC), particularly at the basal segment within the junction area, and a deficient trophoblast invasion observed in 30–50% of spiral arteries of the placental bed. Consequently, there is a decreased and intermittent perfusion of the intervillous chamber generating transient hypoxia. As a result, the placenta is exposed to a chronic low-grade ischemia-reperfusion phenomenon. The mechanisms leading to the failed trophoblastic invasion are not fully understood, and could involve immunological factors or insufficient proteolysis [[2], [3], [4], [5], [6],31,36].
Ischemia/reperfusion and oxidative stress
Ischemia/reperfusion is a powerful oxidative stress inducer, much more powerful than simple hypoxia even when it is prolonged because the placental tissue begins to develop in a physiologically low O2 environment. Oxidative stress gradually stimulates the release into maternal circulation of apoptotic and necrotic trophoblastic placental debris, pro-inflammatory cytokines, and anti-angiogenic factors such as sFlt-1 (soluble fms-like tyrosine kinase 1 or sVEGFR-1, a soluble form of the VEGF type 1 receptor) and soluble endoglin (sEng) [[37], [38], [39]]. The release into maternal circulation of placental compounds and debris, and the recirculation of maternal leukocytes activated during their passage through intervillous chamber of the placenta undergoing ischemia-reperfusion and oxidative stress, may participate in the maternal systemic inflammatory response, endothelial dysfunction and hypertension [[40], [41], [42]].
Endothelial dysfunction and inflammation
The presence of endothelial cell activation markers is a characteristic of PE pathogenesis, characterized by increased levels of von Willebrand factor, endothelin, thrombomodulin and fibronectin in the maternal systemic circulation [[1], [2], [3], [4], [5], [6],[32], [33], [34], [35], [36], [37]]. The identification of sFlt-1 and endoglin, produced in excessive amounts during PE, revealed the links between placental abnormality and endothelial dysfunction [38,39]. sFlt-1 binds its ligands (VEGF and the placental growth factor, PlGF) which are involved in endothelial cell survival, peripheral vasodilation and glomerular endothelial integrity during normal pregnancy. Circulating levels of free VEGF and PlGF usually decrease in PE patients, resulting in an anti-angiogenic imbalance leading to maternal endothelial dysfunction and glomerular nephropathy [37,43].
Endoglin is the receptor for transforming growth factor-β (TGF-β), a protein that acts on vascular homeostasis through eNOS. The soluble form of endoglin (sEng) prevents the binding of TGF-β to its membrane receptors. It potentiates endothelial dysfunction induced by sFlt-1 and contributes to increase vascular permeability and hypertension [9,39,43,44]. In association with sFlt-1, sEng plays a role in the development of severe forms of the disease and would be involved in the pathophysiology of HELLP syndrome [39]. sEng injection to mice increases arterial pressure by increasing vascular resistance, probably through its interaction with TGF-β1 that prevents the TGF-β1-mediated eNOS activation in endothelial cells [39]. Thus it can be postulated that high circulating sEng levels, together with increased production of thromboxane A2 (TXA2) (vasoconstrictors), and on the other hand, a decreased NO and prostacyclin production (vasodilators), modifies the vasomotor response, leading to an increase in total peripheral resistance and hypertension in PE patients [45].
There is also an increase in the peripheral vascular resistance due to the activation of the renin-angiotensin system by placental cytokines [37,38,46]. Cyclooxygenase and eNOS activities are increased during normal pregnancy, and are decreased in PE, leading to vasoconstriction and an altered capillary permeability that is partly responsible for oedema and hypertension [47]. Atherosclerotic lesions could be detected in the spiral arteries of PE patients, together with platelet activation evidenced by the presence of TXA2, fibrin and complement deposits, and foam cells [48]. PE patients are at increased risk of cardiovascular issues, especially after menopause [48]. Finally, in the kidney, endothelial cells of glomerular capillaries accumulate lipids and frequently obstruct the lumen of these capillaries. The characteristic histological defect is glomerular endotheliosis, which suggests that the endothelium has a central role in PE [1,49,50].
PE is characterized by an excessive and progressive activation of the immune system along with an increase in proinflammatory cytokines and antiangiogenic factors, in the fetoplacental unit and in maternal endothelium [37,51,52]. The pathophysiology of PE involves a chronic activation of maternal immune system characterized by a prolonged inflammatory response during pathological pregnancies.
All in all, PE is not limited to hypertension associated (or not) with proteinuria. It is characterized by an endothelial dysfunction and a systemic inflammatory response. A large body of evidence indicates that oxidative stress plays a key-role throughout the pathophysiology of PE, more in early onset than in late onset PE, because the poor uteroplacental perfusion resulting from the defective spiral artery remodeling, generates a large amount of ROS [32]. This places oxidative stress as a major cause of cytokines and anti-angiogenic factors release in the maternal circulation, subsequently leading to endothelial dysfunction and inflammation, together with the decrease in NO bioavailability and the dysfunctional eNOS activity in placenta [[32], [33], [34], [35], [51], [52], [53], [54], [55], [56], [57], [58], [59]]. The causes of these events are not fully identified.
Reactive oxygen species and oxidative stress in PE
Physiological reactive oxygen species (ROS) production during pregnancy
During normal early pregnancy (before the 8-10th week of gestation), the fetoplacental unit develops in a hypoxic environment, because the maternal blood flow is not yet established in the intervillous space and spiral arteries are invaded and plugged by extravillous trophoblasts, which prevents the entry of maternal blood in the intervillous space [54]. O2 and nutrients are supplied by diffusion. The subsequent relative hypoxia is apparently required at this early step of development, probably because of the low level of embryo antioxidant defenses [51].
Concomitantly, an increased expression of protective systems, such as heat shock protein P70 (HSP70), may be observed at the 9th week of gestation in the villous syncytiotrophoblasts in the peripheral zone of the primitive placenta [37,[55], [56], [57], [58]]. Likewise, there is an increased level of thioredoxin-1, an endogenous antioxidant and redox-sensitive protein, which plays a protective role in fetal growth, from implantation to later stages [60].
Then, at the end of the first trimester of normal pregnancy, the maternal blood flow begins to vascularize the intervillous spaces, when the plug of spiral arteries is dissolved and extravillous trophoblast cells remodel spiral arteries to wide diameter “low resistance vessels”. This induces a stable blood flow in the intervillous space.
During normal pregnancy, there is an increase in ROS production, including NO, the radical anion superoxide O2•−, hydrogen peroxide (H2O2), the hydroxyl radical •OH, and peroxynitrite ONOO−. Indeed, placental is constantly exposed to variations depending on posture, diet, exercise and uterine contractions, that may induce mild ROS production [53].
These physiological ROS are associated with the rapid development of placenta [61] and mainly result from the increased mitochondrial activity in villous and extravillous trophoblasts [62]. The placenta is mitochondria-rich and consumes approximately 1% of the pregnant woman's basal metabolism [63]. The placenta requires energy for its own metabolism and remodeling, and for the synthesis of substrates and hormones necessary for fetus development. More than 50–70% of O2 taken up from the uterine circulation are used by the placenta, and energy requirements increase with the growth of the fetus [62].
There is also a decrease in superoxide dismutase (SOD) activity and plasma thiol levels, while ceruloplasmin levels increase, suggesting a certain level of “physiological stress” during normal pregnancy through the production of ROS [33,35,64]. Moderate ROS levels are implicated in proliferation and cell maturation required for pregnancy maintenance and embryo development [65,66]. ROS are also involved in the degeneration of the villous tissue in the peripheral region, which is essential for the formation of placental membranes [32]. When ROS are produced in excess and when antioxidant defenses are overwhelmed, high levels of ROS (i.e. oxidative stress) become pathological, as observed in PE [66].
Oxidative stress in the pathogenesis of PE
Hypoxia/reperfusion, a main cause of oxidative stress in PE
Hypoxia-reoxygenation events, due to reduced organ blood flow (ischemia), followed by reperfusion and reoxygenation, are main sources of ROS, and are associated with antioxidant depletion, oxidative stress, oxidative damages and inflammatory responses [[51], [52], [53],[55], [56], [57], [58]]. Impaired trophoblastic invasion, together with subsequent poor placentation and reduced placental perfusion, lead to repeated hypoxia/reoxygenation waves, that stimulate ROS production in the intervillous chamber [52]. This ROS production involves a stimulation of the mitochondrial respiratory chain and an activation of ROS-producing enzymes including NADPH oxidase and xanthine oxidase [52,57,58,66]. There is an activation of monocytes and neutrophils, which produce pro-inflammatory cytokines, antiangiogenic factors and microparticles, and stimulate ROS production [67,68]. Another source of ROS may originate from the dysfunction of eNOS [[10], [11], [12], [13]]. All these events contribute to the “placental oxidative stress”, possibly involved in the systemic endothelial dysfunction and vascular inflammation that characterize PE pathogenesis [52,[56], [57], [58]]. There is a concomitant proinflammatory response, i.e. a release of inflammatory cytokines particularly the tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), and a (debated) decrease in anti-inflammatory and antioxidant defenses including IL-10, SODs, catalase, glutathione peroxidase (GPx) [33,35,52,69]. Hypoxia/reperfusion promotes the release in the maternal circulation, of placental debris and apoptotic fragments i.e. damaged trophoblastic cells, which potentiate inflammation [52,56,58,70]. Oxidative stress markers, including oxidative modifications of proteins and lipid peroxidation products, could be observed both in maternal circulation and in the placenta, while antioxidant capacity and antioxidant reserves are globally reduced [[33], [34], [35],52,[56], [57], [58],66,71]. Recently Taravati et al. raised the hypothesis that oxidant defenses could increase at the beginning of PE pregnancy, to compensate the outcomes of oxidative stress and protect the fetus. However, the antioxidant capacity of plasma is finally not sufficient to counter oxidative stress in PE PE4 compared with that of a normal pregnancy. Further studies are warranted to investigate the role of dietary supplements in preventing preeclampsia and evaluation of genetic variation of antioxidant enzymes contributing to this morbid condition in women with different ethnics. As discussed by Taravati et al., [72], it is likely that antioxidant defenses could be reinforced at the beginning of pregnancy, as a compensatory mechanism to protect the fetus against oxidative stress, this mechanism being however not enough efficient to counter oxidative stress outcomes in PE.
Oxidative stress promotes the peroxidation of polyunsaturated fatty acids (PUFA), which generates a huge amount of lipoperoxides, hydroperoxides and lipid peroxidation-derived aldehydes, that elicit cellular dysfunction, inflammation and apoptosis [73]. Previous reports from Walsh et al. [74], had shown that placental ischemia may be enhanced by an increased biosynthesis of TXA2, a vasoconstrictor and platelet aggregant eicosanoid, concomitant with a decrease of prostacyclin, another eicosanoid with vasodilating and antiaggregant properties, which counteracts the effects of TXA2 [74,75]. This imbalance of the prostacyclin/TXA2 ratio, could play a role in the decreased uteroplacental blood flow, placental ischemia and endothelium damages [74,75].
Main sources of ROS in PE
NADPH oxidase
NADPH oxidases (NOXs) constitute a ubiquitous multicomponent protein complex, which produces O2•− by transferring one electron to oxygen from NADPH to NADP+ [76]. The NADPH oxidase complex includes cytosolic proteins (p47phox, p67phox, and p40phox), and membrane-associated proteins (p22phox and gp91phox). Several catalytic NOX isoforms have been described, including NOX1, NOX2, NOX4 and NOX5 which are expressed in vascular cells [77]. Note that the NOX4 isoform is constitutively active and only requires the membrane protein p22phox [78].
In pregnancy, moderate doses of O2•−produced by NOXs, may help to regulate the vascular tone, while high O2•− levels generate oxidative stress and contribute to vascular dysfunction [71,79]. Increased expression of p22phox, p47phox, and p67phox have been reported in trophoblasts and placental SMC in the placenta of PE-affected women [80]. Likewise, higher placental NOX activity was reported in women with early-onset compared with late-onset PE [71,81].
NOX2 is the prototypic NADPH oxidase involved in the phagocytic respiratory burst in neutrophils [82]. Otherwise, vascular NOX2 is expressed in endothelial cells and SMC. Its basal activity is low, but it is rapidly activated in response to inflammatory and stress-inducing agents (angiotensin II or AngII, cytokines, interleukin-1 …) or growth factors [80]. NOX2 plays a prominent role in the generation of peroxynitrite (ONOO-), through the reaction of NO with O2•− produced by NOX2, which decreases NO bioavailability, triggers eNOS uncoupling and promotes endothelial dysfunction [83].
Neutrophil levels are significantly increased in the peripheral circulation of PE patients, placing these cells at the center of ROS generating systems during PE [67,68]. NOX2 is largely involved in ROS production by activated neutrophils during PE, suggesting its implication in this disease [58]. The expression and enzymatic activity of NOX2 are elevated in PE, in endothelial cells and placentas, or in small resistance vessels in abdominal fat from PE-affected women [57,58,84,85]. Many factors can activate NOX2 in PE, such as elevated circulating levels of inflammatory cytokines, increased AngII/AngII type I receptor (AT1R) sensitivity, or placental vascular shear stress [86], which stimulates the release by placenta of agents such as activin, an antiangiogenic factor implicated in NOX2 up-regulation and endothelial dysfunction [87].
NOX1 is increased in syncytiotrophoblasts and endothelial cells in placentas from PE patients [88]. It is inducible and activated by inflammatory factors including AngII and cytokines, and may contribute to the decrease in NO bioavailability and hypertension [88].
NOX4 is constitutively active, and has the particularity of producing H2O2, due to its conformational E-loop structure which accelerates O2•− dismutation [89]. Intracellularly, NOX4 would be located in mitochondria [78,90]. Its implication in vascular ROS production in PE is still unclear. A recent article by Choi et al., indicated that endothelial intermediate-conductance KCa3.1 and small-conductance KCa2.3 channels, which are involved in endothelium-derived hyperpolarization and SMC relaxation, could be downregulated in uterine endothelial cells from PE-affected patients, through an increased expression of NOX2 and NOX4, and the subsequent increase in O2•− and H2O2 production [91].
Xanthine oxidase
Xanthine oxidase is another important source of O2•− in PE [92]. In fact, xanthine oxidoreductase exists in two interconvertible but distinct forms, i.e. the constitutively expressed xanthine dehydrogenase (XD), and xanthine oxidase (XO), which is activated by the oxidation of thiol groups, that converts XD to XO [91]. XO is an iron and molybdenum-containing flavoprotein, which oxidizes hypoxanthine from nucleic acid metabolites to xanthine, and xanthine to uric acid, producing O2•− and H2O2 [93]. Its activity is low in normal conditions, but it can be rapidly stimulated by inflammatory cytokines and ischemia/reperfusion conditions, as observed in PE [94]. As hyperuricemia is frequently observed in PE patients, increased XO activity may likely contribute to the production of high O2•− levels and oxidative stress in this disease [92,[94], [95], [96]].
Mitochondria
Placental mitochondrial ROS production and oxidative damages are increased in PE, in response to hypoxia/reoxygenation events and depending on PE severity [97]. Hypoxia/reoxygenation stimulates the production of mitochondrial O2•− by the complexes I and II of the respiratory chain, rapidly dismutated into H2O2 by the mitochondrial manganese SOD (MnSOD) or by copper and zinc SOD (CuZnSOD). H2O2 is then neutralized (reduced to water) by glutathione peroxidases (GPx) or catalase [33,34,52]. As discussed by Holland et al. [97], mitochondrial ROS signaling in moderate PE placenta may stimulate compensatory antioxidant responses, gene expression and uncoupling protein activation, allowing to maintain the mitochondrial function, whereas in more severe PE clinical forms, mitochondrial dysfunction and altered mitochondrial processes are observed in cytotrophoblasts and syncitiotrophoblasts. Electron microscopy studies of mitochondria in PE vs normotensive placentas, showed significant morphological differences, such as degenerative and swollen appearance, suggesting an intermittent anoxia state in PE mitochondria [98]. Comparative proteomic analysis performed on mitochondria isolated from PE vs normotensive placentas, allowed to identify at least 26 mitochondrial proteins differentially expressed in PE, with four proteins upregulated and 22 downregulated, in the field of fatty acid oxidation, the tricarboxylic acid cycle, apoptosis, ROS generation and oxidative stress [98]. As well, an increased mitochondrial activity, associated with a reduction of the mitochondrial mass, and a nitroso-redox imbalance were reported in placentas overexpressing Storkhead box 1 (STOX1), a transcription factor involved in the genetic forms of PE [99], all these observations indicating that mitochondria dysfunction largely contributes to PE pathophysiology.
Decreased antioxidant levels
The cellular redox homeostasis is tightly maintained by a balance between ROS production and their neutralization by enzymatic and non-enzymatic antioxidant systems able to neutralize free radicals or metal ions involved in free radical production [72]. Endogenous antioxidant systems include low-molecular-weight agents such as glutathione, ubiquinol, or uric acid, proteins able to scavenge free radicals, or bind metal ions (serum albumin, lactoferrin, transferrin, ceruleoplamin, ferritin, haptoglobin, hemopexin), and enzymatic systems that regulate the intra- and extracellular ROS levels (SODs, catalase, GPx, thioredoxins, heme-oxygenase) [73,100]. Exogenous sources of antioxidants are found in the diet, mainly vitamin E (α-tocopherol), vitamin C (ascorbic acid), β-carotene, and polyphenols [100,101].
Antioxidant status vs oxidative stress have been largely explored in PE patients. However, there is a wide heterogeneity in plasma and placental levels of oxidative stress markers vs antioxidant status in the literature [[32], [33], [34]]. In normal pregnancy, there is an increase in antioxidant defenses [102], evidenced by an increased antioxidant capacity. It is generally stated that antioxidant capacity and the antioxidant content are lower in PE. However recent studies reported by Ferreira et al., pointed out increased levels of SOD and catalase antioxidant enzymes, increased level of reduced glutathione (GSH) and a higher GSH/GSSG ratio, in placentas from PE-affected women, by comparison with pregnant normotensive women [32,34]. As discussed by the authors, these increased antioxidant parameters could be a compensatory mechanism tending to protect the fetus against aggressive oxidative stress in placenta. By comparison, in the circulating blood, it seems that antioxidant defences are lower in PE. A meta-analysis study investigated the results concerning both oxidative stress markers and enzymatic/non-enzymatic antioxidant systems in the plasma of PE patients [72]. The main conclusions were that circulating levels of antioxidants were globally decreased in PE, with lower plasma levels of glutathione (GSH) and conversely increased glutathione peroxidase (GPx) activity. Most studies indicated a decrease in plasma SOD activity, possibly resulting from its inhibition by an increased accumulation of H2O2, correlated with increased activities of catalase and GPx [72].
Hyperuricemia is frequently observed, and the concentration of serum uric acid in pregnant women with preeclampsia has been suggested to be associated with disease severity [94,95]. Though contradictory data were reported concerning vitamin E and vitamin C plasma concentrations in PE [103,104], the meta-analysis study concluded to their significant decrease, even though a significant heterogeneity was observed through the data [72]. This decrease in vitamin C and vitamin E content, combined with increased ROS production, may exacerbate oxidative stress, lipid peroxidation and cellular damages. In this context, large randomized trials were carried out to evaluate the protective effect of vitamin E and C supplementation, but generally gave controversial and disappointing results in the prevention of PE [105] (and see section 5). However, as reported in a recent meta-analysis, an improvement of pregnancy outcomes could be observed when antioxidants are administered in treatment of confirmed preeclampsia [34].
Accumulation of lipid peroxidation products
Oxidative stress triggers direct damages on lipids, proteins and DNA, causing various pathological responses including activation of the endoplasmic reticulum stress, cellular dysfunction and cell death [73,106,107]. The oxidation of polyunsaturated fatty acids (PUFAs) generates lipid peroxidation products (LPPs), among them lipid oxidation-derived aldehydes including acrolein, 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA), 4-oxo-2-nonenal (ONE), which covalently bind to the nucleophilic sulfhydryl and primary amine groups of proteins, forming Schiff bases, Michael adducts and protein crosslinks [[106], [107], [108], [109], [110]]. The modification of proteins by LPPs depends on their nature, expression and conformation, oxidative stress intensity and duration, cell type, local LPP concentration, and generates various biological responses from the expression of protective and adaptive factors to protein dysfunction, inflammation, senescence and apoptosis [[109], [110], [111], [112], [113], [114], [115]].
HNE and MDA-adducts are detected in PE patients, in the fetal and maternal circulation, syncytiotrophoblasts, endothelial cells and macrophages [52,[114], [115], [116], [117]]. PE placentas exhibit high levels of carbonyl proteins, which are a hallmark of oxidative stress, lipid peroxidation and aging [[111], [117], [118], [119]].
The presence of LPPs in PE placentas, could be indicative of their premature senescence, in agreement with the hypothesis that accelerated placental aging is involved in PE pathophysiology via oxidative stress [120]. Indeed, connections exist between oxidative stress, cell senescence and premature placental aging, with possible implication in PE pathophysiology [[120], [121], [122], [123], [124]]. Placental aging during normal pregnancy, is a physiological mechanism observed in the multinucleated syncitiotrophoblast layer which is continuously undergoing a senescent-associated secretory phenotype (SASP) [120,122]. Physiologically, there is an increased expression of senescence markers, such as the senescence-associated β-galactosidase (SA-βgal), or the cell cycle inhibitor p21 [125,126]. However in pathological pregnancies, including PE, there is an upregulation of senescence markers and molecular pathways associated with SASP, suggesting that premature placental senescence is associated with adverse pregnancy outcomes, including IUGR and PE. Though the causes and mechanisms of premature placental senescence are not yet clarified, a role is expected for oxidative stress as trigger of chronic inflammation and lipid peroxidation which characterize aging [119,120].
In a recent report, Guerby et al. showed that the placental eNOS is targeted by HNE and ONE in PE placentas and in cultured human trophoblasts, with possible consequences on its enzymatic activity and NO production [127]. The accumulation of LPPs is associated with an increased expression of heat-shock protein 70 (HSP70), in both fetal and maternal circulation, which could act as a defense mechanism in tissues with altered antioxidant function [128].
Placental eNOS dysfunction and oxidative stress in PE pathogenesis
Physiological role of NO in pregnancy
NO and NO synthase activity
NO plays an essential role in vascular homeostasis due to its vasodilatory effect. NO is synthesized by nitric oxide synthases (NOS), from l-arginine and molecular oxygen (O2) according to the following reaction:
l-arginine + O2→ l-citrulline + NO.
In short, the reaction allowing NO synthesis could be compared to two mono-oxygenation reactions. The first reaction consists of the oxidation of l-arginine. This reaction produces an intermediate, –OH–l-arginine, which is rapidly oxidized into l-citrulline. These two oxygenation reactions occur in parallel with a concomitant conversion of NADPH to NADP+. The electrons are supplied by NADPH, transferred to flavins (FAD and FMN) and calmodulin, then presented to heme, the catalytic center.
Three NOS isoforms have been characterized, the neuronal NOS (nNOS or NOS1), the inducible NOS (iNOS or NOS2) and the endothelial NOS (eNOS, or NOS3, OMIM 163729, GenBank NM_000603.5), in reference to the tissues in which they were first described. The three isoforms function in a homodimeric state. Each monomer contains an oxygenase domain in the N-terminal section and a reductase domain in the C-terminal section. The oxygenase domain has binding sites for FAD, FMN and NADPH and is linked through a calmodulin recognition site to the reductase domain which has binding sites for heme, tetrahydrobiopterin (BH4), and l-arginine. In functional NOS, electrons are released by NADPH in the reductase domain and are transferred through FAD and FMN to the heme group of the opposite dimer. At this point, in the presence of l-arginine and the cofactor BH4, the electrons enable the reduction of O2 and the formation of NO and l-citrulline [129,130]. The bioavailability of substrates (l-arginine and O2) and the cofactor BH4 are important elements of enzyme activity.
The formation of NO requires electron flow, starting at the flavin level in the reductase domain, and ending at the heme level, on the oxygenase domain of the enzyme. The oxidized heme is able to bind O2 and l-arginine to synthesize NO and l-citrulline [129]. The presence of BH4 is essential for protein coupling and NO formation, as it ensures the “coupling” of the protein in its homodimeric form. BH4 binds to the interface of the two monomers where it is directly involved in the oxidation process by temporarily supplying an electron to the heme. Consequently, in the absence of BH4, the bond between O2 and heme is broken and NOS produces O2•− (“NOS uncoupling").
BH4 also participates to the binding of l-arginine and electron transfer. In the absence of BH4, l-arginine cannot bind to its site, and the terminal electron acceptor becomes O2, thereby forming O2•−, and decreasing NO production and bioavailability. In this context, O2•−and NO may interact to form peroxynitrite, ONOO-. eNOS is constitutively expressed in vascular endothelium, placental vessels and syncytiotrophoblasts [[131], [132], [133]]. Its activity is stimulated by growth factors, estrogens and cytokines [132,133], and is regulated by post-translational modifications, including acylation, phosphorylation, S-nitrosylation or S-glutathionylation, depending on the cellular redox state [134].
The eNOS gene (NOS3) is located at the end of the long arm of chromosome 7 (7q35-36). eNOS includes some polymorphisms, two of which (G894T and T-786C) being possibly associated with a decreased NO production and an increased risk of PE [135].
Inducible NOS (iNOS) is weakly expressed under physiological conditions. Its expression is stimulated by inflammatory factors, sepsis or when oxidative stress is decompensated [136]. Unlike nNOS and eNOS, iNOS is not calcium-dependent and could produce high levels of NO (a hundred times higher than those generated by other NOS isoforms), over long periods of time. NO produced by iNOS can combine with O2•−, to form toxic ONOO-, leading to vasoconstriction and endothelial dysfunction [137].
Physiological properties of NO
NO is a signaling agent involved in many essential physiological functions, such as blood pressure regulation, vasodilation, long-term potentiation of the central nervous system capacities, and immune system activity. NO exerts its effects according to two direct or indirect mechanisms, in endothelium and SMC. The direct pathway is S-nitrosylation, which allows NO to modify the functional properties of the protein to which it binds. The indirect pathway is cGMP (guanosine monophosphate cyclase), which is responsible of NO vasorelaxing properties.
During pregnancy, NO has a primary role in vasodilation and blood pressure regulation, placentation and VEGF synthesis [[10], [11], [12]].
The indirect NO/cGMP pathway
This pathway is involved in vasodilation. Once NO is produced by endothelial cells (via eNOS), it diffuses into adjacent SMC and binds guanylate cyclase, which converts guanosine triphosphate into cyclic guanosine monophosphate (cGMP). cGMP activates protein kinase G (PKG), leading to a decrease in intracellular calcium concentration. This decrease triggers a relaxation of smooth muscle fibers, as well as a reduction in the formation of the calmoduline-Ca2+ complex, thereby inhibiting vasoconstriction [138,139].
NO released by endothelial cells in vivo causes a permanent vasodilation of the arterial tone that helps to regulate arterial pressure. Therefore, eNOS inhibition is associated with a decrease in endothelial-dependent relaxation in vitro and in vivo [[138], [139], [140], [141]].
NO is involved in the arterial wall remodeling, as it stimulates angiogenesis under the control of the hypoxia-induced transcription factor HIF-1α, the mobilization of endothelial progenitor cells and the production of VEGF [13]. In addition to its vasodilatory properties, NO inhibits leukocyte adhesion to endothelium surface, platelet aggregation and SMC proliferation, and is globally considered as antiatherogenic [10,142].
S-nitrosylation by NO
NO may covalently bind to protein cysteine residues, to form nitrosothiol groups (SNO). The S–NO bond can be formed by other NO-derived species, including N2O3, S-nitroso-glutathione or GSNO, and S-nitrosylated proteins [143,144]. S-nitrosylation is rapidly reversible, and involves various mechanisms, such as thioredoxin, GSNO reductase, transnitrosylation or ascorbate [145,146]. The post-translational modification of proteins by S-nitrosylation modifies protein function and cellular signaling, as reported for eNOS, which could be inhibited by NO through the S-nitrosylation pathway [147].
NO in physiological pregnancy
NO is a key-regulator of maternal systemic vasodilation, cardiovascular changes and blood pressure during pregnancy [[10], [11], [12]]. As recently reviewed by Sutton et al. [10], NO plays a critical role throughout pregnancy, including ovulation, implantation, vascular remodeling of spiral arteries, vascular tone, and feto-placental blood flow. In the early stages of pregnancy, NO is required for an optimal migration of trophoblasts, and the remodeling of uterine spiral arteries. The increased release of endothelial NO leads to spiral artery vasorelaxation, via a reduction of free calcium in SMC [148], which could finally facilitate cytotrophoblast invasion, artery remodeling and the regulation of the feto-placental blood flow [149].
Among other properties, NO inhibits the vasoconstriction evoked by endothelin-1 and TXA2 [[150], [151], [152]], and stimulates the production of the vasodilatory prostacyclin. NO is a main effector of VEGF production, and takes part in VEGF, fibroblast growth factor (FGF) and angiopoietin-1 signaling, so that it plays a key-role in vasculogenesis and angiogenesis during pregnancy [13]. A reciprocal relationship exists between NO and VEGF signaling, as NO and hypoxia upregulate VEGF gene expression by enhancing HIF-1α and heme-oxygenase 1 (HO-1) activities, while VEGF stimulates eNOS to produce NO via the activation of several signaling pathways including Akt/PKB, Ca(2+)/calmodulin, protein kinase C, or sphingosine 1-phosphate (S1P), as well as HIF-1α and HO-1 activation, depending on the rate of NO production [13,153,154].
Oxidative stress and NO bioavailability in PE
Conflicting reports exist concerning NO levels in PE. Most reports indicate that PE patients exhibit lower plasma levels of NO, evaluated through the determination of nitrite/nitrates assays, whereas other studies show no difference or even higher NO levels. The role of NO in pregnancy and PE, including NO substrate availability, NOS expression and activity, inhibition or increase in NO synthesis and the consequences for placenta and endothelium, have been recently comprehensively reviewed by Sutton et al. [10]. In the present article, we mainly focus on alterations evoked by oxidative stress on the NO/eNOS pathway.
NO and eNOS are intracellular sensors of the cellular redox state of the cell. In physiological conditions, when ROS production is low, NO acts as a buffer by reacting with O2•− and forming ONOO- [155]. As long as NO production is higher than that of ONOO-, this association has no consequences for cell homeostasis. In contrast, when ROS production exceeds NO production, eNOS becomes uncoupled and unable to produce NO [142,156]. The reduced bioavailability of NO results from the decrease in NO production and/or an increase in reactive oxygen species (ROS) production, leading to an imbalance between NO and O2•−, the formation of ONOO- and the inhibition of eNOS activity [157,158] (Table 1). Oxidative stress may affect NO synthesis substrates and eNOS activity or expression [159].

| Mechanisms | Consequences | Impact in PE | References |
|---|---|---|---|
| Increased O2•−production | Peroxynitrite formation | Increased | [160,161,162,163] |
| Sources:NOX2,Xanthine Oxidase, eNOS uncoupling | Decreased NO bioavailability | ||
| Nitration, inflammation | |||
| Increased arginaseactivity | l-arginine depletion | Increased | [[164], [165], [166], [167]] |
| Increased ADMA levels | eNOS uncoupling | [168,169] | |
| BH4 oxidation | eNOS uncoupling | No variation | [170] |
| Decreased NO generation | (very few studies) | ||
| Increased O2•−production | |||
| eNOS S-glutathionylation | eNOS uncoupling | Increased | [171] |
| Decreased NO generation | |||
| Increased O2•−production | |||
| LPP-adducts on eNOS | eNOS dysfunction | ||
| Decreased NO generation | Increased | [127] | |
O2•−, superoxide anion; NOX2, NADPH oxidase 2; NO, nitric oxide; ADMA, asymmetric dimethylarginine; BH4, tetrahydrobiopterin; eNOS, endothelial nitric oxide synthase; LPP, lipid peroxidation products.
Formation of peroxynitrite (ONOO-)
During PE, the decreased NO bioavailability in placenta may result from an increased production of O2•− which reacts with NO to form peroxynitrite ONOO- [14,83,172]. ONOO- is a powerful pro-oxidant and nitrating agent that causes many cellular damages, particularly on DNA and proteins [173], involved in inflammation, hypertension and toxicity [155,173]. ONOO- may post-translationally modify tyrosine residues on proteins to form 3-nitrotyrosine (protein nitration), that constitute a true “molecular fingerprint” of peroxynitrite formation [160]. The formation of 3-nitrotyrosine could be observed in normal pregnancy, but it is largely increased in the placental villous vascular endothelium, surrounding vascular smooth muscle and villous stroma in PE patients [[160], [161], [162]]. The occurrence of protein nitration in placentas, may lead to a gain or loss of function (loss of catalytic activity, impaired interactions with other molecules, increased degradation and reduced expression) [161,162]. This is of importance for signal transduction enzymes and transporters, which could be inactivated by nitration [174]. For instance, the nitration of phospho-p38 mitogen-activated protein kinase (p38MAPK) has been described in PE placenta, resulting in an inactivation and a reduction of expression, with possible consequences on trophoblast invasion and placental development [163]. Oxidative stress affects NO synthesis, substrate availability and eNOS co-factors.
Inhibition of eNOS enzymatic activity
Arginine depletion
The enzymatic activity of eNOS depends on its cofactors (BH4, FAD, FMN, NADPH), and on the availability of its substrate, l-arginine. Plasma l-arginine (80–120 μM) represents only 1.2% of the total fraction but contributes to approximately 60% of NO formation in endothelial cells [175]. The flow of l-arginine is not the only factor that affects its bioavailability within endothelial cells. In fact, the metabolism of this molecule is complex and involves different factors that can be influenced by oxidative stress. l-arginine can be degraded to ornithine and urea by arginase, an enzyme whose expression increases considerably in the presence of ROS [175]. An increased arginase activity is observed in plasma, platelets and vasculature of PE-affected women [164,165], which contributes to decrease NO levels and promote oxidative damages and endothelial dysfunction when associated with low SOD activity [[166], [167], [168]]. In addition, the arginine protein N-methyltransferase (PRMT) catalyzes the methylation of l-arginine into asymmetric dimethylarginine (ADMA), which is a competitive l-arginine inhibitor that prevents NO synthesis [169]. Significantly lower levels of l-arginine have been observed in PE patients, while plasma levels of ADMA were either not different between normal and PE patients, or more elevated, possibly contributing to oxidative stress and endothelial dysfunction [176,177]. Oxidative stress promotes the expression of PRMT, thereby contributing to the degradation of l-arginine and eNOS inhibition. Dimethylarginine dimethylargino hydrolase (DDAH) enables the breakdown of ADMA molecules into citrulline and dimethylamine [178]. However DDAH is also targeted and inhibited by ROS [179]. It is suggested that reduced levels of l-arginine, rather than increased ADMA levels, may play a role in PE [[180], [181], [182]].
Oxidation of the essential cofactor, BH4
BH4 has a complex metabolism, with two synthesis pathways, one from sepiapterin and one de novo synthesis from guanosine triphosphate (GTP) [182,183]. Dihydrofolate reductase (DHFR) is a key-enzyme involved in the first pathway. It limits the production of BH4 and is therefore decisive for the coupling state of eNOS. BH4 stabilizes the dimeric form of eNOS, allows the binding of the oxygen molecule and participates in the electronic transfer within the enzyme. In the absence of BH4, the heme-ferrous complex dissociates to form O2•−, and a ferric heme. BH4 depletion is generally attributed to its oxidation by ROS and precisely by ONOO-. It is then oxidized to dihydrobiopterin (BH2). In this form it can still bind to eNOS but no longer allows NO formation, and rather participates to the production of O2•−. The BH4/BH2 ratio is decisive for NO production [183,184].
The decrease of BH4 under conditions of oxidative stress seems to be a main cause of eNOS uncoupling [185]. Using a model of pregnant rats rendered hypertensive by treatment with deoxycorticosterone acetate, Mitchell and coll. demonstrated that exogenous BH4 administered as sepiapterin restored the endothelium-dependent relaxation responses of mesenteric arteries, increased vascular NO production, and reduced the generation of ROS (O2•− and ONOO−) [186]. However very few reports are available about BH4 deficiency in human PE. Toth et al., demonstrated that ascorbic acid (vitamin C) concentrations in placenta, are sufficient to stabilize and protect BH4 and the NO/eNOS pathway, so that it can be hypothesized that the reduction in vitamin C concentration during PE, may contribute to BH4 depletion [187]. A previous study from Kukor and coll. pointed out an important variability in BH4 concentrations in placentas, with globally no major differences between physiological and PE pregnancies [170]. Interestingly, this group reported that in some PE-affected patients, eNOS becomes “resistant” to BH4 stimulation, resulting in eNOS uncoupling, decreased NO, and increased radical anion superoxide production [188].
eNOS uncoupling by S-glutathionylation
Thiol groups (SH) on cysteine are highly sensitive to oxidative stress. Two SH groups can be oxidized to form a disulfide bond, found in glutathione disulfide (GSSG), and as mixed disulfides between protein and glutathione (S-glutathionylation). This reaction may occur either non-enzymatically, or via glutathione S-transferase [[189], [190], [191]]. In addition, S-glutathionylation can be promoted by NO, via mechanisms implicating S-nitrosoglutathione (GSNO) and thiyl radicals [191]. S-glutathionylated proteins reversibly accumulate under oxidative stress conditions and could be rapidly reduced by reducing agents and glutaredoxins [[190], [191], [192]]. S-glutathionylation alters the structure, folding and function of proteins, and can be considered as an adaptative and protective mechanism against the irreversible oxidation of cysteine residues during oxidative stress [190,191].
S-glutathionylation may modify the protein function when occurring on critical cysteine residues, as reported for eNOS in the vascular wall of hypertensive rats [193]. In this study, Chen et al. observed that upon oxidative stress conditions, two cysteine residues (Cys 689 and Cys 908) located in the reductase domain and critical to maintain eNOS function, were S-glutathionylated, leading to eNOS uncoupling, decreased NOS activity, increased O2•− generation, and impaired endothelium-dependent vasodilation [194]. This S-glutathionylation of eNOS was also observed in endothelial cells upon hypoxia-reoxygenation conditions [194].
We recently reported that eNOS is highly S-glutathionylated in PE placentas [171]. In this study, S-glutathionylation of eNOS was observed in normal placentas, but it was twice as high in PE placentas [171]. Around 40–45% of eNOS was S-glutathionylated in normal placentas, thus indicating that a moderate oxidative stress occurs in placentas during normal pregnancy. In PE placentas, the level of S-glutathionylated eNOS reached 75–80% (of total eNOS), supporting the occurrence of a higher oxidative stress [171]. Since S-glutathionylated eNOS retains approximately 30% of its activity [193], and since more than 50% of total eNOS was not modified, the rate of NO production should be not affected in normal physiological conditions of pregnancy. In contrast, in PE, the high level of eNOS S-glutathionylation suggests that NO production and bioavailability should be strongly reduced, with possible consequences on NO bioavailability and subsequent placentation, spiral artery remodeling, feto-placental circulation and maternal blood pressure regulation [171]. Moreover, uncoupled eNOS generates O2•–, which may enhance oxidative stress, inactivate NO and reduce placental blood flow. In short, S-glutathionylation results in eNOS uncoupling, which decreases NO generation and increases ROS production, leading to NO inactivation and oxidative stress. Thus, the high S-glutathionylation level of eNOS in PE placentas, may be both cause and consequence of oxidative stress and eNOS dysfunction in this disease [171].
Post-translational modifications of eNOS by lipid peroxidation products
As developed in § 2.2, lipid peroxidation-derived aldehydes (acrolein, HNE, MDA, ONE …), may covalently bind to the nucleophilic sulfhydryl and primary amine groups of proteins, to form adducts which modify protein structure and function, depending on their nature, oxidative stress intensity and duration [[109], [110], [111]]. Post-translational modifications evoked by lipid peroxidation-derived aldehydes, affect a huge number of proteins with consequences on their structure and activity (gain or loss of function) [[109], [110], [111], [112], [113], [114], [115]]. As recently reported, eNOS could be modified by HNE and at a higher extent, by ONE, a highly reactive lipid peroxidation-derived aldehyde, abundantly present in PE placentas [127]. ONE can bind several Lys-residues on eNOS, and inhibits its enzymatic activity by modifying Lys residues involved in cofactors binding sites. Indeed, LC-MS/MS analysis of recombinant eNOS modified by ONE, and 3D-modelling of the enzyme, showed that ONE-modified Lys residues are located close the Ca2+-calmodulin (K519), and the FAD/NADPH (K1085) binding sites, which may hinder the interaction of eNOS with these cofactors [127]. Moreover, the addition of ONE to recombinant eNOS, or to cultured cytotrophoblasts strongly decreased the production of NO and the migration of these cells [127]. HNE-, MDA- and acrolein-adducts were found in PE placentas, but were poorly detected on eNOS, in spite of the ability of HNE to bind several Cys, His and Lys residues, without major consequences for eNOS enzymatic activity [127]. HNE and ONE are chemically close and differ at the C4 position, with a ketone group for ONE, in place a hydroxyl group on HNE [195]. However, ONE is more reactive than HNE on protein nucleophiles, particularly on Lys, on which it rapidly forms readily reversible Schiff base adducts that can be oxidized to stable 4-ketoamide adducts [195,196]. ONE has a higher capacity than HNE to form cross-links on proteins, and particularly Lys–Lys cross-links. In addition, the neutralization of aldehydes by GSH is different for ONE and HNE. HNE can be rapidly neutralized by GSH, so that GSH-HNE-Michael conjugates are unable to react with proteins [197]. In contrast, ONE is not neutralized by GSH which rather increases its ability to modify proteins by irreversible glutathionylation, not reversed by reductant agents [197,198].
Inhibition of the NO/eNOS system in animal models for PE
Many attempts have been tried to generate animal models of PE, partly unsuccessful, because these models do not include the complete pathophysiological features of the PE human disease, which may limit their interest for studying the mechanisms and new therapeutic treatments [[199], [200], [201]]. However, the inhibition of the NO/eNOS system has been largely used, and produces several patterns that resemble human PE. Different models have been developed including mice knock-out for the NOS3 gene which encodes eNOS [202,203]. These mice are hypertensive, but their blood pressure is not particularly increased during pregnancy, when compared to wild type animals [204]. Likewise, the knock-out of other NOS isoforms (neuronal nNOS and inducible iNOS), does not generate hypertension in pregnant mice [204]. Mice knock-out for NOS3 and overexpressing the angiotensinogen gene (Agt(2/2)), exhibit higher blood pressure throughout pregnancy [205], but no renal nor liver alterations. As discussed by Marshall et al., eNOS-KO mice are not a model of hypertension suitable for studying PE, in contrast to other models of hypertension using chronic NOS inhibition [206].
Several studies used a model based on the chronic inhibition of NO synthesis by the administration of L-nitro-arginine methyl ester (l-NAME) to pregnant rats or mice. These models exhibit clinical patterns of PE, including hypertension, fetal growth retardation, renal vasoconstriction, glomerular filtration rate, proteinuria, increased maternal and fetal mortality, and are suitable for studying the pathophysiology of PE or for therapeutical preclinical purposes [[199], [200], [201],[207], [208], [209], [210], [211]].
Therapeutic perspectives targeting oxidative stress and NO/eNOS dysfunction
In PE, there is currently no other effective treatment than fetus delivery, with the risk of major complications for the newborn related to prematurity. A huge number of therapeutic approaches, new and repurposed drugs have been evaluated in clinical trials for PE. Given the importance of oxidative stress and NO, many attempts were carried out to restore the redox imbalance and NO bioavailability. In this article, we present only a summary of studies focused on antioxidant or NO supplementation.
Antioxidants
Most data concerning the use of antioxidants for preventing PE, have been comprehensively reviewed by Salles et al. [105] and more recently by Tenorio et al. [212]. The conclusions of both interventional studies (heterogenous in the inclusion or exclusion criteria, nature and concentration of antioxidants, duration of the treatment), and multicenter, randomized, double-blind clinical trials based on vitamins C and E supplementation in early pregnancy, showed no significant amelioration nor reduction of the adverse maternal or perinatal outcomes in women at high risk [105,[212], [213], [214]].
Several other antioxidants were tested for PE prevention, among them lycopene, a tetraterpene carotenoid, which gave encouraging results by reducing PE and IUGR [215], but with several outcomes for the fetus [212,216]. Supplementation by N-acetylcysteine, a GSH precursor, did not result in any significant benefit [217].
As reviewed by Xu et al., the selenium levels are lower in PE patients compared to healthy pregnant women, so that meta-analysis were carried out to evaluate the efficacy of selenium supplementation in preventing PE, with a tendency to amelioration [218]. However, as discussed by the authors, more prospective clinical trials would be necessary to reach reliable conclusions on selenium supplementation, with better definition of the dose and timing (beginning and duration) of the treatment [218].
These disappointing results for antioxidants, particularly vitamin E and C, could be explained by several hypotheses including the fact that i/PE is a multifactorial disease, and ROS are not the primary cause of the disease. ROS are co-factors and amplifiers of many redundant signaling pathways, which are independently activated by their own systems, thus inhibiting ROS may be not sufficient to block the responses evoked by inflammatory agents in PE; ii/physiological ROS are produced and are necessary for normal pregnancy, so that their inhibition may have unexpected aggravating consequences on pregnancy outcomes iii/there is a redundancy of ROS producing and neutralizing systems, not necessarily targeted by vitamins E and C, iv/possible limits of vitamin E and C (and other antioxidants), could exist concerning their availability and distribution in placentas, explaining their poor efficacy in PE, as observed in coronary patients [100].
Targeting the NO/eNOS pathway
l-arginine supplementation
Several clinical trials were carried out with l-arginine supplementation, and were recently comprehensively reviewed by Weckman et al. [219]. These studies globally showed a significant amelioration of maternal and fetal outcomes, with a reduction of hypertension and higher birth weight [220,221]. However, it seems that these parameters could be not statistically significant [220] and a short-term supplementation in l-arginine seems insufficient to improve maternal hemodynamics, particularly in later pregnancy. Moreover, l-arginine supplementation may generate ONOO- [222].
Inhibitors of type-5 phosphodiesterase (PDE5)
These inhibitors reduce cGMP degradation, thus increase the levels of cGMP, restore a normal function of the NO-cGMP pathway, and generate a sustained vasodilation [223]. Several clinical trials and meta-analysis were carried out with the use of Sildenafil in PE treatment, showing that globally, this agent attenuates oxidative stress and vasoconstriction, and restores eNOS function. Most studies indicate that Sildenafil ameliorates the uterine blood flow, uterine vascular resistance, and fetal weight [223]. However trials based on Sildenafil were recently suspended in view of the unexplained death of newborns from Sildenafil-supplemented women [224].
NO donors
A pilot study carried out by Groten et al., used a supplementation with the NO donor pentaerithrityl tetranitrate (PETN) in pregnant women at risk [225]. This randomized controlled multicenter-trial concluded that PTEN treatment significantly improved the uteroplacental flow, and reduced preterm births, PE outcomes and IUGR, pointing out a potential benefit of NO donors in PE. In contrast, a Cochrane analysis based on randomized trials using NO donors (glyceryl trinitrate) or precursors (l-arginine), concluded to a lack of reliable conclusions about their efficacy to prevent PE complications [226]. Finally, a systematic review is currently under investigation, to evaluate published randomized control trials investigating the ability of different NO agents to prevent PE [227].
Aspirin
So far, it seems that low dose aspirin prophylaxis (60–150 mg/day), may represent an effective preventive treatment, which reduces PE incidence and outcomes when initiated before 16 weeks of gestational age, and given to patients at high risk of developing PE [[27], [28], [29], [30],[228], [229], [230], [231]]. Aspirin (acetyl salicylic acid), has many pharmacological, anti aggregant and anti-inflammatory properties. It inhibits the redox-sensitive transcription factor NF-κB, and the activity of COX1 and COX2, resulting in a strong reduction in the production of prostaglandins and TXA2 [232]. Moreover, aspirin reduces oxidative stress by inducing HO-1 activation [233]. Importantly, aspirin-acetylated COX2 may generate lipoxins from arachidonic acid [234], or “aspirin-triggered lipoxins” (ATLs or lipoxin-A4). These ATLs are potent anti-inflammatory mediators able to decrease oxidative stress and inflammation evoked by the plasma of women affected with PE [235]. ATLs could also stimulate the release of NO, by activating both eNOS and iNOS [235]. In addition, aspirin is able to acetylate and stimulates the enzymatic activity of eNOS, and promotes a release of NO from endothelial cells [236]. Recent studies indicated that aspirin could trigger a vasodilation of small uterine arteries in gravid rats, via an activation of eNOS and an increased NO production [237]. These data suggest that low-dose aspirin may enhance vasodilation and uteroplacental blood flow, by preventing NO reduction and promoting its production. Further studies will be needed to confirm the links between aspirin, eNOS, NO and oxidative stress in the early stages of pregnancy when placentation is developing, knowing that no efficacy is observed with late initiation of aspirin prophylaxis [[27], [28], [29], [30],231].
Conclusion
In this review, we aimed at summarizing the main findings showing the relationships existing between oxidative stress and the dysfunction of the NO/eNOS pathway in PE. Placental oxidative stress strongly alters NO production either by promoting its inactivation via the production of ONOO-, or by inhibiting eNOS activity via eNOS uncoupling. Several mechanisms are involved, including an oxidation of the cofactor BH4, or an accumulation of the arginine inhibitor ADMA, or an increased arginase activity, or S-glutathionylation (oxidized glutathione inducing a post-translational modification of cysteine residues of eNOS that are critical for maintaining eNOS function). The post-translational modification of eNOS by ONE and possibly by other lipid peroxidation aldehydes, could be another cause of eNOS dysfunction directly resulting from oxidative stress. The pathophysiology of PE is complex, with many interconnections between inflammation, oxidative stress and antioxidant defense systems. This may explain why supplementation with antioxidants, or NO donors gave controversial and unconvincing results, and why it is so difficult to find a consensus on treatments targeting oxidative stress and the NO pathway. Additional researchs are needed to deepen the pathophysiology of PE, and develop new innovative therapeutic approaches to prevent this disease and reduce its health outcomes.
Fundings
This work was supported by INSERM (Institut National de la Santé et de la Recherche Médicale), and by University Paul Sabatier Toulouse. Dr Paul Guerby holds a postdoctoral Award from Fonds de recherche du Québec – Santé (FRQ-S) and INSERM.
Declaration of competing interest
None.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
135
136
137
138
139
140
141
142
143
144
145
146
147
148
149
150
151
152
153
154
155
156
157
158
159
160
161
162
163
164
165
166
167
168
169
170
171
172
173
174
175
176
177
178
179
180
181
182
183
184
185
186
187
188
189
190
191
192
193
194
195
196
197
198
199
200
201
202
203
204
205
206
207
208
209
210
211
212
213
214
215
216
217
218
219
220
221
222
223
224
225
226
227
228
229
230
231
232
233
234
235
236
237
 Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia
Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia