
These authors contributed equally to this work.
- Altmetric
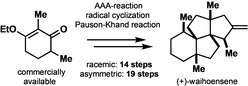
A racemic and scalable enantioselective total synthesis of (+)‐waihoensene was accomplished. (+)‐Waihoensene belongs to the diterpene natural product family, and it features an angular triquinane substructure motif. Its tetracyclic [6.5.5.5]backbone is all‐cis‐fused, containing six contiguous stereocenters, four of which are quaternary. These structural features were efficiently installed by means of a diastereoselective radical cyclization, followed by an intramolecular Pauson–Khand reaction, a diastereoselective α‐alkylation, and a diastereoselective 1,4‐addition reaction. Enantioselectivity was introduced at an early stage, by an asymmetric palladium catalyzed decarboxylative allylation reaction on gram scale.
An efficient, racemic, and enantioselective total synthesis of the diterpene waihoensene is presented. Key steps are a radical cyclization, a Pauson–Khand reaction, and an asymmetric allylic decarboxylative allylation (AAA) reaction to obtain optical active material.
Terpene natural products containing polyquinane structure motifs constitute a diverse structural subclass prominent in sesqui‐ and di‐, as well as sesterterpenes, and ever since have attracted great attention from the synthetic community. [1] Angular triquinanes 2 in particular pose a synthetic challenge, since their principal structure element implies the existence of a quaternary stereocenter [2] (blue dot structure 2 in Figure 1), as opposed to linear triquinanes 3, where a quaternary stereocenter is not a prerequisite.

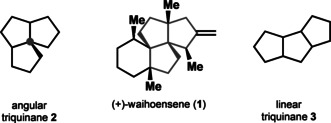
Comparison of the linear and angular triquinane structure element.
Waihoensene (1) harbors such an angular triquinane substructure, and moreover contains altogether four quaternary stereocenters which are aligned in a contiguous fashion across the carboskeleton. The diterpene waihoensene (1) has been isolated from the plant Podocarpus totara var. waihoensis native to New Zealand in 1997 by the Weavers group. [3] Structural analysis of waihoensene (1) reveals a tetracyclic fused ring‐skeleton with six stereocenters embedded of which four are quaternary stereocenters. All stereocenters are contiguously arranged, making it a very densely packed molecule sterically utmost encumbered. In addition, waihoensene (1) does not contain any functional groups beside a single double bond thus representing a pure hydrocarbon. This specific feature might look innocent in the first place, but upon synthetic planning it turns out to represent one of the greatest synthetic obstacles for an efficient synthesis, besides the four quaternary stereocenters present in the natural product. Since most C−C bond forming reactions require functional groups, the lack of functionalization (or in biosynthetic terms—lack of oxygenation of the natural product) inevitably entails over‐functionalization of synthetic intermediates (overshooting molecular complexity), and eventually requires application of additional de‐functionalization reactions (FGIs), lengthening the overall synthetic route. Waihoensene (1) has been synthesized previously by three groups, who devised different synthetic strategies depicted in Figure 2.[ 4 , 5 , 6 , 7 ] The first accomplished total synthesis in racemic form followed a tandem [3+2] cycloaddition strategy, and was published in 2017 by Lee and co‐workers. [5] Recently, in 2020 two further syntheses were published involving a Conia–Ene/Pauson–Khand strategy by the groups of Huang & Zhang [6] and the group of Snyder. [7] Both latter synthetic approaches are enantioselective with a step count ranging of 15 and 16 steps. By contrast Lee's racemic synthesis required 21 steps.

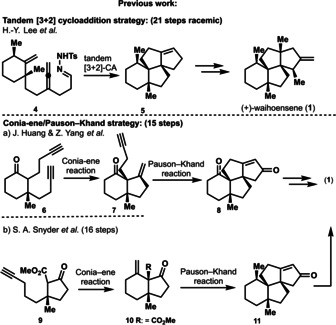
Synthetic strategies of previous syntheses.
Herein we report both, an enantioselective synthesis of waihoensene (1) and in racemic form, with an overall step count of 14 (racemic) and 19 (enantioselective) steps. Our strategy was guided by first establishing one of the four quaternary stereocenters via an asymmetric allylation reaction to give compound 27 (Scheme 3). [8] We then assembled the hydrindane part 18 of waihoensene (1) by a radical cyclization reaction together with establishing the methyl‐group at C7 at the same time. [9] By contrast, all other syntheses do not tie these two transformations together. This bicycle 18 served as rigid template for the Pauson–Khand reaction [10] to give the carboskeleton of 1. Compound 11 was eventually converted to waihoensene (1) in a three‐step sequence.

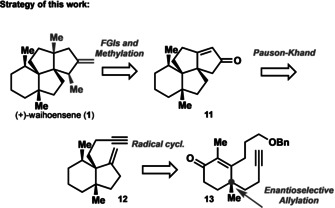
Retrosynthetic analysis this work's synthesis.
On the outset, we started our synthetic racemic sequence from commercially available compound 14 (Scheme 1). Introduction of the quaternary center to 14 was accomplished by an alkylation reaction with homopropargylic triflate to yield 16 in 80 %. This was followed by an addition of lithiated 17 to the vinylogous ester in 16 and in situ elimination reaction to afford enone 13 in 89 % yield. At this point the radical cyclization reaction established the desired hydrindane scaffold 18 in 95 % yield as an inconsequential diastereomeric mixture at C7. Destannylation of 18 and consecutive treatment with sodium hydroxide in methanol established the desired configuration of the C7 methyl group by equilibration to give 97 % of desired all‐cis 19 in 88 % yield. The correct configuration of the methyl‐group and the cis‐fusion of the hydrindane system was confirmed by single crystal X‐ray analysis after converting the ketone into the 2,4‐di‐nitrophenyl hydrazone. [11]

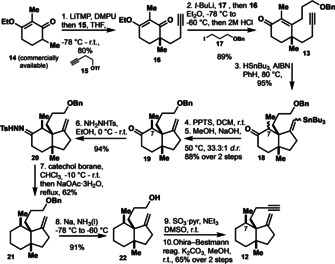
Synthesis of the Pauson–Khand precursor 12.
The high preference for the desired configuration of the C7 methyl group in 19 is attributed to minimization of 1,3‐allyl‐strain. Defunctionalization of the ketone in 19 was accomplished by conversion to hydrazone 20 followed by reduction with catechol borane to give compound 21. This was converted to alkyne 12 by deprotection under Birch conditions, Parikh–Doering oxidation to the corresponding aldehyde, and Ohira‐Bestmann alkynylation. The intermediate aldehyde tends to undergo acid‐mediated Prins cyclization and was therefore used in the next step without purification. Alkyne 12 poses the precursor for the Pauson–Khand reaction, and it was treated with dicobalt‐octacarbonyl to give the corresponding cobalt‐alkyne complex. This intermediate underwent cyclization and CO‐insertion under forcing conditions to yield 46 % of the desired product 11, and thus delivering the fully established core of waihoensene (1; Scheme 2). Fortuitously, we obtained a single crystal [12] of this key intermediate and therefore we were able to unambiguously establish the correct configuration of 11.

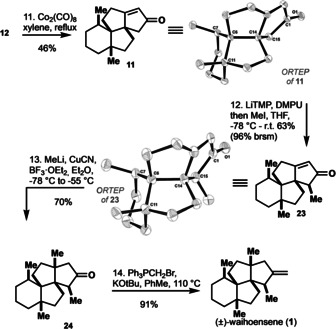
Pauson–Khand reaction of 12 and endgame to waihoensene (1).
The endgame of the synthesis consisted of a three‐step sequence starting with α‐alkylation of Pauson–Khand product 11 to give 23 as a single diastereomer. From this point onward, our synthesis deviates from all previously published ones, who share a common endgame consisting sequentially of a 1,4‐addition of the methyl‐group to enone 11, subsequent α‐alkylation, and final olefination to give waihoensene (1). By contrast to these reports, [5] we encountered no difficulties upon first performing an α‐alkylation of 11, and were able to confirm the desired stereochemistry of the newly formed stereocenter by single crystal X‐ray analysis of product 23. [13] The choice of base and additive in this α‐alkylation of 11 is crucial with regard to the competing formation of kinetic and thermodynamic enolates. Strong bases, that is, LDA (lithium diisopropylamide), LiTMP (lithium 2,2,6,6‐tetramethylpiperidid) and LiICA (lithium isopropylcyclohexylamide) in combination with DMPU give desired methylated 23 as the only product, however incomplete conversion lead to concurrent re‐isolation of starting material in all cases. The use of weaker bases such as LiHMDS lead to the formation of the thermodynamic enolate (deprotonation in γ‐position of the enone moiety). 1,4‐Addition of methyl cuprate to 23 delivered 24 in 70 % yield, and again exclusively gave a single stereoisomer. The synthesis was concluded by Wittig olefination to deliver the natural product waihoensene (1) in 91 % yield and a very concise overall 14 step sequence. For obtaining optically active material, we decided to proceed via an enantioselective allylation reaction [14] to introduce the first of the four quaternary stereocenters (Scheme 3). We thereby started from vinylogous thioester 25, [15] which was required in order to obtain excellent enantioselectivity. Quaternarization of 25 with methyl iodide delivered 26 [14] and palladium catalyzed decarboxylative allylation using Trost's ligand [16] furnished the desired optically active 27 with 96 % ee and 87 % yield. Conversion of the vinylogous thioester to its corresponding methylester, manipulation of the allyl side chain in three steps (hydroboration/oxidation and Ohira–Bestman reaction), and addition elimination reaction of 30 gave synthetic intermediate (+)‐13 in full accordance with the racemic route. The enantioselective route is very robust and was executed on gram scale with no loss of yields and optical activity. Therefore, the enantioselective material was funneled into the racemic route and eventually delivered (+)‐waihoensene (1) in analogous fashion and 19 overall steps.

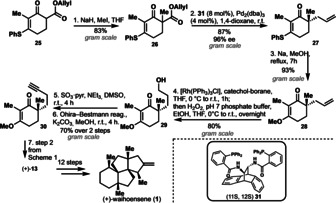
Enantioselective approach to (+)‐waihoensene (1) by an asymmetric allylation reaction.
In conclusion, we have developed the so far shortest route to (±)‐waihoensene (1) consisting of only 14 steps and delivering racemic material. For the enantioselective route we require 19 steps and are able to produce optical active material 30 on gram scale. Our synthetic strategy to assemble the carboskeleton features a radical cyclization to form the hydrindane part 18 of waihoensene as a rigid template for further C−C‐bond forming reactions. The skeleton is constructed via the Pauson–Khand reaction to deliver the final product (+)‐waihoensene (1) in a three‐steps containing endgame which introduces two additional stereocenters.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank the NMR department of the University of Konstanz (A. Friemel and U. Haunz) for extensive analyses, and Heiko Rebmann and Dr. Thomas Huhn for X‐ray analysis and structure refinement. We thank Dr. Gerald Dräger from the Institute of Organic Chemistry of the Leibniz University Hannover and Malin Bein from the University of Konstanz for mass spectroscopic analysis. Open access funding enabled and organized by Projekt DEAL.
References
1
1b
2
2
3
4
4b
5
5
6
7
7
8
9
9a
9b
9c
10
11
12
13
14
14
16
16a
 Total Synthesis of the Diterpene Waihoensene
Total Synthesis of the Diterpene Waihoensene
