
- Altmetric
Acute lung injury (ALI) is a devastating clinical syndrome with no effective therapies. Inflammasome activation has been reported to play a critical role in the initiation and progression of ALI. The molecular mechanisms involved in regulating the activation of inflammasome in ALI remains unresolved, although increases in mitochondrial derived reactive oxygen species (mito-ROS) are involved. Our previous work has shown that the mitochondrial redistribution of uncoupled eNOS impairs mitochondrial bioenergetics and increases mito-ROS generation. Thus, the focus of our study was to determine if lipopolysaccharide (LPS)-mediated inflammasome activation involves the mitochondrial redistribution of uncoupled eNOS. Our data show that the increase in mito-ROS involved in LPS-mediated inflammasome activation is associated with the disruption of mitochondrial bioenergetics in human lung microvascular endothelial cells (HLMVEC) and the mitochondrial redistribution of eNOS. These effects are dependent on RhoA-ROCK signaling and are mediated via increased phosphorylation of eNOS at Threonine (T)-495. A derivative of the mitochondrial targeted Szeto‐Schiller peptide (SSP) attached to the antioxidant Tiron (T-SSP), significantly attenuated LPS-mediated mito-ROS generation and inflammasome activation in HLMVEC. Further, T-SSP attenuated mitochondrial superoxide production in a mouse model of sepsis induced ALI. This in turn significantly reduced the inflammatory response and attenuated lung injury. Thus, our findings show that the mitochondrial redistribution of uncoupled eNOS is intimately involved in the activation of the inflammatory response in ALI and implicate attenuating mito-ROS as a therapeutic strategy in humans.

•
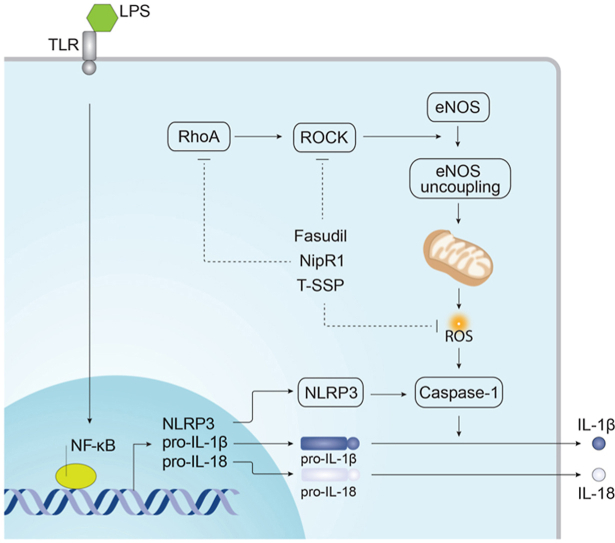
The mitochondrial redistribution of uncoupled eNOS is involved in LPS-mediated activation of the NLRP3 inflammasome.
•Nitration-mediated activation of RhoA is involved in the activation of NF-κb signaling during sepsis.
•Blocking RhoA nitration attenuates the increase in mitochondrial ROS required for NLRP3 inflammasome activation.
•A mitochondrial targeted antioxidant reduces NLRP3 inflammasome activation and sepsis mediated lung injury.
Introduction
Acute lung injury (ALI), and its clinical form acute respiratory distress syndrome (ARDS), are devastating conditions characterized by an excessive inflammatory response associated with alveolar-capillary barrier disruption and pulmonary edema within the lungs [[1], [2], [3]]. Lipopolysaccharide (LPS) is derived from the outer membrane of Gram-negative bacteria, and its exposure can cause sepsis-associated ALI and endothelial barrier dysfunction. The common pathophysiologic processes of LPS-induced ALI are dysregulated inflammation, neutrophil recruitment across the endothelium into the lung, altered permeability of alveolar endothelial and epithelial barriers, pulmonary edema and finally in the impairment of gas exchange [1,4]. Among the numerous signaling pathways involved in LPS-induced ALI, nuclear translocation of nuclear factor-κB (NF-κB) occupies an important position which increases the expression of pro-inflammatory mediators such as NLRP3, IL-1β and IL-18 [5]. NLRP3 can then bind to the adapter apoptosis-associated speck-like protein (ASC) containing a C-terminal caspase recruitment domain (CARD), which induce the autocatalysis of pro-caspase-1 into its cleaved form leading to the maturation and secretion of proinflammatory cytokines IL-1β and IL-18 [6].
Accumulating evidence indicates that Ras homolog gene family (Rho) of small GTPases serve important pathophysiological roles in the regulation of endothelial barrier function [7]. RhoA, through its downstream effector, Rho-kinase (ROCK), promotes actin-cytoskeletal re-assembly and cell contraction, resulting in junction protein remodeling and loss of endothelial barrier integrity [[8], [9], [10]]. Therefore, abnormal activation of RhoA/ROCK is an underlying mechanism of LPS-induced sepsis [[11], [12], [13]]. Increased generation of reactive oxygen species (ROS), resulting in oxidative stress, is also considered to be an important early contributor to the pathogenesis of ALI. Excessive ROS in combination with increased inflammation have been proposed to be major causes of the endothelial and epithelial barrier dysfunctions associated with acute lung injury [14]. Further, our previous studies have shown that endothelial NO synthase (eNOS) derived peroxynitrite and subsequent protein nitration mediates the LPS induced activation of RhoA, the disruption of lung mechanics and the production, and secretion, of pro-inflammatory cytokines [15]. ROS can also stimulate pro-inflammatory cytokine production in response to LPS, which plays an important role in activation of the NLRP3 inflammasome [16]. However, the discrete molecular mechanisms involved in regulating the activation of inflammasome in ALI remains unclear.
Our previous studies have demonstrated that the mitochondrial redistribution of uncoupled eNOS can increase mito-ROS generation secondary to the disruption of mitochondrial bioenergetics [4]. Thus, in the present study, we investigated the role of uncoupled eNOS and the disruption of mitochondrial bioenergetics in inflammasome activation during LPS-mediated ALI. Our data indicate that in human lung microvascular endothelial cells (HLMVEC) exposed to LPS, RhoA-ROCK mediated signaling increases eNOS phosphorylation at T495 leading to its uncoupling and mitochondrial redistribution. The resulting disruption of mitochondrial bioenergetics leads to an increase in mito-ROS levels and the activation of the inflammasome. Further, using a derivative of the mitochondrial targeted Szeto‐Schiller peptide (SSP) attached to the antioxidant, Tiron (T-SSP) we were able to demonstrate that T-SSP treatment significantly attenuated LPS-mediated mito-ROS generation and inflammasome activation in both HLMVEC and a mouse LPS-model of ALI. These data identify a novel pathway involved in LPS-induced inflammasome activation during ALI and suggest that suppressing mito-ROS or RhoA activation may provide a promising therapeutic strategy to prevent ALI progression.
Materials and methods
Cell culture
Primary HLMVEC were purchased for Lonza and cultured in basal VascuLife VEGF Endothelial Medium supplemented with VasculifeVEGF-Mv factors kit (Lifeline cell technology) and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin) according to the manufacturer's instruction and maintained at 37 °C in a humidifier with 5% CO2 and 95% air. Cells were used between passages 5–8.
Mitochondrial bioenergetics
The XF24 Analyzer (Seahorse Biosciences, North Billerica, MA, USA) and XF Cell Mito Stress Test Kit (# 101706-100; Seahorse Biosciences) were used for the mitochondrial bioenergetic analyses. The optimum number of cells/well was determined to be 75,000/0.32 cm2. Cells were exposed to LPS (2 EU/ml, 4 h). The XF24 culture microplates were then incubated in a CO2-free XF prep station at 37 °C for 45 min to allow temperature and pH calibration. For the Mito Stress test, we sequentially injected Oligomycin (1 μM final concentration), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 1 μM final concentration), and Rotenone + antimycin A (1 μM final concentration of each) and measured the oxygen consumption rate (OCR). Using these agents, we determined basal mitochondrial respiration, reserve respiratory capacity and maximal respiratory capacity measurements in pmoles/min of oxygen consumed.
Mitochondrial ROS levels
MitoSOX™ Red mitochondrial ROS indicator (Molecular Probes, Grand Island, NY) a fluorogenic dye for detection of ROS in the mitochondria of live cells was used. Briefly, cells after treatment were washed with fresh serum free medium, incubated with MitoSOX Red (5 μM), for 30 min at 37 °C in dark conditions, then subjected to fluorescence microscopy using an excitation of 510 nm and an emission at 580 nm (for MitoSOX). An Olympus IX51 microscope equipped with a CCD camera (Hamamatsu Photonics) was used for acquisition of fluorescent images. The average fluorescent intensities (to correct for differences in cell number) were quantified using ImagePro Plus version 5.0 imaging software (Media Cybernetics).
Western blot analysis
Protein lysates were extracted from cultured cells or mice lung tissues using RIPA Lysis and Extraction Buffer (Thermo Fisher) containing Halt™ Protease and Phosphatase Inhibitor Single-Use Cocktail (100X) (ThermoScientific). The lysates were centrifuged 13,000 g ×20 min at 4 °C. Protein concentration was determined by using a Pierce™ bicinchoninic acid (BCA) Protein Assay Kit (Thermo fisher) according to the manufacturer's recommendations. Equal amounts of protein (30 μg) from each sample were separated on SurePAGEᵀᴹ, Bis-Tris, gels (Genscript) and subsequently transferred to Immuno-Blot™ PVDF membrane (Bio-Rad Laboratories, Hercules, CA), subsequently blocked with 5% nonfat dry milk in Tris-buffered saline for 1 h. Then the membranes were incubated with anti-rabbit NLRP3, (cleaved) caspase-3, (cleaved) IL-1β, RhoA, pS536 p65, iNOS (Novus), anti-mouse eNOS and pT495 eNOS (BD Biosciences) antibodies overnight at 4 °C. Reactive bands were visualized using chemiluminescence (Super Signal West Femto; Pierce, Rockford, IL) on a LI-COR Odyssey image station (Lincoln, NE). Bands were quantified using LI-COR Image Station software. Loading was normalized by reprobing the membranes with an antibody specific to β-actin.
RhoA activity assay
RhoA activity was performed using a Rhotekin Rho-binding domain pulldown assay kit in accordance with the manufacturer's instructions (Cell Signaling Technology, Inc., Denver, CO). Briefly, 2 x 106 cells were seeded in 10-cm dishes and incubated overnight in VascuLife VEGF Endothelial Medium. The cells were pretreated with Fasudil (10uM) or NirpR1(100 ng/ml) for 30 min then exposed to LPS (2EU/ml, 6 h). The level of active RhoA pulled down by the assay was measured by Western blot analysis.
mRNA analysis
Total RNA was isolated from HLMVECs using QIAshredder (Qiagen) and RNeasy Mini Kit (Qiagen); 2 μg of total RNA were converted to cDNA using SuperScript VILO Master Mix (Thermo Fisher). cDNA samples were diluted 10 times with DNAse-/RNAse-free water, and 2μl of resulted cDNA solution were used for qPCR in MicroAmp EnduraPlate Optical 96-Well Clear Reaction Plates (Thermo Fisher) using QuantiTect SYBR Green PCR Kit (Qiagen) in QuantStudio 3 System (Applied Biosystems). Data obtained were analyzed using the 2-ΔΔCt method to quantitate relative mRNA expressions. β2-microglobulin expression was used as a reference (housekeeper).
Animals
Pyrogen-free, 6-8-week old male C57BL/6 mice were purchased from Jackson Laboratory. Animals were housed under controlled environmental conditions (12/12 h of light/dark cycle, 55% ± 5% humidity, 22–24 °C). Mice were given free access to standard laboratory chow and water. All animal studies were approved by the institutional animal care and use committee at University of Arizona and complied with the animal welfare act. The methods applied in this study were carried out in accordance with the approved guidelines.
LPS model of ALI
Age- and weight-matched male mice (n = 20) were anesthetized by intramuscular injection of ketamine/xylazine (100/2 mg/kg) admixture and randomized into one of the following 4 study groups: Control, T-SSP, LPS, and LPS + T-SSP (n = 5 per group). ALI was induced by intratracheal instillation of LPS (2 mg/kg in 50 μl saline). Mice in PBS and T-SSP groups received intraperitoneal (i.p.) injection of saline and T-SSP (5 mg/kg) 1 h prior to LPS challenge, respectively. The experiment was terminated at 6 h after LPS inhalation, and lung tissues and BALF were harvested and collected thereafter.
Measurement of leukocyte infiltration and protein content in BALF
BALF was collected after the lungs were lavaged with 1 ml PBS via a tracheal cannula. Centrifuge the BALF at 4 °C, 500 g for 10 min. Total leukocyte counts in BALF were carried out using a standard haemocytometer. Total protein concentrations in BALF were determined using a BCA protein assay kit (Piece, Rockford, IL, USA).
IL-1β detection
HLMVEC were pretreated with Fasudil (10uM), NirpR1(100 ng/ml) or T-SSP (100 nM) for 30 min respectively, then exposed to LPS (2EU/ml) for 6 h. Then the cell culture medium of each group was collected for IL-1β secretion content detection by using IL-1 beta Human High Sensitivity ELISA Kit (Invitrogen) according to the manufacturer's instructions. IL-1β concentration in the BALF was determined using a mouse ELISA IL-1β Kit (Invitrogen) according to the manufacturer's instructions. The absorbance at 450 nm was measured using a 96-well plate reader.
Measurement of mitochondrial superoxide levels in mouse lung
To measure mitochondrial superoxide levels in lung tissue, we performed electron paramagnetic resonance (EPR) measurements. Mouse lung tissues was pulverized to form fine tissue powder. Then 4–6 mg of tissue powder was dissolved in 300 μL of EPR buffer (PBS supplemented with 5 uM diethyldithiocarbamate and 25uM desferrioxamine; Sigma-Aldrich) at 0 °C in a temperature-controlled cooling shaker (UltraCruz; Santa Cruz). The dissolved powder (75 μL) was separated between two 1.5 ml tubes containing spin probe CMH (5 mg/ml) only and CMH plus the mitochondria respiratory chain inhibitor, Rotenone (1 mM) and incubated for 2 min at 0 °C. 30 μl of tissue suspension from each tube was transferred to a glass capillary at room temperature. Generation of superoxide in capillary took place for 30 min at room temperature. Superoxide radical generation was measured by reaction with spin probe CMH at room temperature in Magnettech M100 instrument (Magnettech, Germany). EPR spectra were analyzed for amplitude using ANALYSIS 2.0 software (Magnettech). To quantitate the amount of superoxide per milligram of protein, we also performed a standard reaction of the superoxide-generating enzyme xanthine oxidase in the presence of xanthine and CMH. Final superoxide generation rate was expressed as pmol/min/mg of tissue.
Myeloperoxidase staining
Sections (5 μm) were cut from paraffin blocks and mounted on treated slides (Superfrost plus; Fisher Scientific). Slides were air dried overnight, placed in a 60 °C oven for 30 min, deparaffinized in xylene, and run through graded ethanol to distilled water. Endogenous peroxidases were quenched with 0.3% H2O2 for 5 min followed by two rinses with distilled water. Slides were pretreated with target retrieval solution, citrate pH 6, rinsed in distilled water, incubated in Power Block, rinsed in distilled water, placed in 1x PBS for 5 min then incubated with anti-myeloperoxidase (MPO) antibody (1:200 dilution, Abcam, Cambridge, MA, USA) for 30 min at room temperature. The slides were rinsed twice in 1x PBS then incubated with a secondary peroxidase-labeled polymer conjugated to goat anti-rabbit IgG for 30 min, and then finally rinsed again in 1x PBS. Bound antibody was detected with diaminobenzidine (DAB + substrate kit, Dako Corp.). Hematoxylin was used as a counter stain. MPO-stained slides were then evaluated by scoring for the presence of neutrophils within the alveolar and interstitial spaces, as described previously [15].
Myeloperoxidase activity
Lung tissue was homogenized and fluidized in MPO Assay buffer. MPO activity was measured using an activity kit (BioVision, Inc, Milpitas, California, USA) according to the manufacturer's instructions by measuring the absorbance at 412 nm using a 96-well plate reader.
Lung histological examination
The harvested lung tissues were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin. Morphological changes in lung tissues were evaluated by light microscopy in a blinded fashion.
Immunofluorescent microscopy
HLMVEC were grown on cover glass till 100% confluent then exposed to the appropriate intervention. Cells were then fixed with 4% paraformaldehyde (Thermo Fisher Scientific) for 30 min, permeabilized with 100% cold methanol at -20 °C for 5 min. Cells then blocked with 1% BSA for 1 h, and incubated with first antibody overnight at 4 °C overnight, then the secondary antibody at room temperature for 1 h. Finally, cells were mounted on microscope slides using Prolong Gold Anti Fade Reagent (Cell Signaling Technologies®). Immunofluorescent images were observed with a Nikon Eclipse TE2000-U microscope, with Hamamatsu digital camera C11440, and Olympus IX51 microscope with Hamamatsu digital camera C4742-95. The images were analyzed with ImagePro Plus 7.0 to evaluate the colocalization of fluorescent.
Statistical analysis
Statistical calculations were performed using the GrapPad Prism software. The mean ± SEM was calculated for all samples. Statistical significance was determined either by the unpaired t-test (for 2 groups) or ANOVA (for ≥3 groups) with Newman-Keuls post-hoc testing. A value of P<0.05 was considered significant. Data >2 SD from the mean were excluded from further analysis.
Results
LPS induces mitochondrial dysfunction and inflammasome activation in HLMVEC
HLMVEC were exposed or not to LPS for 6h. LPS significantly attenuated mitochondrial bioenergetics in HLMVEC (Fig. 1 A) as demonstrated by significant decreases in maximum respiratory capacity (Fig. 1B) and reserve respiratory capacity (Fig. 1C). This mitochondrial dysfunction was also associated with increased reactive oxygen species (ROS) accumulation (Fig. 1D). In addition to mitochondrial disruption, LPS also activated the NLRP3 inflammasome and induced cellular inflammatory responses as shown by increased protein levels of NLRP3 (Fig. 1E), caspase-1 (Fig. 1F), cleaved caspase-1 (Fig. 1G), and IL-1β (Fig. 1 H). Moreover, LPS stimulated the secretion of IL-1β (Fig. 1I). Taken together, these results demonstrate that the attenuation of mitochondrial bioenergetics and increased generation of mitochondrial ROS are closely related to LPS-induced inflammasome activation.

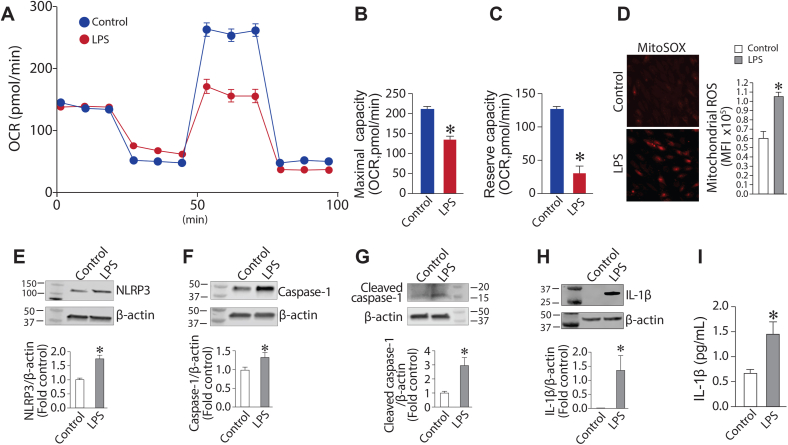
LPS induces mitochondrial dysfunction and inflammasome activation in human lung microvascular endothelial cells. HLMVEC (75,000 cells/0.32 cm2) were exposed to LPS (2EU/ml, 4 h), then the Seahorse XF24 analyzer used to measure changes in oxygen consumption rate (OCR). Oligomycin (1 μM), p-triflouromethoxyphenylhydrazone (1 μM), and rotenone and antimycin A (1 μM each) were added sequentially (A). Both the maximal respiratory capacity (B) and the spare respiratory capacity (C) in HLMVEC were significantly attenuated by LPS. HLMVEC were treated with LPS (2EU/ml, 6h), and mito-ROSlevels, determined using MitoSOX Red, were increased by LPS (D). NLRP3(E), caspase-1 (F), cleaved caspase-1 (G) and IL-1β (H) levels were all increased by LPS as determined by determined by Western blot analysis. The secretion of IL-1β(I), determined by ELISA was also elevated. Values are mean ± SEM; n = 4–6. *P < 0.05 vs. untreated. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
RhoA/ROCK inhibition attenuates LPS induced inflammasome activation in HLMVEC
The RhoA/ROCK signaling pathway plays an important role in LPS-induced sepsis. Thus, we next investigated its potential role in NLRP3 inflammasome activation. We found that the exposure of HLMVEC to LPS significantly increased RhoA activity (Fig. 2A). Fasudil, a nonspecific ROCK inhibitor, was then evaluated for its effect on LPS-mediated activation of the NLRP3 inflammasome. Our data show that fasudil reduced LPS-mediated RhoA activation (Figure 2A). Further, fasudil markedly attenuated the LPS-mediated increase of NLRP3 (Fig. 2B) and IL-1β (Fig. 2C) gene expression. Consistent with the reduction in NLRP3 and IL-1β mRNA levels, fasudil decreased NF-κB activity (Fig. 2D), measured by changes in the phosphorylation of the p65 subunit at Ser536. NLRP3 (Fig. 2E) and cleaved IL-1β (Fig. 2F) protein levels were also significantly reduced. Fasudil also prevented the LPS-mediated increase in mito-ROS levels (Fig. 2G) and reduced IL-1β secretion (Fig. 2H). Together, these data indicate that the RhoA-ROCK pathway is important for LPS-induced inflammasome activation.

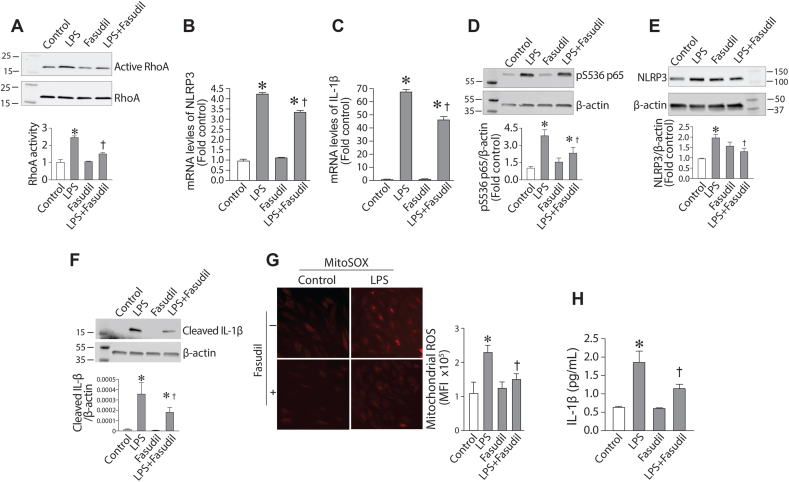
Fasudil attenuates LPS-mediated inflammasome and mitochondrial ROS generation in human lung microvascular endothelial cells. HLMVEC, pretreated with Fasudil (10 μM, 30min), were exposed to LPS (2EU/ml, 6 h). The increase in active RhoA levels induced by LPS was attenuated by fasudil, as determined by Western blot analysis (A). The LPS-mediated increase in NLRP3 (B) and IL-1β (C) mRNA levels was attenuated by fasudil as measured by qPCR analysis. The LPS-mediated increase in pS536-p65 levels (D), NLRP3 (E) and cleaved IL-1β (F) was attenuated by fasudil, as determined by Western blot analysis. The increase in mito-ROS levels was also reduced by fasudil, determined using MitoSOX Red (G). The secretion of IL-1β (H) in medium was also attenuated by fasudil, determined via ELISA. Values are mean ± SEM; n = 4–6. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
LPS induces the mitochondrial redistribution of eNOS via RhoA-ROCK signaling
We have previously shown that uncoupled eNOS contributes significantly to sepsis-mediated ALI [17]. Further, we have shown that uncoupled eNOS can redistributed to the mitochondria where it induces mitochondrial dysfunction and increases mito-ROS generation [4]. Thus, we initially investigated whether LPS-mediated eNOS uncoupling also induced the mitochondrial redistribution of eNOS. Using Immunofluorescence analysis, we were able to demonstrate an LPS dependent mitochondrial this redistribution (Fig. 3A). Further, the mitochondrial redistribution correlated with an increase in eNOS phosphorylation at T495 (Fig. 3B), a phosphorylation we have shown to previously shown to result in eNOS uncoupling and its mitochondrial redistribution [4]. Fasudil blocked the increase in pT495-eNOS induced by LPS (Fig. 3B) implicating ROCK as the kinase responsible for the phosphorylation event.

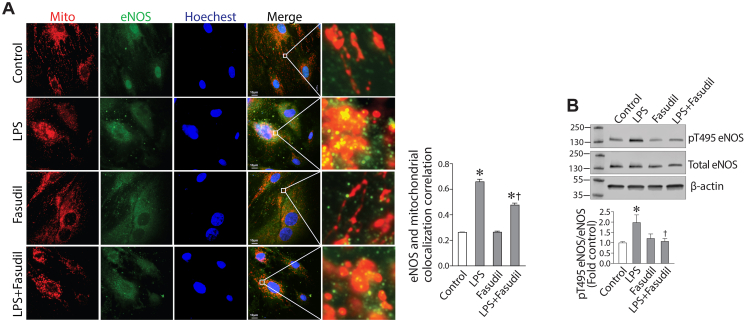
LPS induces eNOS translocation to the mitochondria via ROCK activation in human lung microvascular endothelial cells. HLMVEC, pretreated with fasudil (10 μM, 30min), were exposed to LPS (2EU/ml, 6 h). Immunofluorescence analysis shows that LPS induces the mitochondrial redistribution of eNOS and this is attenuated by fasudil (A). Western blot analysis shows that LPS increases the phosphorylation of eNOS at T495 and again fasudil attenuates this phosphorylation (B). Values are mean ± SEM; n = 4–6. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone.
RhoA nitration is involved in the LPS dependent mitochondrial redistribution of eNOS in HLMVEC
We have previously reported that RhoA-ROCK signaling during sepsis-mediated ALI involves the activation of RhoA through a single nitration at Tyr34 [15]. Our data show that the increase in RhoA activity in HLMVEC exposed to LPS (Fig. 2A) correlates with an increase in RhoA nitration (Fig. 4A). Further, this increase in RhoA nitration is inhibited by the peptide NipR1 (nitration inhibitory peptide for RhoA)) we have developed to specifically block RhoA nitration [15] (Fig. 4A). NipR1 also attenuates the increase in RhoA activity induced by LPS in HLMVEC (Fig. 4B). NipR1 reduces the LPS mediated increase in eNOS phosphorylation at T495 (Fig. 4C) and the mitochondrial redistribution of eNOS (Fig. 4D). The LPS mediated increase in mito-ROS is also significantly reduced in the presence of NipR1 (Fig. 4E). Taken together, these data implicate nitrated RhoA as a key regulator in the LPS induced mitochondrial redistribution of eNOS.

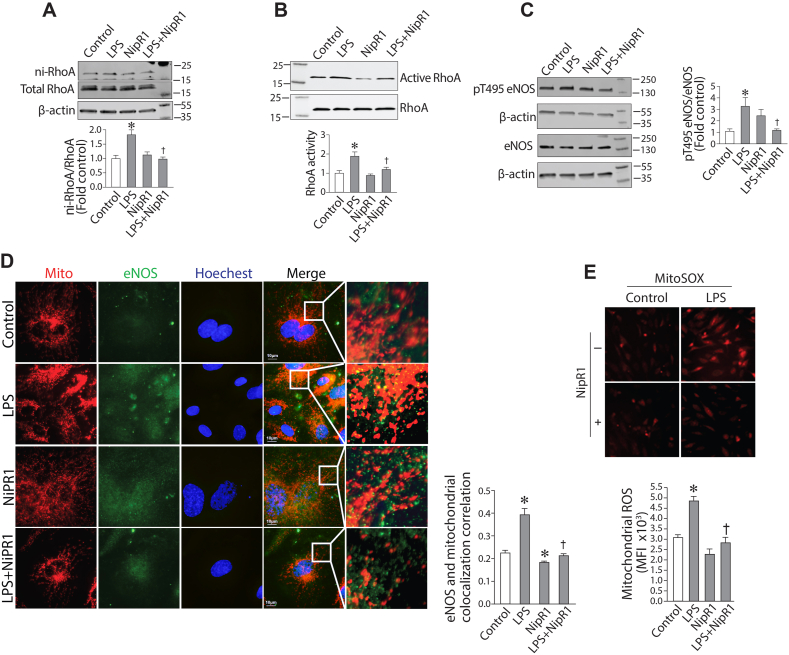
RhoA Nitration is involved in the LPS-mediated mitochondrial redistribution of eNOS in human lung microvascular endothelial cells. HLMVEC were pretreated with NipR1(100 ng/ml, 30min) then exposed to LPS (2EU/ml, 6 h). Nitrated RhoA (A), active RhoA (B) and phophspho-eNOS at T495 (C) levels were determined by Western blot. The LPS-mediated increase in RhoA nitration (A) and RhoA activation (B) was attenuated by NipR1. NipR1 peptide also attenuated the LPS-mediated increase in eNOS phosphorylation at T495 (C). Immunofluorescence analysis shows that NipR1 blocks the mitochondrial redistribution of eNOS induced by LPS (D) and the increases in mito-ROS (E). Values are mean ± SEM; n = 4–6. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone.
The nitration-mediated activation of RhoA is involved in LPS induced inflammasome activation in HLMVEC
We next investigated the effect of NipR1 on the LPS-mediated inflammatory pathway. qPCR analysis revealed that NipR1 significantly attenuated the LPS dependent increase in mRNA levels of NLPR3 (Fig. 5A), Caspase 1 (Fig. 5B) and IL-1β (Fig. 5C). Further Western blot analysis demonstrated that NipR1 decreased NF-κB activation (Fig. 5D), measured by changes in the phosphorylation of the p65 subunit at Ser536. NLRP3 (Fig. 5E), caspase 1 (Fig. 5F) and cleaved caspase 1 (Fig. 5G) protein levels were also significantly reduced. NipR1 also blocked the LPS dependent increase in IL-1β (Fig. 2H) and cleaved IL-1β (Fig. 5I) protein levels in HLMVEC. IL-1β secretion was also significantly attenuated (Fig. 5J). These data provide compelling evidence that nitration mediated RhoA activation plays an essential role in LPS mediated inflammatory signaling.

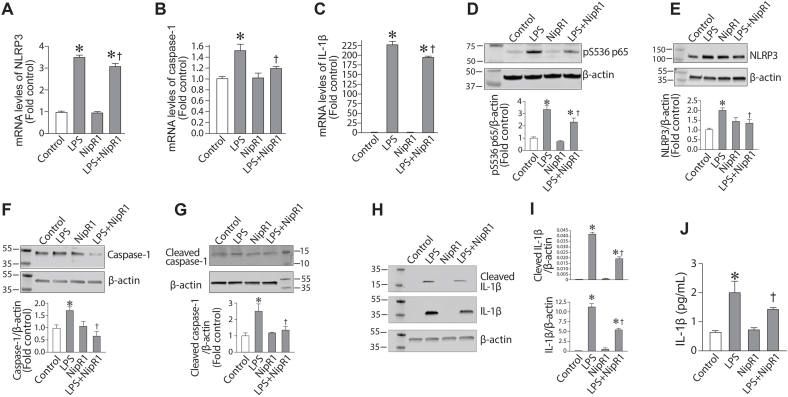
LPS-induces inflammasome activation through the nitration-mediated activation of RhoA in human lung microvascular endothelial cells. HLMVEC were pretreated with NipR1(100 ng/ml, 30min) then exposed to LPS (2EU/ml, 6 h). The LPS-mediated increase in NLRP3 (A), caspase-1 (B) and IL-1β (C) mRNA levels was attenuated by NipR1. Similarly, NipR1 blocked the increases in p-S536p65 (D), NLRP3 (E), caspase-1 (F), cleaved caspase-1 (G), IL-1β (H) and cleaved IL-1β (I) protein levels induced by LPS. The secretion of IL-1β in medium was also reduced by NipR1 (J). Values are mean ± SEM; n = 6. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone.
Targeting mito-ROS levels attenuates LPS induced inflammasome activation in HLMVEC
Utilizing a derivative of the mitochondrial targeted Szeto-Schiller SS peptide in which the powerful antioxidant, Tiron is covalently attached (T-SSP), identified a significant reduction in the LPS-mediated increase in mito-ROS levels in HLMVEC (Fig. 6A). T-SSP also attenuated the increase in NLRP3 (Fig. 6B), IL-1β (Fig. 6C) and cleaved caspase 1 (Fig. 6D) in LPS treated HLMVEC. T-SSP also attenuated the LPS-mediated increase in IL-1β secretion (Fig. 6E). These data indicate that specifically targeting mito-ROS can reduce LPS dependent inflammasome activation.

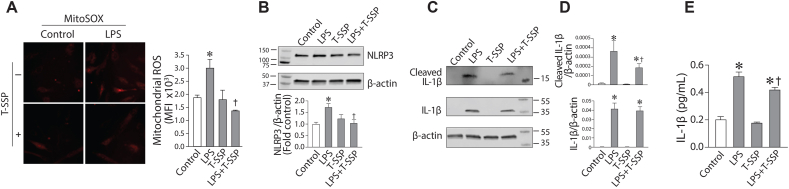
Reducing mito-ROS levels attenuates LPS induced inflammasome activation in human lung microvascular endothelial cells. HLMVEC were pretreated with a mitochondrial targeted antioxidant, T-SSP (100 nM, 30min) then exposed to LPS (2EU/ml, 6 h). The LPS mediated increase in mito-ROS levels is attenuated by T-SSP (A). Similarly, NipR1 blocked the increases in NLRP3 (B), IL-1β (C) and cleaved IL-1β (D) induced by LPS. T-SSP also attenuated IL-1β secretion (E). Values are mean ± SEM; n = 3–6. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone.
Reducing mito-ROS levels attenuates LPS induced ALI in the mouse
Based on the results in Fig. 6, we further explored the therapeutic potential of T-SSP, utilizing a mouse model of ALI induced by the intratracheal distillation of LPS. Our data indicate that LPS increased pT495-eNOS levels in the mouse lung (Fig. 7A) but T-SSP (5 mg/kg), administered i.p. 1h prior to LPS exposure, had no effect on this increase (Fig. 7 A). LPS stimulated mito-ROS levels in the mouse lung (Fig. 7A) and this was significantly attenuated by T-SSP (Fig. 7B). The increase in NLRP3 (Fig. 7C) and caspase-1 (Fig. 7C) protein levels in the mouse lung after LPS exposure was also reduced by T-SSP. The LPS mediated increase in IL-1β secretion into the BALF was inhibited by T-SSP (Fig. 7D) as was the increase in iNOS expression (Fig. 7E), a gene regulated by inflammatory cytokines [[18], [19], [20], [21]]. T-SSP treatment also reduced the number of inflammatory cells (Fig. 7F) and protein levels (Fig. 7G) in the BALF. T-SSP also reduced MPO activity (Fig. 7H). H&E stained lung tissue sections showed LPS caused severe alveolar damage that includes the presence of large amounts of neutrophils and red blood cells in the alveolar and interstitial space, formation of hyaline membranes, septal thickening, and debris accumulation in the alveoli (Fig. 7I). T-SSP again attenuated these conditions (Fig. 7I) resulting in a significant decrease in the lung injury score (Fig. 7J). Together these data show that specifically targeting mito-ROS is a potential therapeutic strategy for LPS-mediated ALI.

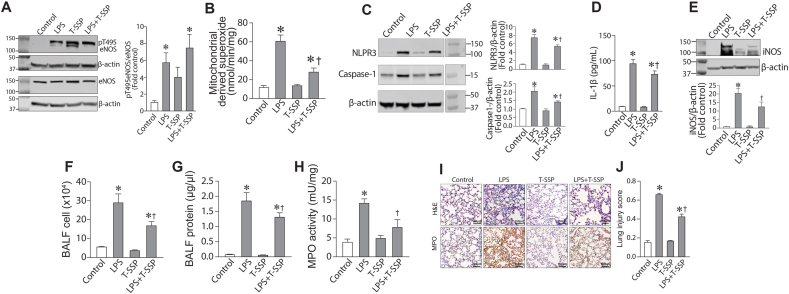
Reducing mito-ROS levels attenuates LPS induced acute lung injury in the mouse. Mice received saline or T-SSP (intraperitoneal, 5 mg/kg) 1 h prior to i.t. installation of vehicle or LPS (2 mg/kg). After 6 h, mice were sacrificed. Western blot analysis showed that LPS increased pT459-eNOS levels in the mouse lung (A). T-SSP had no effect on this increase (A). EPR analysis identified an increase in mitochondrial superoxide levels in LPS treated mice and this was attenuated by T-SSP (B). LPS increased Caspase-1 (C) and NLRP3 (C) protein levels and this were blocked by T-SSP. The LPS-mediated increase in IL-1β levels in the BALF were also attenuated by T-SSP (D). The LPS-mediated increase in iNOS was attenuated (E). Total white blood cell counts (F) as well as protein concentration (G) were significantly increased in BALF of LPS exposed mice, and these increases were attenuated by T-SSP. The increase in MPO activity as also attenuated by T-SSP (H). Lung sections were examined for signs of inflammation after hematoxylin and eosin staining (representative micrographs are shown, I), and scored for lung injury (J). The inflammatory response induced by LPS was reduced by T-SSP, as indicated by significantly lower lung injury score (J). Values are mean ± SEM; n = 5–7. *P < 0.05 vs. untreated; †P<0.05 vs. LPS alone.
Discussion
ALI/ARDS is a complex and devastating disorder [22]. Accumulating evidence has shown that excessive oxidative stress and an overwhelming inflammatory response, via NF-κB signaling, play pivotal roles in the pathogenesis of ALI [17,23,24]. An important aspect in NF-κB mediated inflammatory signaling is the activation of the inflammasome. Inflammasome activation is necessary for the clearance of pathogens during bacterial infection. However, sustained or excessive inflammasome activation may exacerbate pathological inflammation [25]. Inflammasomes are a group of cytosolic protein complexes that regulate the activation of caspase-1, and the processing of pro-interleukin (IL)-1β and pro-IL-18 to their mature active forms [26]. A number of nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family members have been shown to form inflammasomes in response to various stimuli. Among them, NLR family, pyrin domain-containing 3 (NLRP3) has been the most studied as it responds to a multitude of external stimuli including sepsis stimuli [[27], [28], [29], [30]]. The NLRP3 inflammasome consists of the NLRP3 protein, the adaptor protein apoptotic speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 [31]. The activation of the NLRP3 inflammasome is a two-step process: the expression of NLRP3 and pro-IL-1β is induced by transcriptional up-regulation via NF-κB signaling [32], and NLRP3 inflammasome protein components are then assembled, e.g., after exposure to microbial pathogens, in order to form a platform to activate caspase-1, which cleaves pro-IL-1β and pro-IL-18, thereby allowing it to be secreted from cells [26]. Although the regulation of the inflammasome during sepsis is still far from resolved, one of the mechanisms identified for NLRP3 inflammasome assembly is the generation of mt-ROS [31]. However, how the increases in mito-ROS occur is unresolved. Our data demonstrate that this occurs, at least in part, through the mitochondrial redistribution of uncoupled eNOS and the disruption of mitochondrial bioenergetics. Further, we show for the first time that a mitochondrial targeted antioxidant markedly decreased LPS-induced mito-ROS generation and inflammasome activation in vitro but significantly suppressed the LPS-induced inflammatory response in the mouse lung exposed to LPS. Moreover, this alleviated the LPS-induced lung injury, as shown by a significant attenuation of pulmonary neutrophils infiltration, reduced BALF protein content, ameliorated lung pathological changes and decreased MPO activity. In addition, macrophages activation and neutrophils accumulation in the lung, which not only enhances inflammatory cytokines and cells release, but also enhance ROS production [33,34].
It is becoming increasing evident that dysfunctional mitochondria play important roles in a number of human diseases. Thus, mitochondrial targeted therapeutics could be of great clinical benefit. have been proposed as therapeutic targets for a number ischemic heart disease, heart failure, neurodegenerative diseases, and metabolic disorders [35]. However, a number of challenges has limited the development of specific mitoprotective agents, and approved therapies for mitochondrial diseases are limited. Over the last decade a class of mitochondrial targeted agents have been identified based on a highly polar, water-soluble tetrapeptide [36]. These peptides designated Szeto-Schiller peptides (SSP) [37,38] accrue at high concentrations at the inner mitochondrial membrane due to their ability to bind cardiolipin [39] and are able to exert strong antioxidant effects [[40], [41], [42], [43]] and can reverse the mitochondrial dysfunction associated with aging [44]. SSP's can scavenge a number of oxidant and peroxide species including superoxide, hydrogen peroxide and peroxynitrite as well as attenuating lipid peroxidation [45,46]. Utilizing a more potent derivative in which the powerful antioxidant Tiron [47] was covalently attached to the peptide we were able to attenuate the mito-ROS mediated activation of the NLRP3 inflammasome induced by LPS and so reduce cytokine secretion and lung injury. These data confirm the importance of increases in mito-ROS in the activation of the NLRP3 inflammasome during sepsis. The decrease in mito-ROS achieved by SSP's has also been shown to prevent the opening of the mitochondrial permeability transition pore which in turn reduces cytochrome c release, preventing oxidant-induced cell death through apoptosis [48,49]. We have previously shown that LPS exposure induces endothelial cell death in the mouse lung [50]. Thus, it is possible that some of the protective effects of our T-SSP could be related to a reduction in EC apoptosis. However, further studies will be required to test this possibility. In addition, it is clear that other cell types in the lung such as macrophages, neutrophils, and epithelial cells are involved in the development of ALI [51,52]. As T-SSP was given i.p., it is possible that the protection against ALI we have identified could be due to effects on cell types in the lung besides EC. But again, further studies will be required to investigate this possibility. However, it is clear that our data suggest that therapies targeting mito-ROS could have clinical utility against ALI/ARDS. A devastating disorder with an unacceptable mortality of ~30% and that has no specific therapies beyond low tidal mechanical ventilation strategies. Further, we speculate that given the “cytokine-storm” associated with the lungs of patients infected with the novel SARS-2 virus [53] this mitochondrial targeted antioxidant strategy could be of clinical benefit. However, large-scale clinical trials would be necessary to test the efficacy of this strategy.
NF-κB activity is regulated through p50-RelA (p65) dimer interactions with inhibitory IκB proteins such as IκBα [54]. This NF-κB complex localizes to the cytoplasm attenuating the ability of NF-κB to bind to DNA and regulate transcription. Activation of an IκB kinase (IKK) complex leads to IκBα serine phosphorylation targeting degradation [55,56]. The exposed NF-κB complex then enters the nucleus to activate gene expression including IκBα [57] which enters the nucleus and removes NF-κB bound to DNA, restoring the original latent state [[58], [59], [60], [61]]. ARDS, however, is characterized by sustained NF-κB activation and a severe inflammatory response via unresolved mechanisms. We have pioneered studies evaluating the role of protein nitration in the development of ARDS pathobiology and lung EC barrier dysfunction [15,[62], [63], [64]]. Protein nitration is driven by peroxynitrite, formed by the interaction of superoxide with NO [65,66]. We have demonstrated that eNOS is intimately engaged in the protein nitration events involved in the development of ARDS [62,63,67]. Blocking eNOS activity reduces NF-κB inflammatory signaling [15,[62], [63], [64]]. Our previous work has identified eNOS uncoupling as playing an important role in the increased protein nitration associated with ALI [15,50,62,63,[67], [68], [69], [70]] and identified the nitration of RhoA as an important activator of RhoA during ALI [15]. RhoA, plays an important role in the regulation of the actin cytoskeleton [[71], [72], [73], [74], [75], [76]] through the phosphorylation mediated inhibition of myosin phosphatase‐targeting subunit 1 (MYPT1) decreasing myosin light chain phosphatase (MLCP) activity. This in turn allows myosin light chain kinase (MLCK) mediated phosphorylation of the regulatory myosin light chain (MLC-2) to proceed unabated. Phosphorylation of MLC-2 induces actomyosin contraction and the reorganization of the cytoskeleton to favor EC barrier disruption and lung injury. However, RhoA/ROCK signaling also plays a less appreciated role in ALI through its ability to stimulate NF-κB-mediated inflammatory signaling. Studies using the Rho inhibitor, TcdB-10463, an uncovered a previously unknown role for RhoA in the inflammatory cascade associated with the inhibition of NF-κB activity human endothelial cells [77]. RhoA has been shown to be important for TLR4 signaling [78] and adhesion molecule expression [79] through NF-κB. Further, this appears to require the MyD88 pathway and the guanine exchange factor, GEF-H1 [80,81]. Our studies have added to this knowledge base by demonstrating the importance of RhoA mediated nitration at Y34 in the activation of NF-κB-mediated inflammatory signaling. Indeed, we have previously shown that specifically blocking RhoA nitration at Y34 using the NipR1 nitration-shielding peptide dramatically reduces LPS-mediated ALI [15]. Interestingly when we evaluated the effects on inflammatory cytokine levels in the BALF induced by LPS exposure in the mouse, NipPR1 decreased IL-1ß levels [15] supporting the effect of nitration-mediated RhoA signaling in regulating inflammatory signaling and inflammasome activation.
We have previously identified a role for increases in the NOS uncoupler, asymmetric dimethylarginine (ADMA) in the uncoupling process. Here we add to this work by demonstrating that LPS also induces in pT495-eNOS levels, a phosphorylation event that is widely accepted to be associated with eNOS uncoupling [69,82,83]. The mechanism by which pT495 phosphorylation causes eNOS uncoupling has not been resolved but may involve reduced interactions with caveolin-1, as caveolin-1 knockout mice have significantly increased levels of protein nitration compared to wildtype mice [84]. There are a number of kinases that are known to phosphorylate eNOS at T495 including AMP kinase (AMPK), PKC and RhoA kinase (ROCK) [85]. The involvement of ROCK is interesting as the data we present here, and our prior work, have linked the activation of RhoA/ROCK signaling to the nitration mediated activation of RhoA [15,67,86]. This coupled with our data showing that our RhoA nitration shielding peptide decreases the LPS-mediated increase in pT495-eNOS levels suggests that ROCK is the major kinase phosphorylating eNOS during LPS exposure. However, it should be noted that LPS is also known to increase intracellular Ca2+ levels [87] which can activate PKC isoforms [88]. Thus, activated PKC could also be involved in the increases in pT495-eNOS levels. It is also unclear whether the increased ADMA levels associated with ALI synergize with pT495 phosphorylation to stimulate eNOS uncoupling and protein nitration. It is possible that increases in ADMA may be an earlier event than T495 phosphorylation which could lead to an initial uncoupling of eNOS that stimulates RhoA nitration and hence ROCK dependent phosphorylation of T495 leading to enhanced uncoupling, leading to more RhoA nitration, and resulting in a feed-forward cascade. However, further studies will be required to investigate this possibility.
Besides its normal caveolar location, eNOS can be targeted to the mitochondrion through the mitochondrial targeting loop located within aa627-631 (RRKRK) [89]. An increasing body of work has shown that the mitochondria is a site on which eNOS is trafficked. However, the effect of this redistribution on bioenergetics appears to be dependent on whether the enzyme is coupled or uncoupled with the coupled enzyme enhancing mitochondrial health [[90], [91], [92]] while the uncoupled enzyme disrupts mitochondrial bioenergetics [[93], [94], [95], [96]]. However, the mechanism by which eNOS redistribution is regulated is still unresolved. A number of post-translational modifications (PTMs) have been shown to be associated with the mitochondrial redistribution of eNOS in addition to pT495. These include Akt1 nitration and phosphorylation [97,98] as well as the Akt1-mediated phosphorylation of eNOS at S617 [90,98]. These PTMs likely result in changes in the tertiary structure of the eNOS protein as the mitochondrial targeting loop [89] is normally buried within the eNOS protein [90] suggesting structural changes within eNOS are required to expose this sequence and allow the protein to redistribute to the mitochondrion. Further, although our data demonstrate that the redistribution of uncoupled eNOS disrupts mitochondrial bioenergetics the underlying mechanism was not resolved. However, based on prior work it is likely that the loss of mitochondrial bioenergetics is caused, at least in part, by a reduction in ß-oxidation and loss of activity of the carnitine homeostasis enzymes (CPT1, CPT2, and CrAT) [94,96,99,100]. Indeed, prior studies have shown that ß-oxidation is impaired in mice with LPS-induced ALI, at least in alveolar epithelial cells (AECs) [101]. Further, treatment with the ß-oxidation stimulator, l-carnitine has been shown to reduce the ALI associated with experimental biliary obstruction [102] and bleomycin exposure [103].
In conclusion, we have identified a novel mechanism by which the mitochondrial redistribution of uncoupled eNOS is involved in the mito-ROS dependent activation of the NLRP3 inflammasome during sepsis. Further, we demonstrate that mito-ROS scavenging effectively protects against LPS-induced ALI. We speculate that the specific scavenging of mito-ROS using agents such as T-SSP we describe here, could have clinical utility in the prevention of the hyper-inflammatory state associated with ALI/ARDS, a disease with no specific therapies and a high mortality rate.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
1
2
3
4
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
55
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
Acknowledgements
This research was supported in part by HL60190 (SMB), HL137282 (SMB), HL134610 (SMB/TW), HL142212 (SMB), HL146369 (SMB/TW), and the Interdisciplinary Training in Cardiovascular Research T32 HL007249 (to XW) all from the National Institutes of Health. The authors have no conflicts of interest to declare.
 The mitochondrial redistribution of eNOS is involved in lipopolysaccharide induced inflammasome activation during acute lung injury
The mitochondrial redistribution of eNOS is involved in lipopolysaccharide induced inflammasome activation during acute lung injury