
Middle authors are listed in alphabetical order, particularly engaged authors are highlighted with the symbol.
- Altmetric
- Introduction
- Chemistry dictates the drug properties of oligonucleotides
- Delivery systems for oligonucleotides
- Model systems for oligonucleotide development
- Safety assessment of oligonucleotide‐based therapeutics
- Approved oligonucleotide‐based therapeutics
- Concluding comments and future perspectives
- Conflict of interest
- For more information
Nucleic acid‐based therapeutics that regulate gene expression have been developed towards clinical use at a steady pace for several decades, but in recent years the field has been accelerating. To date, there are 11 marketed products based on antisense oligonucleotides, aptamers and small interfering RNAs, and many others are in the pipeline for both academia and industry. A major technology trigger for this development has been progress in oligonucleotide chemistry to improve the drug properties and reduce cost of goods, but the main hurdle for the application to a wider range of disorders is delivery to target tissues. The adoption of delivery technologies, such as conjugates or nanoparticles, has been a game changer for many therapeutic indications, but many others are still awaiting their eureka moment. Here, we cover the variety of methods developed to deliver nucleic acid‐based therapeutics across biological barriers and the model systems used to test them. We discuss important safety considerations and regulatory requirements for synthetic oligonucleotide chemistries and the hurdles for translating laboratory breakthroughs to the clinic. Recent advances in the delivery of nucleic acid‐based therapeutics and in the development of model systems, as well as safety considerations and regulatory requirements for synthetic oligonucleotide chemistries are discussed in this review on oligonucleotide‐based therapeutics.
Recent advances in the delivery of nucleic acid‐based therapeutics and in the development of model systems, as well as safety considerations and regulatory requirements for synthetic oligonucleotide chemistries are discussed in this review on oligonucleotide‐based therapeutics.
Glossary
| Anti‐drug antibodies (ADAs) | Antibody‐mediated immunogenicity elicited in vivo to a given drug. Drug‐specific antibodies can reduce the efficacy of the treatment and even fully inactivate the drug, and/or they can induce adverse effects. |
| Antisense oligonucleotides (ASOs) | Single‐stranded oligonucleotides complementary to RNA target sequences. |
| Aptamers | Single‐stranded oligonucleotides (20‐100 nucleotides) which adopt three‐dimensional structures that allow them to bind very specifically to protein target sites. |
| Blood–brain barrier (BBB) and blood–spinal cord barrier (BSCB) | Selectively permeable membranes of the central nervous system (CNS) vasculature. Only small molecules (molecular weight below 400‐500 Da) and high lipid solubility (logP value of approximately 2.1) can cross these vascular barriers. Generally, oligonucleotides display a molecular weight of approximately 10 kDa and are hydrophilic; hence, they are too large and hydrophilic to cross biological barriers by passive diffusion. |
| Cell‐penetrating peptides (CPPs) | Short cationic and/or amphipathic peptides (usually less than 30 amino acids) capable of translocating different types of cargoes across biological barriers and cell membranes. CPPs can be directly conjugated to oligonucleotides (ONs) or used to encapsulate ONs into nanoparticles. |
| European Medicines Agency (EMA) | Agency of the European Union in charge of the evaluation and supervision of medicinal products. The EMA facilitates development and access to medicines, evaluates applications for marketing authorisation and monitors the safety of human and veterinary medicines. |
| Food and Drug Administration (FDA) | The federal agency of the United States Department of Health and Human Services, responsible for protecting public health by ensuring the safety, efficacy and security of human and veterinary drugs. |
| Gapmer | Chimeric antisense oligonucleotides (ASOs) that contain a central block of DNA nucleotides, flanked by modified sequences, usually containing 2′‐O‐modified or locked nucleic acid (LNA) chemistries. Gapmers are used for gene silencing by stimulating RNA cleavage through the recruitment of RNase H. |
| Lipid nanoparticles (LNPs) | Delivery systems based on LNPs are composed of one or several lipid components, often an ionisable cationic lipid used for complexation of polyanionic DNA/RNA and stabilising helper lipids such as distearoylphosphatidylcholine (DSPC) and cholesterol. In addition, LNPs may be stabilised sterically by surface coating with polyethylene glycol (PEG). LNPs have a complex internal lipid architecture that is well suited for stable and efficient encapsulation of DNA/RNA cargoes. |
| MicroRNAs (miRNAs) | Small noncoding RNAs (∼22 nt), which regulate gene expression at the post‐transcriptional level by degrading target mRNAs, when complementary to the sequence, or inhibiting their translation when not fully complementary. Each miRNA can influence the expression of hundreds of mRNAs. |
| Pharmacodynamics (PD) | The relationship between the drug concentration at the site of action and the observed biochemical response and its efficacy. |
| Pharmacokinetics (PK) | The time course of drug absorption, distribution, metabolism, excretion and toxicity (ADMET), as well as the liberation of a drug from its formulation. |
| Phosphorodiamidate morpholino oligonucleotides (PMOs) | Oligonucleotides containing uncharged chemistry. The nucleic acid backbone has been replaced with 6‐membered morpholino rings and phosphorodiamidate linkages, while retaining standard nucleobases. |
| Peptide nucleic acid (PNA) | Uncharged oligonucleotide chemistry with amide bond linkages between the nucleobases. PNAs are manufactured by peptide synthesis. |
| RNAse H cleavage | RNAse H hydrolyses the phosphodiester bonds of RNA when hybridised to DNA. |
| Small interfering RNA (siRNA) | Double‐stranded RNA (~21 nt) composed of a guide strand complementary to the target mRNA and a passenger strand. siRNAs act within the endogenous RNA‐induced silencing complex (RISC) to degrade mRNA. |
| Toll‐like receptors (TLRs) | Pattern‐recognition receptors usually found on the plasma or endosomal membranes of sentinel cells such as macrophages and dendritic cells (DCs). Activation of TLRs can promote an inflammatory response. For example, TLR9 is activated by unmethylated cytidine‐phosphate‐guanosine (CpG) dinucleotides present in bacterial and viral DNA. |
Introduction
Synthetic oligonucleotides (ONs) are small, single‐ or double‐stranded pieces of modified nucleic acids that have been exploited as therapeutic modalities in different ways (Table 1). The unique characteristic of ONs is that they bind to their target via Watson–Crick base pairing, enabling intervention at a genetic level by targeting RNA in a specific manner (Zamecnik & Stephenson, 1978). ONs encompass many types of nucleic acid‐based therapeutics, including antisense oligonucleotides (ASOs), small interfering RNA (siRNA), anti‐miRNA (antagomirs), miRNA mimics (agomirs), aptamers and unmethylated CpG‐containing ONs. Depending on their mechanism of action, treatment with therapeutic nucleic acids may cause decreased, increased or restored protein expression. Currently, 11 ON‐based drugs across many disease areas have received regulatory approval by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and/or the Japanese Ministry of Health, Labour and Welfare. However, further therapeutic development is challenged by unfavourable absorption, distribution, metabolism, excretion and toxicity (ADMET) properties for most clinical applications (Godfrey et al, 2017). This review mainly focuses on the development of single‐stranded ONs and covers (i) the numerous methods developed to date to deliver ONs across biological barriers, (ii) the model systems used to test ONs and (iii) the hurdles existing for translating laboratory breakthroughs to the clinic. The content represents the joint efforts of members of the EU Cooperation of Science and Technology (COST) network Delivery of RNA Therapeutics (DARTER, COST Action 17103, www.antisenserna.eu), which aims to facilitate RNA‐targeting nucleic acid‐based drugs to reach their full potential.

| Modality | Mechanism | Example(s) |
|---|---|---|
| RNase H | RNase H‐mediated cleavage of target transcript | Gapmers |
| Steric Blockage | Interference with post‐transcriptional RNA‐binding elements, e.g. splicing modulation and blocking endogenous miRNA | 2nd and 3rd generation ASOs and antagomirs |
| Protein Binding | Bind target proteins in a structure‐specific manner | Aptamer |
| Innate Immunity | Inhibits protein expression via target‐specific mRNA degradation | Unmethylated CpG‐containing ONs |
| RNAi | Inhibition of gene expression via target‐specific mRNA degradation | siRNAs, microRNAs |
Chemistry dictates the drug properties of oligonucleotides
Therapeutic nucleic acids are chemically modified in several ways to endow them with properties such as increased resistance to nucleases and improved target binding affinity (Jarver et al, 2014) (Fig 1). Each modification confers the ON different properties, and some may be combined, but other modifications are not compatible or may modify the ON in ways that complicate their synthesis or interfere with the mechanisms by which they exert their effect. First‐generation chemistries include the widely used phosphate backbone modifications, e.g. phosphorothioate (PS), which imparts resistance to endonucleases and improves bioavailability by reducing renal clearance due to increased affinity for serum proteins (Eckstein, 2014). However, this modification also reduces the affinity for the target RNA. Second‐generation chemistries include ribose modifications at the 2′‐O position of RNA and 2′ position of DNA, of which the 2ʹ‐O‐methyl (2ʹ‐OMe), 2ʹ‐O‐methoxy‐ethyl (2ʹ‐MOE) and 2ʹ‐fluoro (2ʹ‐F) modifications are the most commonly used types. These modifications increase the binding affinity to RNA and further improve the nuclease resistance. An even greater binding affinity chemistry is the conformationally constrained DNA analogues locked nucleic acid (LNA) and tricyclo‐DNA (tcDNA). LNA contains a methyl bridge between the 2′‐O and 4′ position of the ribose ring (Koshkin et al, 1998; Obika et al, 1998). The backbone considerably changed for tcDNA via introduction of an ethylene bridge with a cyclopropane ring between the ribose 3' and 5' carbon positions (Renneberg & Leumann, 2002). The bridge imposes a locked conformation on the ribose ring, which is ideal for binding to RNA. All first‐ and second‐generation chemistries are compatible with nucleic acid synthesis and can easily be mixed with DNA and RNA in ON chimeras. Third‐generation chemistries include changes in the nucleobase, e.g. phosphorodiamidate morpholino oligomers (PMO) (Summerton & Weller, 1997) and peptide nucleic acid (PNA) (Nielsen et al, 1991; Hanvey et al, 1992). For PMOs, the nucleic acid backbone has been replaced with a 6‐membered morpholino ring and phosphorodiamidate linkages, while retaining standard nucleobases. The nucleobases of PNAs are linked by amide bonds, which are synthesised similarly to peptides. Both PMO and PNA are uncharged, very resistant to nucleases, and display variable affinity for the target RNA (Smulevitch et al, 1996; Summerton & Weller, 1997). The choice of chemical modifications is largely dictated by the modality and the target tissue.

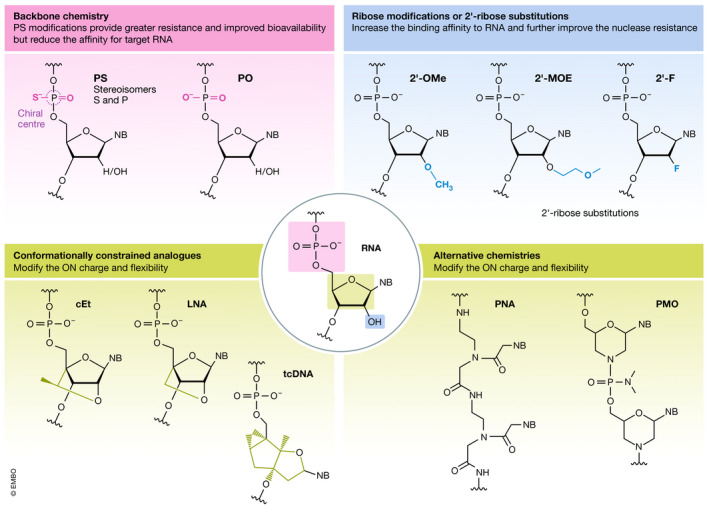
Oligonucleotide chemistries
Commonly used nucleic acid chemistries. The often used phophorothioate (PS) backbone replaces the natural phosphodiester (PO). Modifications to the ribose at the 2ʹ‐O position of RNA and 2ʹ‐position of DNA include the 2ʹ‐O‐methyl (2ʹ‐OMe), 2ʹ‐O‐methoxy‐ethyl (2ʹ‐MOE) and 2ʹ‐fluoro (2ʹ‐F) are the most commonly used. Conformationally constrained DNA analogues, locked nucleic acid (LNA), constrained 2′‐O‐ethyl (cEt) and tricyclo‐DNA (tcDNA), provide greater binding affinity. LNA and cEt are constrained by a methyl bridged from the 2′‐O and 4′ position of the ribose. tcDNA introduces of an ethylene bridge with a cyclopropane ring between the 3′ and 5′ carbon positions of ribose. Alternative chemistries include changes in the nucleobase, e.g. phosphorodiamidate morpholino oligomers (PMO) and peptide nucleic acid (PNA).
Single‐stranded ASOs complementary to target RNA were first utilised therapeutically by exploiting RNase H cleavage of DNA/RNA hybrids (Stein & Hausen, 1969; Wu et al, 2004) (Fig 2). RNase H‐inducible ASOs are designed as gapmers, where central DNA nucleotides are flanked by RNase H‐resistant modified nucleotides (Wahlestedt et al, 2000). The modified sequences improve target affinity while the central DNA sequence forms the DNA/RNA hybrid for RNase H recognition and cleavage (Monia et al, 1993). Fully modified second‐ and third‐generation ASO chemistries act through RNase H‐independent mechanisms (Fig 2) (Jarver et al, 2014). Steric blocking ASOs can inhibit or activate translation through the binding to regulatory elements, e.g. upstream open reading frames (Liang et al, 2016b; Liang et al, 2017). A common therapeutic modality is the modulation of pre‐mRNA splicing (Arechavala‐Gomeza et al, 2014), which is used to induce or suppress exon inclusion. In Duchenne muscular dystrophy (DMD) patients, ASO‐induced exon skipping of mutated dystrophin pre‐mRNA restores the reading frame and allows for the production of partially functional, rather than non‐functional, dystrophin protein (Mitrpant et al, 2009). In contrast, for spinal muscular atrophy (SMA) patients, ASOs increase the level of exon 7 inclusion in survival motor neuron 2 (SMN2) mRNA, leading to increased levels of SMN protein (Singh et al, 2006). Similarly, ASOs can also induce the skipping of pseudoexons (Collin et al, 2012) or block RNA‐splicing factors from recognising cryptic splice sites (Rivera‐Barahona et al, 2015). ASOs can also sterically block the union of RNA‐binding factors to repeat expansion regions of pathogenic mRNAs (Fig 2). In myotonic dystrophy 1, expanded microsatellite repeats sequester RNA‐binding factors within nuclear expansion RNA foci (Miller et al, 2000). ASOs targeting the CUG repeat expansion mRNA release the sequestered RNA‐binding factors and reverse the phenotype (Klein et al, 2019). RNA interference (RNAi)‐based therapies, i.e. double‐stranded siRNA and single‐stranded microRNA (miRNA), exploit the endogenous RNAi pathway in the cytosol (Fire et al, 1998) to silence or modulate the expression of specific proteins (Fig 2). Commonly used chemical modifications for siRNA, including 2ʹ‐OMe and 2ʹ‐F modifications, decrease RNase recognition and are well tolerated throughout the entire siRNA duplex (Watts et al, 2008). In addition, these modifications are widely used to decrease immune stimulation (Judge et al, 2006). ASOs can influence miRNA function, either by sequestering a miRNA (antagomir) or by generating a miRNA mimic (agomir). Notably, a single miRNA generally regulates the expression of multiple genes in a given pathway; hence, antagomirs and agomirs have the potential to mediate increased or decreased expression of multiple genes, respectively (Friedman et al, 2009). Finally, two types of ONs which do not work through Watson–Crick base pairing are aptamers and unmethylated CpG‐containing ONs. Aptamers are single‐stranded ONs (20–100 nucleotides) selected from randomised libraries based on their high‐avidity binding to specific targets (Ellington & Szostak, 1990; Tuerk & Gold, 1990). They adopt three‐dimensional structures that bind to protein target sites through attractive electrostatic interactions and pocket‐like structures (Ellington & Szostak, 1990), and they display binding affinities to their receptor targets which are comparable to those of monoclonal antibodies (Jayasena, 1999). Unmethylated CpG‐containing ONs include a cytosine‐guanine motif coupled with a phosphodiester (PO) or PS backbone. Unmethylated CpG motifs are commonly found in bacterial DNA and activate the immune system through Toll‐like receptor 9 (TLR9). Unmethylated CpG‐containing ONs have been tested clinically as vaccine adjuvants and for cancer immunotherapy (Krieg & Davis, 2001; Krieg, 2006, 2007).

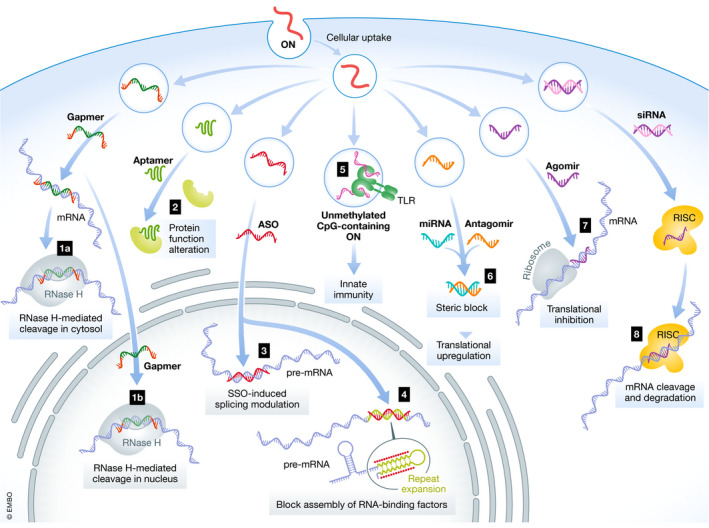
Mechanisms and location of action for oligonucleotides
Representative mechanisms of action and intracellular localisation for (1) gapmer and mRNA degradation, (2) aptamer, (3) nuclear steric blockage for splice switching, (4) blockage the assembly of RNA‐binding factors, (5) TLR activation of innate immunity, (6) miRNA and antagomir, steric block, translational upregulation, (7) agomir, translational inhibition, and (8) siRNA, RISC, RNAi silencing ONs.
Delivery systems for oligonucleotides
The sites of action for ONs lay within the intracellular space. Consequently, they need to overcome several biological barriers to reach their pharmacological targets in vivo. PS‐modified ONs bind reversibly to plasma proteins, e.g. albumin, which increases their plasma half‐life and facilitates their distribution and accumulation in the liver, kidneys, spleen, lymph nodes and bone marrow (Geary, 2009). Targeting tissues beyond these organs has had clinical success for local delivery to the eye, brain and spinal cord via intravitreal (IVT) and intrathecal (IT) administration, respectively (Hache et al, 2016; Cideciyan et al, 2019) (Fig 3). Both routes bypass renal clearance and maintain high ON exposure to the cellular microenvironment for efficient uptake. Additionally, significant advances for pulmonary delivery of RNA therapies have been extensively reviewed elsewhere (Chow et al, 2020; Shaffer, 2020). However, systemic administration of ONs has been less successful due to poor tissue uptake. Cellular uptake of ONs occurs predominantly via different types of endocytosis. ONs are subsequently trafficked into the endolysosomal system, from where they need to escape to avoid degradation in the lysosomal environment (Crooke et al, 2017). Only a very small ON dose fraction escapes the endosomes and becomes available at the site of action (Gilleron et al, 2013). Single‐stranded ONs, such as PS ASOs, which are relatively small, uncharged and/or hydrophobic, can productively enter cells and escape the endosomes into the cytoplasm and nucleus without the need for a delivery agent (Liang et al, 2016a) in a process referred to as gymnosis (Stein et al, 2010), but relatively high ON doses are required for this process to take place. However, most of RNA‐based therapeutics, e.g. double‐stranded siRNA, are too large and charged to enter cells unassisted and require a delivery agent. Accelerating the rate of cellular uptake, intracellular trafficking and endosomal escape has been a driving force behind advances in many chemical modifications and delivery agents (Juliano et al, 2018; Biscans et al, 2020). A wide variety of delivery approaches improve the transport and bioavailability of ONs (Fig 4) (Roberts et al, 2020). These include (i) direct conjugation to carriers and (ii) incorporation into nanoparticulate carriers, both with the aim of improving the ADMET properties.

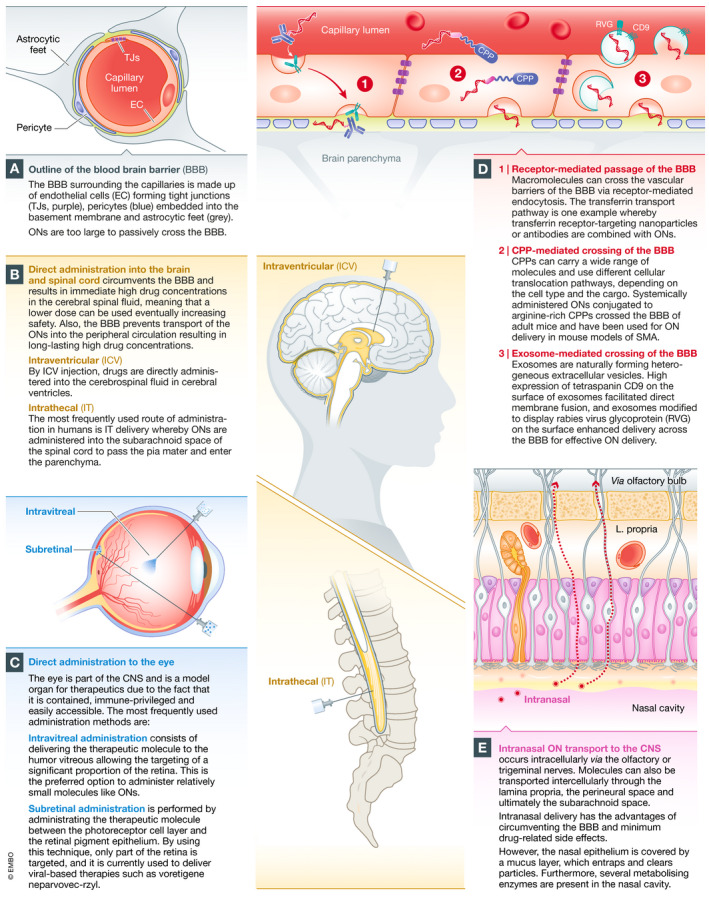
Delivery of oligonucleotides to the brain and eye
(A) ONs are prevented from passive diffusion into the central nervous system (CNS) by the vascular BBB. (B) ONs without a delivery reagent require direct administration into the brain or spinal cord. The most frequently used CNS administration route in humans is intrathecal (IT) administration, where ONs are administered into the subarachnoid space of the spinal cord to pass the pia mater and enter the parenchyma. This results in an immediate high ON concentration in the cerebral spinal fluid, meaning that a lower dose can be used, which reduces side effects. Also, the BBB prevents transport of ONs into the peripheral circulation resulting in long‐lasting high ON concentrations. (C) The eye is a contained and immune‐privileged organ of the CNS that allows local delivery. ONs are effective and well tolerated when administered directly by intravitreal injection. Subretinal delivery is also possible, but the treated area will be reduced. (D) Certain macromolecules can cross the vascular barriers via receptor‐mediated endocytosis after systemic administration (Pardridge, 2007). The transferrin transport pathway has been exploited in several rodent studies to carry ONs into the brain parenchyma (Lee et al, 2002; Kozlu et al, 2014). Systemically delivered ONs covalently conjugated to arginine‐rich CPPs have been shown to cross the BBB in mice (Du et al, 2011) and have been used for ON delivery in mouse models of SMA (Hammond et al, 2016). Several studies have shown exosome‐mediated delivery of small RNAs across the vascular barriers into the CNS (Alvarez‐Erviti et al, 2011; Yang et al, 2017). (E) Drugs dosed by intranasal administration can be transported into the brain along the olfactory, trigeminal nerve and rostral migratory stream (Curtis et al, 2007).

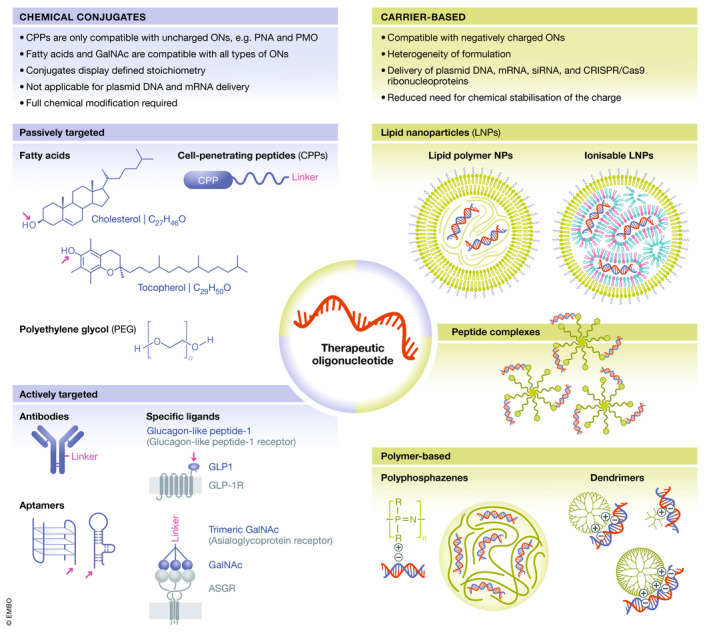
Delivery technologies for oligonucleotides
Delivery technologies used to improve the ADMET properties of ONs, including chemical conjugates (left) and nanoparticulate carriers (right). Polymers, cell‐penetrating peptides (CPPs) and lipids represent examples of molecules used for covalent conjugation to ONs for passive targeting, whereas covalent conjugation of ONs to antibodies, receptor ligands and aptamers are applied for active targeting. Drug conjugates display a defined stoichiometry. CPP conjugation is only compatible with uncharged ONs, e.g. PMOs and PNAs, whereas lipids and GalNAc are compatible with all types of ONs. Nanoparticulate carriers can be used to encapsulate negatively charged ONs and can be based on lipids, e.g. lipid nanoparticles (LNPs) and exosomes, polymers, e.g. dendrimers, poly(lactide‐co‐glycolic acid) (PLGA) and polyphosphazenes, and peptides, or on hybrid systems composed of several different types of compounds. The complexity of these systems poses new challenges in the development with respect to cost, manufacturability, safety, quality assurance and quality control.
Chemical conjugates
Chemical conjugation of molecules to therapeutic ONs is an attractive strategy for improving ADMET properties. As chemical conjugates, ONs are exposed to serum, and therefore, full chemical modification of ONs is needed to protect them from degradation. Polymers, peptides, lipids, receptor ligands and aptamers represent examples of molecules used for conjugation (Fig 4).
Polymers
Covalent conjugation of polyethylene glycol (PEG) improves the ADMET properties of drugs. PEGylation has been applied mainly for therapeutic proteins, but more recently also for ONs, e.g. the marketed aptamer‐PEG conjugate pegaptanib directed against vascular endothelial growth factor (VEGF) (Ng et al, 2006). PEG is a highly flexible, non‐charged and hydrophilic polymer with end groups available for functionalisation. PEG shields the conjugated drug cargo via formation of a hydration shell, which sterically blocks other biomacromolecules from binding to the drug. Also, PEGylation prolongs the circulation time by reducing renal excretion and increasing ON stability. The ADMET properties of PEGylated ONs are dependent on the physicochemical properties of the PEG moiety, including the molecular weight, the type of end group modification and the PEG architecture (linear or branched). For example, pegaptanib contains a 40 kDa Y‐shaped PEG, which causes the aptamer binding affinity to decrease fourfold compared with the parent aptamer, whereas the antiangiogenic efficacy is increased, which is attributed to prolonged tissue residence time due to increased half‐life (Ng et al, 2006).
Peptides
Cell‐penetrating peptides (CPPs) are short cationic and/or amphipathic peptides, usually less than 30 amino acids long, capable of translocating different types of cargoes across biological barriers and cell membranes (Foged & Nielsen, 2008; Pooga & Langel, 2015; Lehto et al, 2016). CPPs can be used as direct conjugates or to encapsulate oligonucleotides into nanoparticles, which is discussed further in the next section. Once inside the cells, CPPs may also improve endosomal escape (Cleal et al, 2013). However, the cationic charge often restricts their covalent conjugation to charge‐neutral ON chemistries (PNAs and PMOs) due to electrostatic interactions between anionic ONs and cationic CPPs that result in aggregation. For systemic diseases, CPP‐ONs circumvent cell‐specific receptors, allowing for pharmacological activity across multiple tissues, and they have been developed for uptake into particularly impervious tissues, e.g. skeletal muscle, heart and CNS (Hammond et al, 2016; Betts et al, 2019), as well as targeting viral and bacterial infections (Burrer et al, 2007; Geller et al, 2013; Geller et al, 2018). At the time of this review, a phase I clinical trial for safety and tolerability of an arginine‐rich CPP‐ASO conjugate for DMD (SRP‐5051) has been completed and a phase II is recruiting to determine the optimal dose.
Lipids
Conjugation of hydrophobic compounds such as cholesterol to ONs can improve delivery in vitro by promoting endosomal release (Wang et al, 2019) and results in longer plasma half‐life and accumulation in the liver upon systemic administration (Osborn et al, 2019). Such modifications may enhance delivery, mainly to the liver, but also to peripheral tissues such as muscle (Prakash et al, 2019), via passive targeting by increasing the binding affinity of ONs to plasma proteins and/or via active targeting by hijacking endogenous lipid transport pathways (Osborn et al, 2019).
Receptor ligands
Tissue‐specific active targeting may be achieved through conjugation of ONs to receptor ligands that facilitate specific binding to receptors on the target cells and mediate tissue‐specific delivery. A wide variety of receptor ligands have been investigated, including carbohydrates, peptides/proteins, aptamers, antibodies/antibody fragments and small molecules), and several feasible receptor‐ligand systems have been identified.
Perhaps the most successful tissue targeting ligand is trimeric N‐acetyl galactosamine (GalNAc) (Lee et al, 1984). GalNAc binds to the asialoglycoprotein receptor (AGPR), which is abundantly expressed in the liver (Schwartz et al, 1980). This high affinity‐binding ligand has been directly conjugated to ONs and siRNA and provides highly specific and effective delivery to hepatocytes (Matsuda et al, 2015; Janas et al, 2018; Debacker et al, 2020). Another striking example is the glucagon‐like peptide‐1 (GLP1) receptor (GLPR1) system for specific targeting of pancreatic β cells (Muller et al, 2019). Recent studies showed that GLP1‐ON conjugates are specifically taken up by GLPR1‐expressing cells in the pancreas, including isolated pancreatic islets, and induce strong accumulation and activity in pancreatic β cells in a ligand‐dependent manner upon systemic delivery in mice (Ammala et al, 2018).
Antibodies
A promising recent development in chemical conjugates is antibody–RNA conjugates (ARCs). ARCs typically include monoclonal antibodies, or antibody fragments, with functional ONs, and they have been used for imaging and protein detection. However, antibodies can also be used as a delivery agent for therapeutic ONs. An antibody fragment specific for the transferrin receptor, which is involved in intracellular transport of iron‐laden transferrin, has been used to target siRNA towards skeletal and cardiac muscle tissues (Sugo et al, 2016). Companies are taking this technology forward for diseases such as myotonic dystrophy and Duchenne muscular disease.
Aptamers
Aptamers have been shown to mediate delivery of therapeutic ONs as aptamer‐ON conjugates, or within nanoparticle formulations (Catuogno et al, 2016; Soldevilla et al, 2018). The first aptamer‐siRNA chimeras targeted prostate‐specific membrane antigen‐expressing cancer cells to deliver apoptosis‐inducing siRNAs (McNamara et al, 2006). Further development of aptamer‐ONs involved chemical modifications to protect the ONs from nuclease degradation and increase their plasma half‐life. Aptamer‐ONs have since shown effective in vivo delivery of miRNAs, antagomirs, ASOs and bi‐modular miRNA‐antagomirs within preclinical cancer models (Catuogno et al, 2015; Esposito et al, 2016; Soldevilla et al, 2018).
Carrier‐based delivery systems
The pharmacological properties of carrier‐based delivery systems are largely independent of the physicochemical properties of the ON cargo, and instead depend on the properties of the delivery system. Therefore, the desired properties can be built into them via formulation design, resulting in multifunctional advanced drug delivery systems. These delivery systems may serve (often simultaneously) many different purposes, including (i) protecting the ON cargo from premature degradation, (ii) increasing the effect duration and (iii) enhancing the targeting. This improved targeting can either occur via passive or active targeting. Passive targeting exploits the microanatomical features of tissues, for instance, tissues with enhanced permeability and retention, or tissues with discontinuous/fenestrated epithelium. For active targeting, delivery systems are decorated with active targeting ligands. Particulate carrier‐based delivery systems also facilitate intracellular delivery by enhancing cellular uptake, intracellular trafficking and endosomal escape. In that way, the dose reaching non‐target tissues and/or the toxicological targets may be reduced, whereas the dose reaching the pharmacological target may be increased. The net result is an improved drug therapeutic index. The complexity of these systems leads to new challenges in the development, for example with respect to cost, manufacturability, safety, quality assurance and quality control.
Reflecting the immense interest in delivery of therapeutic ONs, a plethora of nanocarrier types have been investigated for delivery purposes, such as gold nanoparticles (Ding et al, 2014; Morgan et al, 2019), mesoporous silica (Steinbacher & Landry, 2014; Cha et al, 2017) and other inorganic nanocarriers (Malmsten, 2013). Yet, the current focus seems to be on lipid‐, polymer‐ and peptide‐based delivery systems and hybrids of these, which are described further below (Fig 4).
Lipid‐based delivery systems
The recent approval of patisiran (Table 3) (Suhr et al, 2015), together with improvements in manufacturability brought about by the introduction of microfluidics, has reinforced interest in lipid‐based delivery systems to the scientific community and pharmaceutical industry. The term lipid nanoparticles (LNPs) is used generically below to describe ON‐loaded lipid‐based delivery systems, because the structural complexity of most lipid‐based nanocarriers complicates their further classification into, for example, liposomes and solid lipid nanoparticles.
Cationic lipids entrap ONs via attractive electrostatic interactions (Felgner et al, 1987), and highly efficient commercial in vitro transfection reagents are based on cationic lipids. However, as systemic toxicity of cationic lipids is often dose‐limiting for in vivo application, ionisable lipids that are positively charged at low pH, e.g. during LNP manufacture, and typically neutral at physiological pH, are favoured (Semple et al, 2001). Today, a vast number of ionisable lipids have been developed covering a wide range of different structures. These include, among others, lipidoids (Akinc et al, 2008; Dong et al, 2014) and the ionisable lipid DLin‐MC3‐DMA (Jayaraman et al, 2012), which is considered the gold standard of ionisable cationic lipids. In general, they display headgroups containing tertiary amines, which are protonated under acidic conditions and uncharged at neutral pH. The hydrophobic lipid tails stabilise the LNP structure during formation and in formulation via hydrophobic interactions.
Clinically approved patisiran contains DLin‐MC3‐DMA, helper lipids (Kulkarni et al, 2019) and PEG‐lipid encapsulating siRNA directed against transthyretin (TTR) mRNA (Adams et al, 2018). The PEG lipid stabilises the LNPs during manufacturing and storage, and it increases the circulation half‐life. However, PEG lipids inhibit cellular transfection; hence, they are designed to rapidly diffuse from the LNPs after IV administration (Chen et al, 2016). The LNPs are passively targeted to the liver (Shi et al, 2011), and the size of the LNPs permits delivery through the fenestrated endothelium in the liver to the underlying hepatocytes (Chen et al, 2016). In addition, active hepatocyte targeting has been shown to occur via surface adsorption of apolipoprotein E, which targets the LNPs to the internalising low‐density lipoprotein receptor expressed on hepatocytes (Akinc et al, 2010; Chen et al, 2016). After cellular uptake, endosomal escape of siRNA into the cytosol may be facilitated via interactions between the re‐protonated ionisable cationic lipid in the acidic endosomal environment and anionic endogenous lipids in the endosomal membrane (Habrant et al, 2016).
Exosomes are particular lipid‐based nanocarriers (Barile & Vassalli, 2017). These nanosized vesicles are shed from the cells, encapsulating part of the cellular cytoplasm in the process (Pathan et al, 2019). They are remarkable in their biocompatibility and potential for highly specific active targeting through surface display of endogenous cellular ligands. The main challenges for using exosomes as delivery systems are (i) reproducible, large‐scale production and (ii) effective loading of drugs. Additionally, exosome heterogeneity is caused by their natural content of proteins and nucleic acids derived from the host cell (Willms et al, 2018; Jeppesen et al, 2019). This complicates their use as therapeutic delivery agents. The therapeutic promise of exosomes has been extensively reviewed elsewhere (Wiklander et al, 2019).
Polymer‐based delivery systems
Although less clinically advanced, polymer‐based systems are also interesting carriers for ON delivery, largely due to the chemical flexibility of polymers, in particular synthetic polymers (Fig 4) (Freitag & Wagner, 2020). Both monomer sequence and side/end group functionalities can be engineered. Additionally, polymeric nanocarriers exhibit high structural integrity and stability during storage.
One polymer with high biocompatibility that has been studied and used extensively is the copolymer poly(lactic‐co‐glycolic acid) (PLGA) (Rezvantalab et al, 2018). For small‐molecule drugs, highly efficient encapsulation in polymeric nanoparticles can be achieved, e.g. through miniemulsion‐based synthesis, followed by in situ polymerisation (Fusser et al, 2019). However, due to their negative charge, anionic ONs cannot be encapsulated using this approach. Instead, encapsulation can be achieved through attractive electrostatic interactions between the anionic ONs and polycationic polymers. Dendrimers are hyperbranched polymers, which are well suited for this purpose because they can complex many ON molecules. Several cationic polymers have been used, including poly(amidoamine), poly(propyleneimine) and poly(L‐lysine) [reviewed by (Mignani et al, 2019)].
Among the synthetic polymers, polyphosphazenes are notable in their high biocompatibility and chemical flexibility, and they have successfully been used to deliver therapeutic ONs (Peng et al, 2016; Hsu et al, 2020). Polyphosphazenes can be tailored to exhibit responsivity to external (bio)chemical stimuli (Teasdale, 2019), e.g. local pH. This allows for a targeted release of the cargo at the desired site of action. Complementing the use of synthetic polymers, there is long‐standing interest in the use of naturally occurring biopolymers for ON encapsulation; the most notable example is the use of the polycation chitosan, often in complex with another, anionic polymer, e.g. PLGA (Taetz et al, 2009) or alginate (Lee & Mooney, 2012).
Recently, there has been significant interest in lipid–polymer hybrid nanoparticles (Thanki et al, 2017). These hybrids combine desirable properties from both nanoparticle types, i.e. the serum stability of PLGA‐based matrix system with the biocompatibility and high loading capacity of ONs in delivery systems based on cationic lipids.
Peptide‐based delivery systems
CPPs represent another group of compounds that have been also successfully used as a carrier‐based drug delivery system (Lehto et al, 2016). In this context, formation of CPP/ON nanoparticles is driven by electrostatic and hydrophobic interactions between cationic CPPs and anionic ONs. Compared with directly conjugated CPP‐ONs, peptide‐based vectors are more amphipathic and usually carry additional chemical modifications that make them compatible with encapsulating ONs. Commonly, such modifications include incorporation of various hydrophobic modifications, such as fatty acid derivatives, to the CPP sequences, which increase the stability of the formulation and enhance their cellular uptake and endosomal escape. Various types of CPPs have demonstrated considerable potential for ON delivery in a nanoparticle‐based format, including MPG and PepFect peptide derivatives [reviewed in (Boisguerin et al, 2015; Lehto et al, 2016)].
Antibody complexation delivery systems
Antibodies are another promising form of carrier delivery system used both as direct conjugates or unconjugated carriers. As unconjugated carriers, antibodies or antibody fragments have been fused with either avidin or protamine peptide. Taking advantage of the natural avidin–biotin complexation system, antibody–avidin fusion molecules bind to biotinylated ONs (Penichet et al, 1999). The peptide protamine is a positively charged RNA‐binding peptide, which binds to siRNA and condenses it into antibody–siRNA complex (Song et al, 2005). This system has been used to link cytotoxic siRNAs with Her2‐positive cancer cell‐targeted antibodies (Yao et al, 2012). Like all complexation systems, these two systems have the advantage of an established target‐specific antibody carrier, which can easily be complexed with any siRNA.
Model systems for oligonucleotide development
Successful development of ON‐based drugs depends on detailed knowledge about pharmacokinetic (PK) and pharmacodynamic (PD) properties. PK/PD analyses describe the relationship between PK (drug concentration in the organism) and PD (the organism's biological response to the drugs) in a time‐dependent manner (Negus & Banks, 2018). PK/PD modelling and simulations are used to rapidly characterise the efficacy and safety of drugs, and PK/PD simulation models containing in vitro and in vivo preclinical studies can anticipate potential risks in humans (Li et al, 2016). The use of predictive model systems for PK/PD analyses save time, costs and minimise the need for in vivo studies, facilitating the translation from bench to bedside.
Methodologies for in vitro testing of oligonucleotides
In vitro models can be implemented to test pharmacological activity, transfection efficiency, hepatotoxicity and intracellular half‐life. However, it is usually difficult to correlate in vitro findings to preclinical and clinical in vivo findings (Table 2). Novel technologies, such as reprogramming patient‐derived cells into induced pluripotent stem cells (iPSCs) (Takahashi & Yamanaka, 2006; Takahashi et al, 2007) and genome editing techniques to make isogenic cell lines, have revolutionised the field (Ran et al, 2013). Two‐dimensional (2D) and three‐dimensional (3D) cell cultures, including organoids, are used to improve the understanding of pathological disease mechanisms, as well as ON efficacy studies. One example of successful translation from a 3D‐model to a clinical trial is sepofarsen for the treatment of the inherited retinal disease Leber congenital amaurosis (LCA) (Collin et al, 2012; den Hollander et al, 2006). Combining patient‐derived retinal organoids with toxicity studies in non‐human primates (NHPs) was sufficient to initiate a phase I/II clinical trial (NCT03140969, NCT03913143) (Cideciyan et al, 2019). The eye is an exceptional target organ, given its isolated and immune‐privileged status, which allows for translation of results from organoids in culture to the human eye. However, for other (multi‐)organ diseases, establishing predictive cellular models to mimic the functions of entire organs remains a challenge.

| In vivo | In vitro | 3D Organoids | Organs‐on‐chips | |
|---|---|---|---|---|
| Human‐derived tissue | No | Yes | Yes | Yes |
| Personalised medicine | No | Yes | Yes | Yes |
| Realistic microenvironment | Yes | No | Yes | Yes |
| Organ‐level function | Yes | Limited | Potentially/Limited | Potentially |
| Real‐time readouts | No | Limited | Limited | Yes |
| High‐throughput testing | No | Yes | Limited | Possibly |
| Pharmacodynamics / ‐kinetics | Yes | No | No/Limited | Potentially |
An interesting alternative to 2D and 3D tissue culture techniques is the microfluidics‐based organ‐on‐chip technology (van der Meer & van den Berg, 2012), which consists of micro‐engineered iPSC‐derived models that combine the advantages of current in vitro and in vivo models. The technology breaks down organs into the most essential components, including biological barriers, for drug delivery, efficacy, toxicity and PK/PD studies. Organ‐on‐chips reproduce the interaction between cultures of multiple tissue types using microfluidic channels and chambers (Huh et al, 2010; Kim et al, 2012; Westein et al, 2013). This interaction can be monitored in real time to study the PK/PD of a specific drug as well as drug–drug interactions (Lee et al, 2017; Shinha et al, 2020). For instance, the PK/PD evaluation of terfenadine (a type of antihistamine) has been assessed by using a cellular model combining heart and liver cells in two interconnected chambers. This model, combined with microelectrode arrays, also contributed to predict the potential cardiotoxicity of the drug (McAleer et al, 2019). Interestingly, recent drug permeability studies in blood–brain barrier (BBB)‐on‐chip models were found to be more predictive compared with existing in vitro models (van der Helm et al, 2016). Other cellular models under development include retina‐on‐chip (Achberger et al, 2019; Seo et al, 2019) and lung‐on‐chip (Huh et al, 2010) models. Mimicking the function of entire organ(s) in a dish by combining several cell types in a single device may have valuable potential for drug screening and development, as well as PK/PD and toxicity studies. In the future, organ‐on‐chip models might, to some extent, replace experimental animal models.
Investigation of PK/PD properties in vivo
In vivo models have been extensively used for dose‐finding studies. PK properties are largely comparable across multiple species including mouse, rat, NHP and human (Yu et al, 2009; Geary et al, 2015). Hence, cross‐species PK/PD relationships are very valuable for the prediction of human dosing. Animal models have been vital for determining in vivo efficacy of ONs, tissue‐specific delivery, and optimising the route of administration for systemic and neurological diseases (Schoch & Miller, 2017; Buijsen et al, 2019). Preclinical in vivo testing in a transgenic mouse model for SMA predicted the enhanced benefit of treating pre‐symptomatic stages of the disease, which was later validated in the clinic.
However, detailed knowledge of the disease model is vital for interpreting data: A study in the mdx mouse model for DMD of the PK/PD of 2ʹ‐OMe ONs for DMD revealed higher ON levels in dystrophin‐deficient muscle fibres than in healthy fibres, as well as an enhanced exon skipping efficiency (Heemskerk et al, 2010). However, ON efficiency was lower in clinical trials in DMD patients, potentially due to a better regenerative capacity in mice. Also, animal models may not always reciprocate the human condition due to the different genomic context of the mutations, even when using humanised animal models. This is evident for pre‐mRNA splicing, which is differentially regulated between tissues, organs and species (Rivera‐Barahona et al, 2015). Between tissues, DNA variants have been observed to affect pre‐mRNA splicing, complicating the interpretation of in vitro studies. An example is the aforementioned deep‐intronic change underlying LCA: while lymphoblastoid and fibroblast cells derived from patients suggested a hypomorphic effect (Garanto et al, 2016), reprogrammed patient‐derived iPSCs differentiated towards a retinal fate revealed that the percentage of aberrantly spliced mRNA was highly increased in photoreceptor cells, explaining the retinal phenotype observed in LCA patients (Parfitt et al, 2016). Follow‐up studies revealed that a pseudoexon present in humans was differentially recognised in cell lines derived from other species (Garanto et al, 2015). Thus, care is warranted when selecting a model system for assessing the effects of a certain genetic variant, as well as for the development of splice‐modulation therapies.
Safety assessment of oligonucleotide‐based therapeutics
While new chemistries and delivery technologies might lead to higher efficacy, it is important to screen for potential side effects in early phases of preclinical development to avoid subsequent failure. Toxicological aspects of therapeutic ONs have been comprehensively summarised previously (Andersson & den Besten, 2019). The Oligonucleotide Safety Working Group (OSWG) has also published extensive guidelines for assessing the various aspects of ON safety. Our understanding of ON‐mediated toxicity increases as more preclinical and clinical data become available. While the concept of class toxicity appears nuanced in the light of the expanding knowledge on various chemistries, ON‐related side effects still falls under two main categories: (i) hybridisation‐dependent effects, including on‐ and off‐target effects, and (ii) hybridisation‐independent effects, mostly caused by protein‐binding properties (Fig 5).

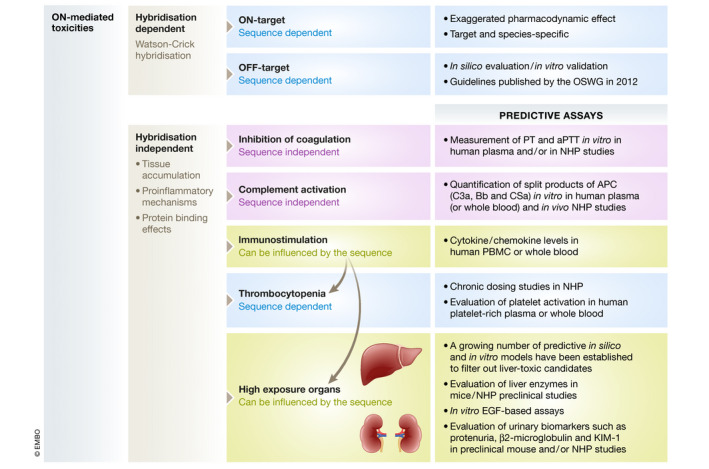
ASO mediated toxicities
Schematic representation of the most common ON‐mediated toxicities, which are mainly classified as hybridisation‐dependent (Watson–Crick hybridisation) or hybridisation‐independent effects (tissue accumulation, proinflammatory mechanisms and/ or protein binding effects). Some of them are strictly class specific (sequence independent), while others can be influenced by the sequence (sequence specific).
Hybridisation‐dependent effects
On‐target safety, also referred to as exaggerated pharmacology, relates to the possible toxicities induced by excessive or prolonged activity of the ON in target or non‐target organs. These effects are considered rare and are generally discovered in preclinical studies. However, due to the sequence‐specific action of ON‐based drugs, target sequences may not be conserved across species. Therefore, human sequences might not display efficacy in rodents or NHPs; hence, species‐specific surrogates are needed for on‐target risk assessment (Levin & Henry, 2008).
Off‐target effects correspond to the potential toxicities associated with ON hybridisation to unintended RNA targets (complete or partial complementarity). They have increased with the development of high‐affinity chemistries, e.g. LNA, tcDNA and constrained ethyl (cEt), which allow the use of much shorter sequences. Off‐target effects are of particular concern for gapmer ONs and siRNA, which aim at downregulating their targets, as they could downregulate the expression of unintended ones (Fedorov et al, 2006; Burel et al, 2016). Several studies have characterised off‐target effect‐associated mechanisms and described elegant ways to reduce risks and improve the design of specific gapmers and siRNAs (Hagedorn et al, 2017; Janas et al, 2018). In contrast, splice‐switching ASOs must bind specific splicing regulatory elements to be efficacious, and they are therefore less likely to induce off‐target effects. With the development of more stable ONs and efficient delivery systems, systemic administrations might distribute to target but also non‐target tissues; hence, off‐target effects should be carefully evaluated during preclinical development. The guidelines published by the OSWG for assessing off‐target effects recommend (i) in silico evaluation, (ii) interpretation of in silico hits using auxiliary data (e.g. time‐ and spatiotemporal‐dependent expression of off‐target RNA) and (iii) in vivo evaluation of ON drugs (Lindow et al, 2012).
Hybridisation‐independent effects
Most ON‐mediated toxicities are not caused by Watson–Crick base pairing to RNA, but are rather a result of ON–protein interactions and therefore depend on the chemistry and/or the delivery system. Single‐stranded PS‐modified ON display particularly high protein binding affinities, and the majority of the hybridisation‐independent effects have thus been reported for this class of ON, as opposed to siRNA containing less PS‐modified residues.
Inhibition of blood coagulation
Inhibition of the intrinsic blood coagulation pathway is a well‐documented side effect of the PS chemistry (Henry et al, 1997b; Echevarria et al, 2019). It is considered a class effect, modulated by interactions of the ON with plasma proteins in a sequence‐independent way. The PS modification selectively prolongs the partial thromboplastin time at low plasma concentrations by inhibiting the tenase complex. However, at high plasma concentrations, both the intrinsic and extrinsic pathways are affected, suggesting additional inhibitory effects (Sheehan & Lan, 1998). Prolongation of clotting times is correlated with the maximal plasma concentration (C max) of circulating ONs, and it has not been associated to relevant clinical signs, as it can be controlled by dose reduction or by extending infusion times. Nevertheless, it should be included in screening studies, which can be performed both in vivo and in vitro in mouse, NHP and human serum, respectively, since results can be extrapolated across species (Andersson & den Besten, 2019).
Complement activation
Systemic administration of PS‐modified ONs has been reported to activate the alternative complement pathway as a consequence of plasma protein binding (Henry et al, 2002). Although this hybridisation‐independent effect is mainly related to the ON chemistry (class effect), unexpected complement activation has been observed with some sequence specificity, as in the case of tcDNA (Aupy et al, 2020). Activation of the alternative complement pathway has been thoroughly studied in NHP models, which are particularly sensitive (Henry et al, 2016). The effect is dependent on the plasma concentration and can be controlled by increasing the IV infusion time to reduce the Cmax. PS‐modified ONs have been shown to interact directly with plasma factor H, which is a negative regulator of the complement cascade that reduces the free levels of inhibitor, permitting uncontrolled amplification of the cascade and release of split products such as Bb and anaphylotoxins C3a and C5a (Henry et al, 1997a). Complement can be activated similarly at every dose; hence, chronic administration of toxic ONs can result in C3 depletion, eventually leading to altered complement function, secondary inflammation and vasculitis (Engelhardt et al, 2015; Shen et al, 2016; Andersson & den Besten, 2019). Although humans appear less sensitive to complement activation, it is recommended to routinely evaluate complement activation in preclinical safety studies of new ON‐drug candidates in NHPs.
Complement activation can be assessed in vitro in NHP or human serum, or whole blood, to measure split products of the alternative complement pathway (Bb, C3a and C5a). Nevertheless, one should be cautious when interpreting the results, as it is difficult to extrapolate and predict dose–response relationships (Andersson & den Besten, 2019).
Immunostimulation
ON‐induced immunostimulation is a complex side effect that depends on several aspects, including chemistry and nucleotide sequence (Krieg, 1998; Agrawal & Kandimalla, 2004). ONs can activate the innate immune system through binding to pattern‐recognition receptors (PRRs) such as the Toll‐like receptors (TLRs). Activation of the innate immune system by CpG‐containing ONs is comparable to that observed for bacterial DNA and CpG‐containing ONs are used for cancer and autoimmune disease therapies as well as vaccine adjuvants (Krieg & Davis, 2001; Krieg, 2006; Kline & Krieg, 2008). However, the immunostimulatory activity of ONs designed for antisense purposes constitutes a potential side effect. In this regard, modified ONs with 2ʹ‐ribose modifications, 5‐methyl cytosine residues, or without CpG motifs, have been designed to avoid TLR9 activation. Additional studies have demonstrated that CpG‐free, PS‐modified ONs can also elicit proinflammatory responses, although the molecular mechanism is still debated (Vollmer et al, 2004; Senn et al, 2005; Younis et al, 2006). Of note, immunostimulatory effects have never been reported for ONs with neutral backbones, e.g. PMOs (Zhang et al, 2015). Rodents are particularly sensitive to immune stimulation. Mice treated with high doses of PS‐ONs display increased levels of circulating cytokines (IL‐1b, IL‐6, interferon, tumour necrosis factor‐α) and chemokines, as well as proliferation of B‐lymphocytes (Monteith et al, 1997). Although generally less critical, some significant inflammatory responses, e.g. vasculitis, related to complement activation mediated by PS‐ONs, have been described in NHP studies (Levin & Henry, 2008; Engelhardt et al, 2015; Frazier, 2015; EMA, 2016). Differences in immune response between species have been attributed to the differential sequence, expression and function of the germline‐encoded PRRs (Barchet et al, 2008).
In clinical trials, inflammatory adverse effects may manifest as flu‐like symptoms and injection site reactions following subcutaneous (SC) administration (Rudin et al, 2001; Thomas et al, 2013; Voit et al, 2014). Understanding the underlying mechanisms of therapeutic ON‐mediated induction of proinflammatory adverse effects has facilitated the design of safer and more potent sequences that are efficacious at lower doses. Nevertheless, some sequences still display unexpected toxicity, and specific screening for immunostimulatory adverse effects is recommended. In addition to in vivo studies in rodents and NHPs, proinflammatory evaluation is usually performed in vitro using human peripheral blood mononuclear cells or whole blood (Apter et al, 1990; Lankveld et al, 2010).
The potential immunogenicity of ONs is poorly documented but recent data show that anti‐drug antibodies (ADAs) are present in NHPs and humans (Andersson & den Besten, 2019). More than 30 and 70% of patients treated with drisapersen and mipomersen, respectively, were found positive for ADA after 24 weeks of treatment. Recently, ADA plasma levels were shown to increase both in monkeys and humans, while no impact on efficacy and safety was reported (Bosgra et al, 2019; Yu et al, 2020).
Formulations based on nanoparticles administered IV may also induce infusion‐related reactions (IRRs), e.g. hypersensitivity, evident as flu‐like symptoms and even cardiac anaphylaxis (Szebeni, 2018). Hence, before IV infusion of patisiran, patients are required to be premedicated with IV antihistamines (H1/H2 blockers), IV corticosteroid and oral acetaminophen or paracetamol to suppress IRRs. Mild to moderate IRRs were observed in a phase III trial of patisiran in approximately 20% of the patients, who were all premedicated, the incidence of which decreased over time (Adams et al, 2018). In contrast, premedication is not required before administration of ONs and GalNAc–siRNA conjugates.
Thrombocytopenia
ON‐associated thrombocytopenia is an occasional event that has been observed in rodents and NHPs, as well as in three recent clinical trials with unrelated PS‐ONs [volanesorsen (FDA, 2018), inotersen (Benson et al, 2018; Mathew & Wang, 2019) and drisapersen (EMA, 2016; Goemans et al, 2016)]. The exact underlying mechanisms of thrombocytopenia is still debated, and several immune and non‐immune mediated mechanisms have been proposed. Direct activation of platelets by PS‐ONs through the binding to platelet receptors has been demonstrated (Flierl et al, 2015; Sewing et al, 2017). In addition, a heparin‐induced thrombocytopenia‐like mechanism through the induction of anti‐platelet factor 4 IgG antibodies has also been proposed, based on the binding of nucleic acids to platelet factor 4 (Jaax et al, 2013), although contradictory results have been reported. A recent study suggests that sequestration of platelets in the liver and spleen occurs through the activation of monocytes, but not platelets, and is accompanied by increased serum IgM levels (Narayanan et al, 2018). In most cases, thrombocytopenia after treatment with ONs is mild to moderate and reversible. The number of platelets does not drop below the normal limit during treatment and normalises after withdrawal from treatment. However, a concerning and severe decline (< 50,000 platelets/µl) has been observed in NHP studies after repeated dosing (Henry et al, 2017). To date, severe thrombocytopenia has not been reported for siRNA drugs, neither in preclinical studies nor in clinical trials, but encapsulation of siRNA to LNPs has been shown to cause thrombocytopenia in rats, presumably induced by the cationic lipid molecules themselves (Chi et al, 2017).
High‐exposure organs
Following IV administration and independently of the chemistry, the highest concentrations of ONs are found in the liver and the kidneys, which are considered high‐exposure organs (Fig 5). The toxicities observed in these organs are not necessarily associated with the accumulation of ONs per se but can also be due to sequence‐specific effects. Accumulated ONs are often apparent as basophilic granules (ONs in lysosomal compartments) in tissue sections. However, these effects are regarded as non‐adverse because of their reversible nature upon termination of treatment. In contrast, acute toxicities characterised by large areas of necrosis, pronounced elevation of liver enzyme levels, morbidity and mortality have been reported for some high‐affinity gapmers after a single or few doses in mice (Hagedorn et al, 2013; Burdick et al, 2014). The mechanisms underlying these sequence‐specific acute toxicities may be accumulation of RNase H‐cleaved mRNA products and/or protein interactions (Burel et al, 2016; Sewing et al, 2016; Shen et al, 2019). While the screening for these acute toxicities previously relied on in vivo studies assessing levels of liver enzymes following IV administration in rodents, a growing number of predictive in silico (Hagedorn et al, 2013; Burdick et al, 2014) and in vitro models (Sewing et al, 2016; Dieckmann et al, 2018) have been established.
Renal lesions are generally restricted to the proximal tubules and appear only in animals treated with much higher ON doses than the clinically relevant doses. No clinically significant renal dysfunction was reported in a large retrospective study of 2ʹ‐MOE gapmer trials (Crooke et al, 2018). Renal toxicity was mostly regarded as accumulation‐related toxicity and primarily sequence unspecific until more acute tubular lesions were reported with high‐affinity ONs, e.g. LNAs (Engelhardt et al, 2015). Beyond the classical biomarkers for renal injury, e.g. increased excretion of β2‐microglobulin and kidney injury molecule‐1, a predictive epidermal growth factor‐based assay has recently been developed to exclude this type of nephrotoxic candidates (Moisan et al, 2017).
Approved oligonucleotide‐based therapeutics
Advances in therapeutic ON technology in recent decades provide a unique opportunity for addressing previously inaccessible drug targets (Bennett et al, 2017). Since the approval of fomivirsen in 1998 by the FDA for treating cytomegalovirus (CMV) retinitis (Marwick, 1998), 11 ON‐based drugs have received marketing authorisation to be used in humans, and two additional ON drugs have received positive opinion for marketing by the EMA (Table 3). Here, we discuss approved ON therapeutics according to their functional modalities.
Fomivirsen (Vitravene) is a 21‐mer PS DNA‐based ON developed for treating CMV retinitis patients, especially those with acquired immunodeficiency syndrome (AIDS) (Vitravene Study G, 2002). This first‐generation ASO targets the human CMV major immediate‐early gene mRNA for RNAse H degradation (Geary et al, 2002). Fomivirsen is delivered locally by IVT administration and hence does not require a delivery agent. While a second‐generation 2ʹ‐MOE‐based gapmer sequence (ISS 13312) was in clinical development (Henry et al, 2001), Novartis discontinued development and withdrew marketing (Wathion, 2002). The number of CMV retinitis cases had decreased dramatically due to the development of highly active antiretroviral therapy. Nevertheless, fomivirsen was a success and established ON therapies as viable for clinical development.

| Approval date | Drug name | Disease | Target | ASO sequence 5′–3′ | Administration route/target tissues | |
|---|---|---|---|---|---|---|
| FDA | EMA | |||||
| RNaseH | ||||||
| 26 August 1998 | 29 July 1999 | Fomivirsen (Vitravene) | Cytomegalovirus retinitis in immunocompromised patients | CMV major immediate‐early gene mRNA | dGs‐dCs‐dGs‐dTs‐dTs‐dTs‐dGs‐dCs‐dTs‐dCs‐dTs‐dTs‐dCs‐dTs‐dTs‐dCs‐dTs‐dTs‐dGs‐dCs‐dG | IVT / eye |
| 29 January 2013 | refused authorisation | Mipomersen (Kynamro) | Homozygous familial hypercholesterolemia | Apolipoprotein B‐100 | Gs*‐mCs*‐mCs*‐Ts*‐mCs*‐dAs‐dGs‐dTs‐dmCs‐dTs‐dGs‐dmCs‐dTs‐dTs‐dmCs‐Gs*‐mCs*‐As*‐mCs*‐mC* | SC / liver |
| 05 October 2018 | 05 July 2018 | Inotersen (Tegsedi) | Hereditary transthyretin amyloidosis | Transthyretin | mTs*‐mCs*‐mTs*‐mTs*‐Gs*‐dGs‐dTs‐dTs‐dAs‐dmCs‐dAs‐dTs‐dGs‐dAs‐dAs‐dAs‐mTs*‐mCs*‐mCs*‐mC*‐3′ | SC / liver |
| Under review | 03 May 2019 | Volanesorsen (Waylivra) | Familial chylomicronemia syndrome, hypertriglyceridemia and familial partial lipodystrophy | Apolipoprotein CIII | As*‐Gs*‐mCs*‐Ts*‐Ts*‐dmCs‐dTs‐dTs‐dGs‐dTs‐dmCs‐dmCs‐dAs‐dGs‐dmCs‐Ts*‐Ts*‐Ts*‐As*‐T* | SC / liver |
| Splice modulation | ||||||
| 01 June 2019‡ | N/A | Milasen | Neuronal ceroid lipofuscinosis 7, Batten's disease | MFSD8 exon 6 | As*‐As*‐Ts*‐Gs*‐Ts*‐Ts*‐As*‐Gs*‐Ts*‐Gs*‐mCs*‐Ts*‐Ts*‐Gs*‐Ts*‐Ts*‐Gs*‐As*‐Gs*‐Gs*‐Gs*‐mC* | IT / CNS |
| 19 September 2016 | refused authorisation | Eteplirsen (Exondys51) | Duchenne muscular dystrophy | Dystrophin exon 51 | CTCCAACATCAAGGAAGATGGCATTTCTAG | IV / skeletal muscle |
| 12 December 2019 | Under review | Golodirsen (Vyondys 53) | Duchenne muscular dystrophy | Dystrophin exon 53 | GTTGCCTCCGGTTCTGAAGGTGTTC | IV / skeletal muscle |
| August 2020 | Under review | Viltolarsen† (Viltepso) | Duchenne muscular dystrophy | Dystrophin exon 53 | CCTCCGGTTCTGAAGGTGTTC | IV / skeletal muscle |
| 23 December 2016 | 30 May 2017 | Nusinersen (Spinraza) | Spinal muscular dystrophy | Survival motor neuron 2 exon 7 | mTs*‐mCs*‐As*‐mCs*‐mTs*‐mTs*‐mTs*‐mCs*‐As*‐mTs*‐As*‐As*‐mTs*‐Gs*‐mCs*‐mTs*‐Gs*‐G* | IT / CNS |
| Aptamer | ||||||
| 17 December 2004 | 31 January 2006 | Pegaptanib (Macugen) | Age‐related macular degeneration | Vascular endothelial growth factor | 40 kDa PEG‐5′‐CF‐G^‐G^‐A‐A‐UF‐CF‐A^‐G^‐UF‐G^‐A^‐A^‐UF‐G^‐CF‐UF‐UF‐A^‐UF‐A^‐CF‐A^‐UF‐CF‐CF‐G^‐3′‐3′‐dT‐5′ | IVT / eye |
| RNAi | ||||||
| 10 August 2018 | 27 August 2018 | Patisiran (Onpattro) | Hereditary transthyretin amyloidosis | Transthyretin |
5′‐G‐U^‐A‐A‐C^‐C^‐A‐A‐G‐A‐G‐U^‐A‐U^‐U^‐C^‐C^‐A‐U^‐dT‐dT‐3′ 3′‐dT‐dT‐C‐A‐U^‐U‐G‐G‐U‐U‐C‐U‐C‐A‐U^‐A‐A‐G‐G‐U‐A‐5′ LNP formulated | IV / liver |
| 20 November 2019 | 02 March 2020 | Givosiran (Givlaari) | Acute hepatic porphyria | Aminolevulinate synthase 1 |
5′‐Cs^‐As^‐G^‐A^‐A^‐A^‐GF‐A^‐GF‐U^‐GF‐U^‐CF‐U^‐CF‐A^‐U^‐C^‐U^‐U^‐A^‐3′ 3′‐Us^‐Gs^‐G^‐UF‐C^‐UF‐U^‐UF‐C^‐UF‐C^‐AF‐C^‐AF‐G^‐AF‐G^‐UF‐A^‐GF‐AsF‐AsF‐U^‐5′ GalNAc Conjugate | SC / liver |
| 16 October 2020¥ | Lumasiran (Oxlumo) | Primary hyperoxaluria type 1 | Hydroxiacid oxidase 1 |
5′‐As^‐Cs^‐C^‐U^‐G^‐A^‐A^‐AF‐G^‐UF‐A^‐G^‐G^‐A^‐CF‐CF‐U^‐UF‐U^‐A^‐Us^‐AsF‐U^‐3′ 3′‐Gs^‐As^‐Cs^‐U^‐U^‐U^‐CF‐A^‐UF‐CF‐CF‐U^‐G^‐G^‐A^‐A^‐A^‐U^‐A^‐U^‐A^‐5′ GalNAc Conjugate | SC / liver | |
| 16 October 2020¥ | Inclisiran | Atherosclerotic cardiovascular disease | Proprotein convertase subtilisin‐kexin type 9 |
5′‐As^‐CFs‐As^‐AF‐AF‐AF‐G^‐CF‐A^‐AF‐A^‐A^‐C^‐AF‐G^‐GF‐U^‐CF‐U^‐A^‐Gs^‐As^‐A^‐3′ 3′‐U^‐G^‐U^‐U^‐U^‐U^‐C^‐G^‐U^‐U^‐dT‐U^‐GF‐U^‐CF‐C^‐A^‐G^‐As^‐Us^‐C^‐5′ GalNAc Conjugate | SC / liver | |
s, phosphorothioate linkage; *, 2′‐MOE; d, 2′‐deoxy; m, 5‐methyl; F, 2′‐F; ^, 2′‐OMe; italicised, PMO; † Viltolarsen approval by Japanese Ministry of Health Labour and Welfare, 25 March 2020; ‡ Milasen approved by FDA for clinical testing only, ¥, lumasiran and inclisiran received positive opinion for marketing by the CHMP.
RNase‐dependent second‐generation ASOs targeting the liver have been approved for polyneuropathy of hereditary transthyretin‐mediated amyloidosis (hATTR) (inotersen) as well as familial chylomicronemia syndrome (FCS), hypertriglyceridemia and familial partial lipodystrophy (volanesorsen), and familial hypercholesterolemia (mipomersen). The rare disease hATTR is linked to missense mutations in the TTR gene, which result in TTR protein misfolding. The TTR protein is secreted into the blood and cerebral spinal fluid, and accumulation of amyloid deposits (both wild type and mutant) in tissues causes polyneuropathy, multiorgan dysfunction and cardiomyopathy. Inotersen targets the hepatic expression of both wild‐type and mutant TTR mRNA. Patients treated with inotersen display a reduction in serum TTR protein levels and enhanced quality of life (Benson et al, 2018). Volanesorsen, although still awaiting FDA approval at the time of writing this review, was awarded EMA approval in May 2019. By targeting the 3ʹ UTR of apolipoprotein C3 mRNA, volanesorsen reduces the levels of triglycerides and apolipoprotein C3, which represent two known risk factors for cardiovascular disease, while increasing the levels of low‐density and high‐density lipoprotein cholesterol and apolipoprotein B in patients with FCS and hypertriglyceridemia (Graham et al, 2013; EMA, 2019). Mipomersen also targets apolipoprotein B‐100 to reduce circulating low‐density lipoprotein cholesterol, which constitutes another major risk factor for cardiovascular disease (Wong & Goldberg, 2014). In contrast to inotersen and volanesorsen, mipomersen was given FDA approval, but EMA authorisation was denied due to safety concerns related to liver toxicity and severe cardiovascular events (EMA, 2012). It has since been discontinued by the FDA and is only available through a restricted risk evaluation and mitigation strategy. All three ON therapies are dosed by SC administration without a delivery agent due to the natural uptake of ONs by the liver. In 2004, the aptamer pegaptanib (Macugen) was approved by the FDA for the prevention of the eye‐related disorder age‐related macular degeneration (Ng et al, 2006). Pegaptanib is a covalent conjugate of a highly modified single‐stranded aptamer and two 20‐kDa PEG units. It binds with high specificity and affinity to the extracellular VEGF isoform 165 and blocks its neo‐angiogenic activity (Ruckman et al, 1998). Patients dosed with pegaptanib demonstrated reduced vision loss compared with placebo controls (Gragoudas et al, 2004). Common to degenerative diseases, early treatment results in improved therapeutic outcome (Gonzales CR & Group VISiONCT, 2005).
Several splice modifying ON‐based drugs are approved to treat the paediatric disorders DMD and SMA, focussing on splice modification and targets tissues beyond liver. The first approved drug, i.e. eteplirsen (Exondys51), is a PMO‐based splice‐switching ASO that interacts specifically with DMD exon 51, and is used in DMD patients with dystrophin deletions amenable to exon 51 skipping (~14% of patients) (Cirak et al, 2011). Dystrophin expression is limited mainly to skeletal and cardiac muscles, and eteplirsen is expected to be most efficacious in skeletal muscle. However, conducive with all PMOs, high accumulation in the kidneys and rapid urine excretion is also expected (Heemskerk et al, 2009). The approval of eteplirsen by the FDA was accompanied by controversy due to the trial design and difficulties in quantifying increased expression of dystrophin, which leaves doubt on the efficacy of eteplirsen (Aartsma‐Rus & Arechavala‐Gomeza, 2018). As a result, it was not approved by the EMA. Strengthened by improved clinical trial designs, two additional PMO‐based ASOs have recently been approved for DMD patients amenable for dystrophin exon 53 skipping, i.e. golodirsen and viltolarsen, by the FDA, and both the FDA and Japanese Ministry of Health Labour and Welfare, respectively (Dhillon, 2020; Heo, 2020).
The only ON‐based therapeutic approved for a neurological disease is nusinersen, used for the treatment of SMA (Aartsma‐Rus, 2017; Finkel et al, 2017; Mercuri et al, 2018). Nusinersen targets the alternatively spliced exon 7 of SMN2 pre‐mRNA, increasing exon inclusion and producing a functional SMN protein. It is administered directly to the cerebral spinal fluid surrounding the spinal cord by IT injection (Hache et al, 2016). IT administration directs uptake into the CNS, allowing low doses and circumvention of liver metabolism and kidney excretion. Patients, especially young pre‐symptomatic patients, report extended survival and reaching motor milestones over the expected natural history of the disease. Controversy related to nusinersen is not over efficacy but rather the exceedingly high cost, which has delayed approval and prevented marketing in countries with national health services (Starner & Gleason, 2019).
The success of nusinersen has led to the use of ONs as personalised medicines, exemplified in the development of milasen, which targets a mutation specific to a single patient with a form of Batten’s disease (Kim et al, 2019). In this case, the insertion of an SVA (SINE‐VNTR‐Alu) retrotransposon altered the splicing of the major facilitator superfamily domain containing 8 (MFSD8) exon 6 into a cryptic splice‐acceptor site. Clinicians followed the preclinical studies and trial designs from the nusinersen studies to accelerate the FDA approval of the clinical study: milasen dosing was initiated 14 months after clinical diagnosis and just 4.5 months after identification of a therapeutic ASO. The patient’s rate of deterioration meant that dosing had to be initiated as soon as possible; hence, the patient was dosed in parallel to toxicology studies in animals. Although therapeutic efficacy in a single patient cannot be defined, milasen reduced the frequency and duration of seizures and potentially diminished the neurodegenerative decline.
Two ON therapeutics based on RNAi, i.e. patisiran and givosiran, were approved by the FDA in 2018 and 2019, respectively. Patisiran represents an important milestone, because it is the first marketed drug based on siRNA, launched only 20 years after the discovery of the RNAi mechanism (Fire et al, 1998). Like inotersen, patisiran inhibits hepatocyte expression of TTR in patients with hATTR (Adams et al, 2018). Patisiran consists of siRNA directed against TTR mRNA formulated as LNPs, which are administered systemically by IV infusion. The latest breakthrough is givosiran, which represents the first approved GalNAc‐siRNA conjugate. Givosiran inhibits hepatic synthesis of delta aminolevulinate synthase 1 (ALAS1) in patients with acute hepatic porphyria (AHP), which is a rare inherited disease of haem biosynthesis (Sardh et al, 2019). Monthly subcutaneous administration of givosiran results in hepatocyte‐specific distribution and downregulation of elevated ALAS1 mRNA in the liver.
Recently, two new liver‐targeting GalNAc‐siRNA drugs received positive opinion for marketing in Europe, i.e. lumasiran and inclisiran (Fitzgerald et al, 2017; McGregor et al, 2020). Lumasiran targets hydroxyacid oxidase 1 (HAO1) for the treatment of primary hyperoxaluria type 1 (PH1), which is a rare inherited disorder characterised by the overproduction of oxalate. Targeting HAO1 reduces the substrate needed for oxalate production in the liver (McGregor et al, 2020). Inclisiran targets the proprotein convertase subtilisin–kexin type 9 (PCSK9) to reduce low‐density lipoprotein (LDL) cholesterol. PCSK9 is a serine protease, which binds to the LDL receptors to induce their lysosomal degradation. Therefore, silencing PCSK9 enhances the half‐life of LDL receptors responsible for cholesterol clearance (Fitzgerald et al, 2017). Inclisiran reduces more than 50% LDL cholesterol levels in treated patients with minimal side effects (Khvorova, 2017; Ray et al, 2020). Approval of Inclisiran will expand the indications for ONs to not only include rare but also common diseases.
Concluding comments and future perspectives
In 1978, it was demonstrated that a 13‐mer DNA‐based ON binding to Rous sarcoma virus RNA could inhibit protein expression in cell culture (Zamecnik & Stephenson, 1978), but it was not until 20 years later (1998) that the first ON‐based therapeutic drug fomiversen was approved. By 2016, only two additional drugs (pegaptanib and mipomersen) had been approved (Table 3) but since then the development pace of ON‐based drugs accelerated with 11 ON‐based drugs currently approved (Aartsma‐Rus & Corey, 2020). Yet, many of these drugs display limited efficacy (eteplirsen, golodirsen, viltolersen), and the more efficacious drugs take advantage of local administration (nusinersen). However, GalNAc conjugation and the LNP technology represent delivery breakthroughs that have completely changed the perspective for therapies targeting hepatocytes: in one stroke, this tissue is now accessible for treatment with ONs. These examples of how delivery technologies can be used to overcome delivery hurdles has provided the whole field with a new impetus that will accelerate discoveries for targeting of tissues beyond the liver.
Design and manufacturing of efficient delivery systems is not the only hurdle: the safety of these and their combination with ONs is also paramount. Testing ON safety has not been easy, primarily because many of these drugs have been developed to treat rare diseases. This implies an abundance of preclinical models but limited clinical data. Being a whole new class of drugs, this makes stakeholders wary of missing any step of the development. A striking exception to this is the recent n‐of‐one clinical studies: the development of milasen (Kim et al, 2019) was achieved in record time, but it took a high risk/high reward gamble, relying on the safety of IT administration of a chemistry already approved for nusinersen.
A likely leapfrog in the clinical application of therapeutic ONs may come from the results of current clinical trial (NCT04023552; testing APO(a)‐LRx, a GalNAc3‐conjugated ASO) for the lowering of lipoprotein (a) in cardiovascular disease. This trial includes 7,680 patients and the large data set that will be generated is due to change in the landscape for these drugs. By then, many new delivery technologies may have successfully been developed for other targets making this decade the era of ON therapeutics.
Pending issues
A vast array of delivery systems could be used to deliver ONs; however, the majority target the liver or deliver throughout the body without specificity. Further advances are needed to enhance tissue‐specific delivery.
Our understanding of ON‐mediated toxicities has improved, and many predictive in vitro tests have been developed to exclude toxic candidates early in development. Since some toxicities are sequence dependent, it will be important to implement toxicity screening early in the preclinical development of ONs.
Following the example of milasen, it is anticipated that bespoke ON therapies will be developed for additional brain diseases. A process to guide this development is required.
Very limited clinical data have been available for many therapeutic ONs, as most target rare disorders and have been tested in dozens or, at best, a few hundred patients. However, this may be about to change thanks to new ON drugs targeting common disorders, such as hyperlipidaemia, which could produce clinical data from thousands of patients and further accelerate development of future therapeutic ONs.
Conflict of interest
A.A‐R discloses being employed by LUMC which has patents on exon skipping technology. As co‐inventor of some of these patents, AAR is entitled to a share of royalties. AAR further discloses being ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, Eisa Pharmaceuticals, WaVe Life Sciences, Alpha Anomeric, CRISPR Therapeutics, BioMarin Pharmaceuticals Inc., Global Guidepoint and GLG consultancy, Grunenthal and BioClinica, being a member of the Duchenne Network Steering Committee (BioMarin) and of the scientific advisory boards of ProQR and Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics and BioMarin Pharmaceuticals. A.G and R.W.J.C are inventors of several patents describing the use of antisense oligonucleotides for the treatment of inherited retinal diseases. C.F is ad hoc consultant for Lundbeck Pharma A/S, Valby, DK. W.vR.M discloses being employed by LUMC which has patents on exon skipping technology for brain disorders. As co‐inventor of some of these patents WvRM is entitled to a share of royalties. T.L is a consultant for and has equity interests in Evox Therapeutics Ltd., Oxford, UK. L.E is an employee of SQY therapeutics developing tcDNA antisense oligonucleotides. M.A.D and G.C are co‐inventors of patent WO2016/151523 (RNA interference mediated therapy for neurodegenerative diseases) filed by the University of Trento and are entitled to a share of royalties. S.M.H is an inventor on a patent describing cell‐penetrating peptides and is employed by Oxford Biomedica Plc, Oxford UK. Co‐authors, R.A.M.B, G.G, S.A, L.R.D, S.K, V.A‐G, S.E.B and A.T.G declare no conflict of interests.
Acknowledgements
This work was supported by funding from Cooperation of Science and Technology (COST) Action CA17103 (networking grant to V.A‐G). V.A‐G holds a Miguel Servet Fellowship from the ISCIII [grant reference CPII17/00004] that is part‐funded by the European Regional Development Fund (ERDF/FEDER) and also acknowledges funding from Ikerbasque (Basque Foundation for Science). S.M.H is funded by the Medical Research Council and Muscular Dystrophy UK. A.A‐R receives funding from amongst others the Duchenne Parent Project, Spieren voor Spieren, the Prinses Beatrix Spierfonds, Duchenne UK and through Horizon2020 project BIND. A.G and R.W.J.C are supported by several foundations including the Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, Stichting Blinden‐Penning, Landelijke Stichting voor Blinden en Slechtzienden, Stichting Oogfonds Nederland, Stichting Macula Degeneratie Fonds, and Stichting Retina Nederland Fonds (who contributed through UitZicht 2015‐31 and 2018‐21), together with the Rotterdamse Stichting Blindenbelangen, Stichting Blindenhulp, Stichting tot Verbetering van het Lot der Blinden, Stichting voor Ooglijders, and Stichting Dowilvo; as well as the Foundation Fighting Blindness USA, grant no. PPA‐0517‐0717‐RAD. R.A.M.B is supported by Hersenstichting Nederland Grant DR‐2018‐00253. G.G. is supported by Ministry of Research and Innovation in Romania/National Program 31N/2016/PN 16.22.02.05. S.A is supported by Project PTDC/BBB‐BMD/6301/2014 (Fundação para a Ciência e a Tecnologia—MCTES, Portugal). L.R.D. is supported by Fundación Ramón Areces Grant XVII CN and Spanish Ministry of Science and Innovation (MICINN, grant PID2019‐105344RB‐I00). T.L is supported by Estonian Research Council grant PSG226. S.K is supported by the Friedrich‐Baur‐Stiftung. C.F is funded by The Danish Council for Independent Research, Technology and Production Sciences (grant number DFF‐4184‐00422). W.vRM is supported by ZonMw Programme Translational Research 2 [Project number 446002002], Campaign Team Huntington and AFM Telethon [Project number 20577]. S.E.B is supported by the H2020 projects B‐SMART, Grant number 721058, and REFINE, Grant number 761104. A.T.G is supported by the Institut National de la santé et la recherche médicale (INSERM) and the Association Monegasque contre les myopathies (AMM). L.E. is founded by the Association Monegasque contre les myopathies (AMM).
 Delivery of oligonucleotide‐based therapeutics: challenges and opportunities
Delivery of oligonucleotide‐based therapeutics: challenges and opportunities
