
Edited by Nils Chr. Stenseth, University of Oslo, Oslo, Norway, and approved February 24, 2021 (received for review October 8, 2020)
Author contributions: E.D.L., C.P.K., and G.B.W. designed research; E.D.L., C.P.K., and G.B.W. performed research; E.D.L. contributed new reagents/analytic tools; E.D.L. and C.P.K. analyzed data; and E.D.L., C.P.K., and G.B.W. wrote the paper.
- Altmetric
Although termite mounds stand out as an example of remarkably regular patterns emerging over long times from local interactions, ecological spatial patterns range from regular to random, and temporal patterns range from transient to stable. We propose a minimal quantitative framework to unify this variety by accounting for how quickly sessile organisms grow and die mediated by competition for fluctuating resources. Building on metabolic scaling theory for forests, we reproduce a wide range of spatial patterns and predict transient features such as population shock waves that align with observations. By connecting diverse ecological dynamics, our work will help apply lessons from model systems more broadly (e.g., by leveraging remote mapping to infer local ecological conditions).
Population-level scaling in ecological systems arises from individual growth and death with competitive constraints. We build on a minimal dynamical model of metabolic growth where the tension between individual growth and mortality determines population size distribution. We then separately include resource competition based on shared capture area. By varying rates of growth, death, and competitive attrition, we connect regular and random spatial patterns across sessile organisms from forests to ants, termites, and fairy circles. Then, we consider transient temporal dynamics in the context of asymmetric competition, such as canopy shading or large colony dominance, whose effects primarily weaken the smaller of two competitors. When such competition couples slow timescales of growth to fast competitive death, it generates population shocks and demographic oscillations similar to those observed in forest data. Our minimal quantitative theory unifies spatiotemporal patterns across sessile organisms through local competition mediated by the laws of metabolic growth, which in turn, are the result of long-term evolutionary dynamics.
Ecological niches display a wide variety of spatial and temporal patterns ranging from random to regular and from transient to long lived. In Fig. 1, we show a small sample from such diversity, including fairy circles in semiarid environments (1), regular and random tiling of termite mounds (2, 3), and more randomly spaced ant nests and trees (4, 5). This variation is not limited to between taxa but also varies between different plots in the same region. These systems also operate on different timescales, where fairy circles have estimated lifetimes of around half of a century compared with days or weeks for nascent ant nests and centuries for trees in unperturbed forests. In the extreme, transient growth is maximized for agricultural crops, which are then razed at maturity before demographic stability (6, 7). Overall, fast and slow dynamics of sessile organisms are characterized by a range of spatial distributions, from the random to the regular, that reflect underlying forces of growth, death, and competition.

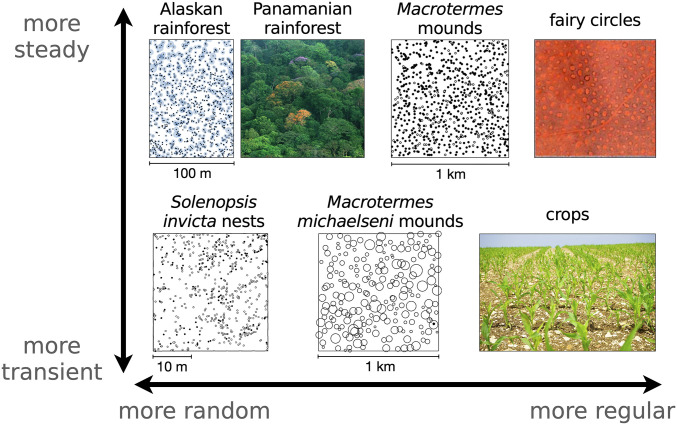
Regular to random spatial distributions and transient to slow temporal evolution in sessile organisms. (Upper) Trees in Alaskan rainforest (circles indicate basal stem diameter of cm increased by a factor of five) (5), view of the Panamian rainforest canopy, semiregularly packed termite mounds reprinted from ref. 3 (empty circles are inactive mounds), and hexagonally packed fairy circles reprinted from ref. 1. (Lower) Newly built ant nests (4), termite mounds with size shown by circles (2), and perennial agricultural crops. Dynamics range from transience dominated, in the case of crops razed at the end of the season or newly built ant nests that die within days as indicated by open circles, to long-lasting structures such as fairy circles, which can live individually for decades or forests at demographic equilibrium lasting millennia. Scale is unavailable for fairy circles, but they range from 2 to 12 m in diameter, meaning that the shown plot covers some hundreds of meters on a side (1). Panamanian rainforest image credit: Christian Ziegler (photographer). Termite mounds image reprinted from ref. 3, which is licensed under CC BY 4.0. Fairy circles image reprinted from ref. 1, which is licensed under CC BY 4.0. Solenopsis invicta nests image reprinted by permission of ref. 4: Springer Nature, Oecologia, copyright 1995. M. michaelseni mounds image reprinted by permission of ref. 2: Springer Nature, Insectes Sociaux, copyright 2010. Crops image credit: Pxhere.
The mechanisms underlying such pattern formation have been a source of robust debate, especially in the context of vegetation (8, 9). Following Turing’s seminal work on scale-dependent feedback, namely local activation and long-range inhibition, similar principles of pattern formation with local density dependence have been considered (101112–13), touching on the more general question of how multiple scales of time and space emerge (1415–16). More recent work has connected these principles with mechanisms of biological interaction and environmental feedback (1718–19). For spatial patterning, approaches to mechanism range from using perturbations like cascades of tree death to explore self-organized criticality in forests (2021–22) to applying Turing-like activation-inhibition concepts to scale-dependent plant processes (15, 16), which could be modulated by environmental conditions (23), to considering how ecosystem engineers modify the local environment to generate bare and densely vegetated patches (18, 24). Demographic theories, in contrast, focus on variables that aggregate across species and space such as age and size (2526–27) and build on allometric dependence of growth, mortality, and resource acquisition (2829303132333435–36). In an alternative set of approaches, mechanism-free maximum entropy principles can capture demographic patterns by fixing a few population “state variables” to predict measured properties (37). Across these examples, forests are particularly well-studied empirically across diverse species, sizes, and environments (38, 39) and grounded on predicted theoretical regularities in space and demography such as in the context of metabolic scaling (404142–43) and mechanical or hydraulic limits (444546–47).
Here, we build on previous work on forest growth and structure to consider sessile organisms more broadly in the context of both spatial structure and demographic dynamics. We propose a minimal dynamical model that integrates timescales of individual growth and mortality with competitive attrition on a background of fluctuating resources. With the model, we study the emergence and erasure of spatiotemporal order in ecological systems. We show how competition alone is insufficient to generate strong spatial regularity and that growth and death must flatline for the largest organisms to stabilize spatial order. Since most ecological systems are out of equilibrium, we extend our model to consider transient phenomena and predict population shock waves from competitive interactions when there is metabolic growth. This minimal framework serves to unify at a conceptual level the role of various timescales for pattern formation in distinct ecological settings.
As the starting point, we consider how metabolism determines individual growth and death. Metabolic scaling theory describes the origins of scaling laws in organism growth across a large range of body sizes derived from energetic constraints (40, 48, 49). Given constraints on average resource consumption per unit area, individual growth follows power law, allometric scaling relations connecting accumulation of biomass
One surprising prediction of metabolic scaling theory is that it is not necessary to explicitly include local competitive interactions to explain steady-state population distributions (33, 34), even if local competition is one of the major factors that drives long-term evolutionary dynamics to optimize fundamental energetic constraints (57). However, competition coupled with other timescales can introduce complex dynamics (58, 59) such as in response to exogenous perturbations (60, 61), which goes beyond steady-state assumptions. Other than mechanistic additions to metabolic scaling theory (44), competition, perturbation, and other dynamics present potential explanations for significant and sometimes substantial deviations from predictions (39, 42). Here, we present a minimal model to account for these missing factors.
We start with allometric scaling theory of forest growth in section 1 and connect deviations from metabolic scaling theory to organism density, resource variability, and competitive interactions in section 2. We explore the implications of competition through space in section 3 and time in section 4, concluding with section 5. Although we explicitly develop our framework using the language of forests, referring, for instance, to individuals as trees and dimensions as stem radii, our formulation is straightforwardly generalizable to other sessile organisms (62). As an example, we extend our model beyond allometric assumptions to consider the emergence of spatial order (SI Appendix, section D).
1. A Size-Class Model for Population Growth
The fractal structure of a forest exists both at the level of the physical branching of individual trees and at the level of self-similar packing of differently sized individuals. This fractal structure reflects energetic constraints that have shaped the long-term evolutionary dynamics of forest life (33, 50, 63). Connecting energy expenditure with the physical limits of how vasculature distributes energy leads to an allometric scaling theory of growth. For the rate of basal stem radius growth,
Building on the metabolic picture of growing individuals, we consider size classes labeled by radial dimension
Although population is typically binned into discrete size classes in both observation and theory, tree growth is in reality a function of continuous radius
Taking predictions from allometric scaling theory that relate mass growth function
From this minimal model of tree growth under the scaling assumptions of individual tree allometry, we obtain a wide range of possible steady states encompassing both predictions consistent with metabolic scaling theory as well as virtually any other population number scaling. This reflects the fact that space filling in forests, when
2. Competition for Fluctuating Resources
Resource collection in sessile organisms is fundamentally connected to local area (62). Examples include 1) local foraging by ants and termites (18); 2) diffusive depletion zones that regulate biofilm and microbial mat growth and spatial patterning (6566–67); and 3) nutrients, water, and sunlight collection in trees through overlapping root or canopy volumes (49, 68), where competition is largely determined by area overlap between neighbors (19). Overlapping canopies in particular reduce light available to shorter trees but not to taller ones (36), an example of asymmetric competitive interaction that we discuss later. As a general formulation of the consequences of symmetric competitive interactions (4), we consider how resource availability is modulated by overlap and environmental fluctuations relative to basal metabolic need.
All organisms have some basal resource budget
Putting these together, incoming resource rate depends on the amount of sharing that tree i with resource area
Averaging over many spatial arrangements over a long period of time, we consider the mean-field effect from such competition (SI Appendix, section C has details):
Combining metabolic growth and mortality from Eqs. 4 and 5 and competition from Eq. 10, we obtain the generalized size-class model
We show numerical simulations of an explicit two-dimensional (2D) simulation of trees in a large plot in Fig. 2A in comparison with the mean-field approximation from Eq. 12. The mean-field approximation does not exactly capture the tail of the distribution, but it does surprisingly well. Furthermore, it captures the qualitative intuition that for large trees,

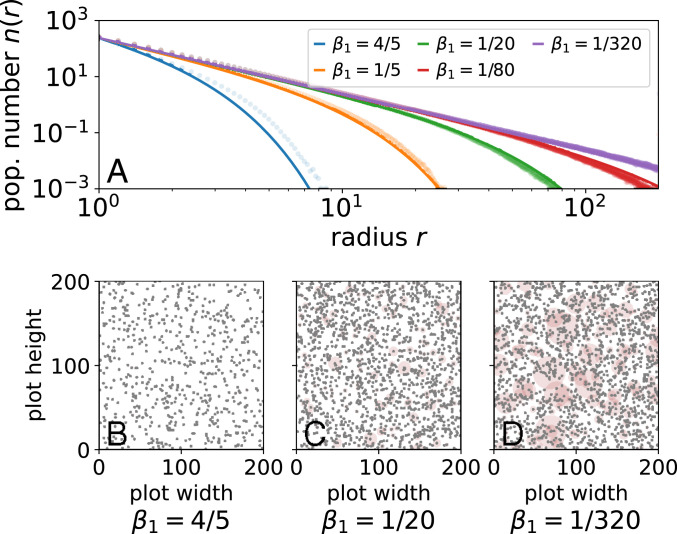
(A) Population number
Importantly, the mean-field argument clearly links resource area with resource fluctuations. This means that deviations from metabolic scaling theory may result from different combinations of resource-area growth and fluctuations in a way that make effects hard to disentangle (31). Beyond the particular form of competitive interactions we consider, this framework is naturally extensible by, for example, modifying resource-sharing fraction
3. From Random to Regular Spatial Patterns
Different organisms, and even the same organism in another environment, may show systematic variation in spacing (70). Such variation reflects individual growth dynamics and the strength of resource area-based competition due to the local properties of competitor species or the environment (7, 12, 71). Returning to Fig. 1, we again point out randomness in spatial surveys of an Alaskan rainforest along with Macrotermes michaelseni mounds and ant nests. In contrast, the spacing between Macrotermes falciger mounds is more regular. Other than intertaxonomic variation, there is also evidence of random and systematic variation between different plots in nearby regions.§ Thus, natural spatial distributions of sessile organisms may be attributable to the assorted effects of individual allometries and local competition in our model.
We survey such variety in Fig. 3 along a schematic region of spatial patterns generated by our model. We vary the rates of growth, death, and competition in Eq. 11. The planes jutting out from the back corner in Fig. 3A all correspond to theories where one of the terms is negligible. When competitive attrition is negligible, or

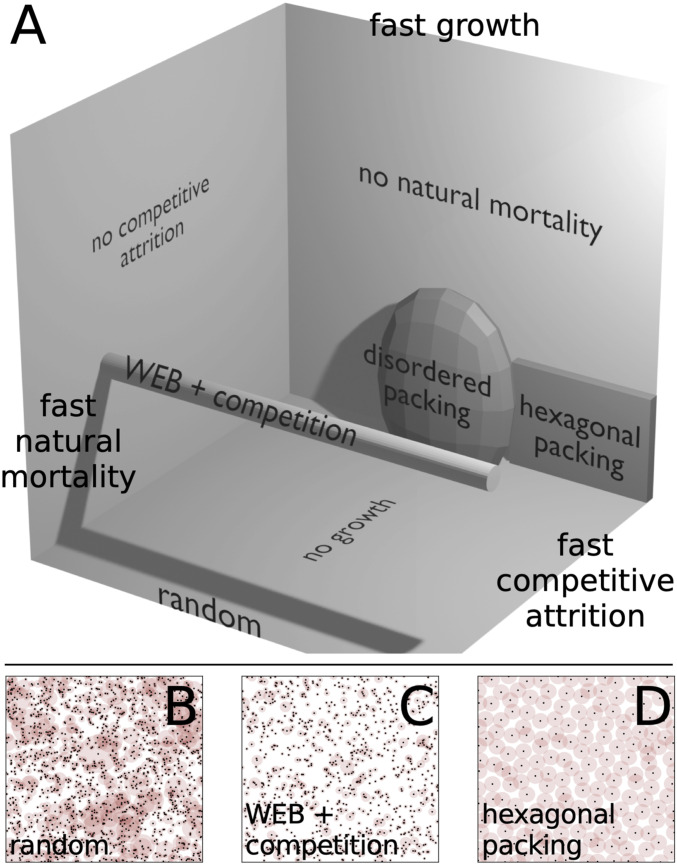
(A) Schematic 3D realm of models defined on rates of growth, death, and competitive attrition (shadows are generated by a single-point light source at the upper right). WEB theory of allometric forest growth corresponds to fixing population number exponent
In the limit of weak interaction, the spatial distribution of individuals is random. Then, the probability of not encountering any neighbors within a distance
Moving across the gray cylinder extending out from WEB theory in Fig. 3A corresponds to strengthening competitive interactions by increasing competitive attrition rate

![Characterizing trajectories through realm of models. (A) Location of cutoff to population number n(r) decreases as competitive costs increase, rcutoff≡[F/(κ+2/3)]−1/(κ+2/3), tracing the gray cylinder in Fig. 3. (B and C) Deviations from randomness indicate emergence of order measured by Kullback-Leibler (KL) divergence of the nearest-neighbor distance distribution for simulation p(rmin) from random points q(rmin). B shows randomness-dominated phase when growth rate is significant, 3cm2/3ā/8=0.3, and mortality rate Ā is small, while competitive attrition rate s varies. C shows signs of ordering when mortality rate is negligible Ā=0, while growth rate ā is driven to zero. Around s=1, we find a “liquid” phase, where organisms are densely packed but without long-range order. Dashed black lines indicate KL divergence measured at the “solid” phase. Bin size is set to Δr=1/20. (D) Normalized neighbor density at distance r, <f(r)/r>, indicates solid, hexagonally packed phase. Function f(r) counts all neighbors at distance r with f(0)=1 and is plotted against distance normalized by average spacing 1/πσ given density σ. For comparison, we show the neighbor density for Namibian termite mounds from ref. 18, which are more tightly packed than in our simulation. Small s corresponds to the weak attrition limit, where the spatial distribution is nearly random.](/dataresources/secured/content-1766061166401-fcc66331-74f0-4514-b43d-73424990aff7/assets/pnas.2020424118fig04.jpg)
Characterizing trajectories through realm of models. (A) Location of cutoff to population number
Such an example manifests in the semiregular packing of the fairy circles shown in Fig. 1. Such spacing entails a relatively narrow and peaked distribution of mound areas at some maximum size, a phenomenon incompatible with scale-free growth. Instead, this distribution implies that mounds that approach the maximum size are stable and that strong competitive interactions inhibit the formation of new smaller mounds. We can approximate such dynamics by driving growth and natural mortality to zero and vastly enhancing competitive mortality. This ensures that mounds are fixed at a typical size, with rigid boundaries delineated by strong competitive interactions and close to hexagonal spacing that minimizes survival of randomly placed colony seeds.¶ As we draw in black dashed lines across Fig. 4 B and C, the simultaneous limits of slow growth (
4. Transient Dynamics and Population Shock Waves
Demographic stability among a population of sessile organisms is not guaranteed since ecosystems are buffeted by a wide range of endogenous and exogenous perturbations (20, 60, 74). For example, local competition is negligible in a young plot until individuals reach a size where they impinge upon neighbors, a phenomenon known as self-thinning (75). This dynamic is especially prominent in agriculture, where spacing is regular, plants are genetically identical, and competition onset is almost uniform (6, 7). Natural stands also vary with plot age, but they are more stochastic, and height differences can be prominent (34, 39). Remarkably in previous measurements, we find oscillations in population number with radius as depicted in Fig. 5 A, Inset taken from ref. 39, suggesting the presence of long-lived transience not captured by the steady state. Inspired by this observation, we consider how competitive asymmetry, specifically forces that increase mortality of smaller organisms, could excite population waves.

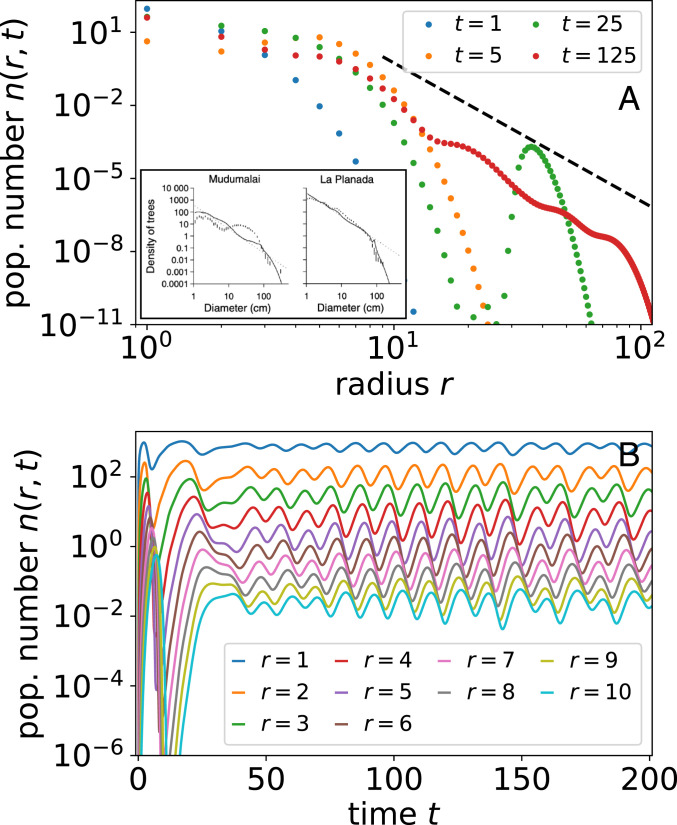
Oscillations in population number
Asymmetric competition takes various forms such as how canopy shading reduces light incident on shorter plants lying underneath or around the larger ones with little cost to the latter (35, 43, 76). Large termite colonies are much more likely to destroy incipient colonies adjacent to their borders than face a threat (8). Susceptibility to exogenous disturbances like wind also depends on size, although sometimes to the benefit of smaller individuals (77, 78). As with symmetric competition, we formulate asymmetric competition in terms of its effects on population growth
As we show in Fig. 5B, canopy competition is negligible during initial forest growth but matters strongly when tall trees reach some critical density at which point the difference between the height of the tallest trees and the shortest ones is
Remarkably, we find oscillations in population number curves across data on tropical forests. Fig. 5 A, Inset displays two examples from ref. 32. Similar oscillations are visible across other tropical forests. For the example from La Planada, the widths of undulations seem consistent with an example from our simulation in Fig. 5A, although this is not the case for Mudumalai, which shows intriguingly wide oscillations. An unanswered question is whether such oscillations are internally generated from tuning of competitive parameters or originate from widespread and repeated exogenous perturbations (74). After all, competitive dynamics between organisms at different points of maturity can generate oscillatory cycles perhaps influenced by or affecting other classic ecological dynamics (25, 58). Although qualitatively similar curves in demographic data are presented as evidence against metabolic scaling—indeed, space filling yields poor explanation—our model shows that such deviations may arise due to dynamics overlaying metabolic scaling.
When considering asymmetric competition with resource fluctuations, we find important differences from symmetric competition. Resource constraints impose a limiting cutoff in maximum size and dampen population waves. As in the case of symmetric competition, accounting for resource constraints introduces an exponentially decaying tail that dominates near the point where resource limitations delimit the largest sustainable size. When resource limitations are sufficiently weak that there exists a wide scaling region in the population number that goes as
5. Discussion
The physical structure of a tree is a beautiful fractal not only along its visible constituents, trunk to branches to twigs, but down to the microscopic vasculature that shuttles products of photosynthesis from its self-similar canopy to a branching network of roots. It is remarkable then that even groups of trees seem to obey this pervasive fractal law such that the trees of a particular size “branch off” into trees of a smaller size and so on in such a way that we can consider, over some range, the set of large trees as a scaled set of smaller trees (34). This self-similar structure, reflected in power law scaling, emerges from consideration of energetic constraints translated into the requirement that trees fill the available canopy space (33). Yet, other sessile organisms fill space in a variety of ways determined by analogous mechanisms of growth, death, and competition (8, 62). Inspired by the forest picture, we propose a minimal model of sessile organism growth incorporating aspects of allometric scaling theory and area-based competition. From these basic principles, we obtain a general framework for competitive forces driven by metabolic requirements and fluctuating resources. When interaction with the environment dampens resource fluctuations (e.g., niche construction) or changes competitive interactions (e.g., symbiosis), these perturbations will be reflected in the spatial distribution of organisms (8) (Fig. 1). In this sense, the spatial distribution may serve as an indicator not only of changing conditions but also of how competition evolves in altered environments (18, 60).
We explore such variation by tuning competitive forces in our model with a tractable mean-field theory that succinctly relates metabolic and competitive effects in exponent relations. In the context of resource-area competition, competitive effects are most prominent in the population statistics of the largest organisms. This is because area-delimited competition must scale superlinearly with radius such that it dominates for the largest organisms (Eq. 11). In comparison, individual metabolic growth and mortality scale sublinearly (41), indicating two different regimes of population number for symmetric competition: individual-dominated scaling and competition-mediated cutoffs (Fig. 2). Asymmetric competition, however, exacts a toll in a scale-free way because relatively larger competitors grow, die, and compete the same at every level. Then, competition is manifest in the population number exponent (Eq. 4), affecting both scaling and cutoffs. Our formulation of competitive interactions establishes a basis to be extended to capture environment- or organism-specific variation in resource stress response or sharing such as by incorporating a distribution of diverse allometries. Yet, it also highlights how such diversity maps to universal features summarized by exponents that quantitatively link environmental fluctuations and metabolic scaling (Eq. 12). Although real systems are noisy, finite, and constantly perturbed, our idealized assumptions about allometries serve as a way to organize models incorporating many more realistic features, a mapping that can be made exact by taking the limit to large systems (80, 81).
Other than indicating limitations of metabolic scaling theory—namely that it may be more accurate in forests with weaker local competition and smaller environmental fluctuations—our work suggests limitations of spatial correlation-based measures of regularity when varying organism size introduces disordered spacing (2). As we show by comparing the form of the nearest-neighbor distance distribution with the KL divergence (Eq. 13), this measure changes weakly with competitive strength, suggesting that statistical approaches to measuring competition are limited. Instead, an integrated framework considering deviations from predicted scaling in demographics as well as spatial patterning may better specify the range of competitive forces acting across environments (4, 18, 60, 82, 83).
Beyond competitive forces, we find strong additional constraints necessary to stabilize spatial order in models with metabolic growth (Fig. 3). Whereas metabolic scaling tends to inject spatial disorder by constantly changing organism size and by opening free space upon organism death, regular tiling such as seen for fairy circles and some termite mounds requires the elimination of unbridled growth along with slow natural mortality and overwhelming competitive attrition in a background of sparse newcomers. This emergence of order starting with individuals and their metabolic dynamics presents a complementary perspective to field-theoretic derivations of vegetation patterns that start instead with densities (17, 8485–86). In a similar vein, some field-theoretic models, such as those introduced by Martínez-García et al. (87, 88), have incorporated competition with local (logistic) limits on growth. That hexagonal packing occurs only in a corner of the much broader model space encompassed by our theory (Fig. 3) reflects the extraordinary nature of such regular patterns.
Complementary to the connection between spatial patterns and asymmetric competition (79, 89), we explore transient dynamics in the context of a size-based competitive hierarchy (58). Asymmetric competition can couple different timescales to one another and lead to oscillatory modes in size classes and thus, population number (Fig. 5)—although touching on the topic of self-thinning, our model extends beyond the typical focus on monoculture stands (6, 7, 71, 75). When there is a threshold at which such effects become important, such as with canopy light competition, we expect to find similar population shock waves. Remarkably, oscillatory modes manifest in multiple datasets of tropical forest demography (39). Such die-offs also may be observable in other systems or directly measurable if future data collection permits highly resolved temporal data on organism death. Furthermore, the lifetimes of these transient phenomena, indicated by width evolution, may allow us to distinguish internal competitive forces by leveraging demographic perturbation (20, 90). Oscillatory modes and instabilities are a widely studied feature of biological populations [for example, with the classic logistic equation (91, 92)], and metabolic growth in sessile organisms presents an unexplored mechanism by which they can arise.
Growth, death, and competition are essential characteristics of life. Yet, for particular ecosystems, assumed allometric forms for these forces will need to be refined. More specifically, we assume that demographic diversity is summarized in terms of individual age, which then determines individual properties like size and growth rate. Notably, the relations we derive do not have to be fixed by principles like space filling as is assumed for metabolic scaling of forests (33), and so, they relay a universe of possible allometries and environments. Nevertheless, power law scaling relations are an approximation of a population, and individuals will deviate from them (42). Accounting for such variation may be important in high-resolution models (93, 94). More fundamentally, organism growth or death may not neatly abide by allometric scaling relations with respect to age. We show one extreme in the example of hexagonal packing, where growth is truncated. Although technically, this is still a form of allometric scaling (if a trivial one), the analogy is only mathematically exact in the limit where individuals live for a long time, and thus, the growth period is short. Individual organisms that do not obey such behavior, perhaps displaying persistent oscillations in size, would present a new set of dynamics extending beyond the canonical picture of metabolic growth. We emphasize that such extensions would be natural and interesting to incorporate into the framework we present here.
The most striking ecological patterns occur when local interactions generate large-scale regularities, propagating information coherently over large scales and long times (95, 96). Fairy circles and termite mounds are a breathtaking example. Although forests, fairy circles, and termite mounds all seem to obey forces driving the cycle of birth, growth, and death at the level of the individual, population-level structure varies widely. Even among forests, some are characterized by randomly spaced trees, such as the examples we show here, but others, such as the pinyon–juniper ecosystem of the US Southwest, are more spatially regular. To connect the wide range of spatial patterns shaped by competitive forces in sessile organisms, we build on theoretical foundations of metabolic scaling. The resulting realm of models may frame analogies between organisms across species, environments, and times in the language of competitive forces acting on top of individual properties constrained by metabolic principles.
Previously published data were used for this work from Tschinkel (1), Grohmann et al. (2), Muvengwi et al. (3), Adams and Tschinkel (4), and Schneider et al. (5).
Acknowledgements
E.D.L. and G.B.W. were supported by NSF Grant PHY1838420. C.P.K. and G.B.W. thank Toby Shannan and Charities Aid Foundation of Canada (CAF) for supporting this work. We acknowledge useful discussions with Sungho Choi about forest data and thank Deborah Gordon for bringing up self-thinning.
Data Availability
Code for reproducing the results shown in this work is available at https://github.com/eltrompetero/simple_sessile.
References
1
2
3
4
5
6
7
8
9
10
12
13
14
15
16
17
18
19
20
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
43
44
45
46
47
48
49
50
51
52
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
 Growth, death, and resource competition in sessile organisms
Growth, death, and resource competition in sessile organisms
