
Edited by Tallie Z. Baram, University of California, Irvine, CA, and accepted by Editorial Board Member Renée Baillargeon February 22, 2021 (received for review June 23, 2020)
Author contributions: R.F. conceived and designed the longitudinal study and conducted the twenty-year follow-up; A.U.Y. and S.W. performed experiments; A.D. performed regressions and statistical analysis; O.S.-R. contributed to fMRI paradigm validation; A.U.Y. analzyed data; R.S. supervised the analysis; A.U.Y., R.S.,and R.F. wrote the paper; and R.F. and R.S. edited versions of the paper.
1A.U.Y. and R.S. contributed equally to this work.
A birth-to-adulthood study tested the effects of maternal–newborn contact and synchronous caregiving on the social processing brain in human adults. For two decades, we followed preterm and full-term neonates, who received or lacked initial maternal bodily contact, repeatedly observing mother–child social synchrony. We measured the brain basis of affect-specific empathy in young adulthood to pinpoint regions sensitive to others’ distinct emotions. Maternal–newborn contact enhanced social synchrony across development, which, in turn, predicted amygdalar and insular sensitivity to emotion-specific empathy. Findings demonstrate the long-term effects of maternal caregiving in humans, similar to their role in other mammals, particularly in tuning core regions implicated in salience detection, simulation, and interoception that sustain empathy and human attachment.
Mammalian young are born with immature brain and rely on the mother’s body and caregiving behavior for maturation of neurobiological systems that sustain adult sociality. While research in animal models indicated the long-term effects of maternal contact and caregiving on the adult brain, little is known about the effects of maternal–newborn contact and parenting behavior on social brain functioning in human adults. We followed human neonates, including premature infants who initially lacked or received maternal–newborn skin-to-skin contact and full-term controls, from birth to adulthood, repeatedly observing mother–child social synchrony at key developmental nodes. We tested the brain basis of affect-specific empathy in young adulthood and utilized multivariate techniques to distinguish brain regions sensitive to others’ distinct emotions from those globally activated by the empathy task. The amygdala, insula, temporal pole (TP), and ventromedial prefrontal cortex (VMPFC) showed high sensitivity to others’ distinct emotions. Provision of maternal–newborn contact enhanced social synchrony across development from infancy and up until adulthood. The experience of synchrony, in turn, predicted the brain’s sensitivity to emotion-specific empathy in the amygdala and insula, core structures of the social brain. Social synchrony linked with greater empathic understanding in adolescence, which was longitudinally associated with higher neural sensitivity to emotion-specific empathy in TP and VMPFC. Findings demonstrate the centrality of synchronous caregiving, by which infants practice the detection and sharing of others’ affective states, for tuning the human social brain, particularly in regions implicated in salience detection, interoception, and mentalization that underpin affect sharing and human attachment.
Being born a mammal implies that the brain is immature at birth and develops in the context of the mother’s body, lactation, and caregiving behavior (1). Infants rely on the provisions embedded in the mother’s body, sensory stimuli (2), and the expression of well-adapted caregiving for maturation of neurobiological systems that sustain participation in the social world. Extant research in animal models has shown that breeches in the mother’s continuous presence and variability in the consistency of caregiving carry long-term effects on brain structure and function, particularly on systems that underpin sociality, and these effects are maintained throughout life, altering the adult animal’s capacity to coordinate social bonds, manage hardships, and parent the next generation (3, 4). However, while the human brain is slowest to mature and requires the most extended period of dependence (5), the long-term consequences of caregiving for the human social brain are largely unknown. The current birth-to-adulthood study examines the effects of maternal–newborn skin-to-skin contact (Kangaroo Care, KC) and parent–child social synchrony experienced across development on the brain’s empathic response to others’ emotional states in young adulthood. Social synchrony describes the coordination between the parent’s and child’s nonverbal behavior and communicative signals during social interactions in ways that enhance positivity, reciprocity, and mutual engagement (6, 7), and we tested its longitudinal impact on the brain basis of empathy, a core feature of the social brain.
The human social brain integrates activity of subcortical, paralimbic, and cortical structures to sustain human social life, which requires rapid processing of social inputs, top–down regulation of intention and affect, and coordination of the two into the present moment (8). The social brain has undergone massive expansion across primate evolution to support humans’ exquisite social skills, communicative competencies, and mindreading capacities. It has been suggested that Homo sapiens’ success over other hominin owes to their unique empathic abilities, which allow humans to quickly identify and mentally share others’ affective states (9). Such multifaceted empathy, which integrates automatic identification of others’ distinct emotions with the ability to use interoceptive signals to detect others’ specific affect and the capacity to reflect on the changing emotional states of social partners, marks a fundamental achievement of the human social brain. The empathic social brain, in turn, enabled humans to coordinate actions for survival, fine-tune communicative signal systems, and partake in the joys and sorrows of others (10). Yet, while empathy is a core feature of human sociality that is tuned in mammals by patterns of parental care, the relational precursors of the neural empathic response have not been fully explored in human studies.
Social synchrony is first observed in the third month of life when parents begin to coordinate with the infant’s nonverbal signals and interactive rhythms. Synchrony continues to mature across childhood and adolescence with the parent’s and child’s increasing reciprocity and adaptation to each other’s verbal and nonverbal communications, affective state, and pace of dialogue and is considered a prototypical experience that prepares children to life with others (11, 12). Through ongoing adaptation first to the infant’s nonverbal cues and then to the older child’s verbal and affective communications, parents orient children to social moments, practice rapid assessment of distinct emotional states, and, over time, enable children to simulate others’ mental states, fine-tuning the social brain and its capacity for empathy (13). Social synchrony undergoes maturation across development and evolves from nonverbal matching to a verbal dialogue that acknowledges others’ emotions, engages multiple perspectives, and reflects on feelings while retaining the interactive rhythms of the familiar dialogue from infancy to adulthood (1). The early experience of synchrony plays a key role in children’s social–emotional development and has been shown to predict the child’s later ability to engage with peers (14, 15), regulate emotions (4), exhibit cognitive control (16), manage stress (17), and display empathic understanding (18), indicating that improvements in mother–infant synchrony during its early stages may have long-term effects on the capacity for empathy and its neural underpinnings.
The development of synchrony is highly sensitive to initial conditions. Conditions that compromise maternal–infant bonding bear long-term negative consequences for the development of social synchrony and, consequently, for maturation of human social abilities (19, 20). When infants are born prematurely and full maternal–infant bodily contact is initially lacking, the development of synchrony is halted and socioemotional competencies compromised. Notably, when we provided structured maternal–infant skin-to-skin contact (KC) to premature neonates during the postpartum period, the intervention improved not only social synchrony but also the functioning of regulatory support systems, such as circadian rhythmicity, autonomic maturity, stress responsivity, and exploratory behavior, the same systems that are shaped in young mammals by contact with the mother’s body (17, 18) and consistent presence (21).
What may be the effects of maternal–newborn skin-to-skin contact and synchronous caregiving across development on the social brain in young adulthood? Utilizing our unique cohort, we imaged the neural empathic response in three groups of healthy young adults who were recruited at birth: infants born at full-term (FT), preterm infants receiving kangaroo contact (KC), and demographically and medically matched preterm infants receiving standard incubator care (SC) who were followed in our laboratory for two decades (Fig. 1A). We focused on the neural basis of empathy, particularly on the brain’s capacity to detect, affectively share, reflect, and empathize with the different emotions of others (22). Two key hypotheses were tested. First, we expected that the provision of maternal bodily contact in the neonatal period would enhance the expression of social synchrony in infancy and across development. This hypothesis is based on research in animal models which shows that maternal bodily contact, consistent presence, and sensory stimuli improve maternal caregiving and have long-term effects on brain and behavior (21, 2324–25). Second, we hypothesized that the experience of synchrony would augment the brain’s capacity to differentiate among others’ emotional states. Synchrony is a dyadic experience by which infants practice the identification and sharing of others’ emotions and, as they develop, learn to imbue others’ feelings with meaning and representations (26). We expected that such practice would tune the brain of young adults to empathize with others’ distinct emotions, particularly in areas that have been linked with parent–child synchrony in the parental brain, the amygdala (27, 28) and insula (29).

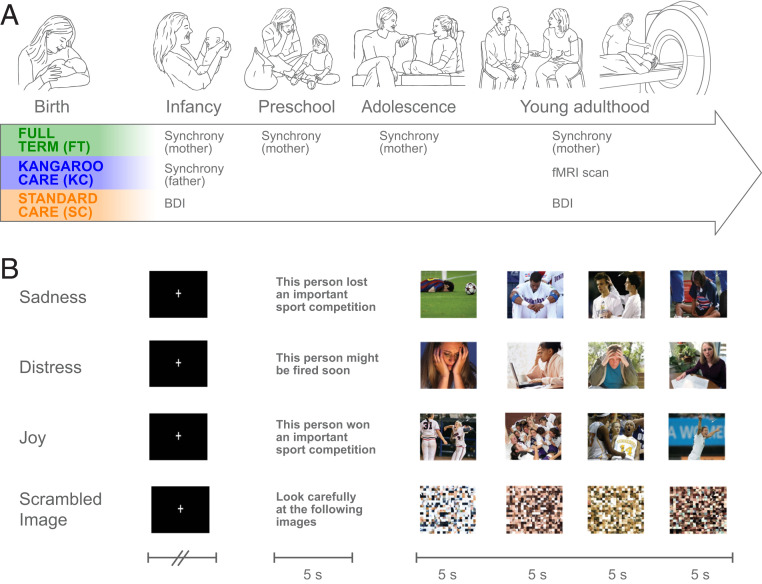
Birth-to-adulthood longitudinal study design and fMRI paradigm. (A) Three cohorts of infants and parents recruited at birth: full-term (FT) infants and two case-matched neurologically intact premature infants assigned to either Kangaroo Care (KC: infants receiving skin-to-skin contact with mother) or matched controls receiving standard incubator care (SC). Mother–child social synchrony was assessed at 4 mo (SD =1.14), 3 y (SD = 1.38), 12 y (SD = 1.62), and 20 y (SD = 2.01). (B) fMRI empathy paradigm. Example illustrates a pseudorandomized design in which participants were presented with an emotional probe followed by four photos depicting this probe. Participants were asked to empathize with the protagonists, and five blocks per condition were presented.
Using a longitudinal sample of n = 96 young adults who were followed from infancy, we first examined the neural basis of affect-specific empathy. We employed a validated functional MRI (fMRI) paradigm that exposed participants to others’ distinct emotions (joy, sadness, and distress) and asked them to mentally empathize with the protagonists (30) (Fig. 1B). Consistent with prior imaging studies on the brain regions activated during empathy tasks (3132–33), we focused on a network of regions sustaining empathy. This included limbic regions: the amygdala, a key player in emotion detection (34), and the parahippocampal gyrus. Also included were the anterior insula, superior temporal sulcus (STS), and temporal pole (TP) that have been repeatedly implicated in human empathy research (32, 35). We also examined the ventromedial prefrontal cortex (VMPFC), precuneus, and inferior parietal gyrus, known as hubs of the default mode network, which is related to self-referential processing, perspective-taking, and theory of mind tasks (36, 37) and plays a key role in social understanding (38).
We used Representational Similarity Analysis (RSA), a multivariate brain pattern analytic technique, to differentiate brain areas that show a distinct neural pattern while empathizing with specific affective states from those generally activated by the empathy task but without a unique response to each emotion. By using RSA, we aimed to compare the distinct neural patterns activated during empathy to different emotions and characterize the brain basis of affect-specific empathy (39). A recent study employing RSA to pinpoint the neural signature of basic emotions indicated that the amygdala, insula, medial prefrontal cortex, frontal pole, and precuneus showed distinct representations for different emotional states (40). Consequently, and in light of research highlighting the role of the amygdala in fear (41) and empathy for negative affective states (42), we focused on the amygdala as a key area that may present differential response during empathy to positive versus negative emotions. Similarly, the insula exhibits similar activations during empathy for physical pain and emotional distress (43, 44), and we expected the insula to show differential activations during empathy to distressing versus nondistressing affective states. Areas including the dorsomedial prefrontal cortex (DMPFC), VMPFC, insula, TP, and precuneus have been shown in research using multivoxel pattern analysis to display specific activation patterns to emotion-related actions and mentalization (36), and we expected these areas to exhibit specific activation patterns during empathy with others’ distinct emotions.
Utilizing a birth cohort of infants born between 1996 and 1999, we recruited mother–infant dyads in three groups: 1) infants born at FT, 2) healthy premature infants who received maternal–infant skin-to-skin contact in the neonatal period (KC), and 3) case-matched premature infants who received SC. Mother–child naturalistic interactions were videotaped in the home ecology at four time points: infancy, preschool, adolescence, and adulthood (Fig. 1), and mother–child synchrony was coded by blind raters, taking into consideration the age-specific expression of synchrony as described in previous research (see Materials and Methods).
To test our first hypothesis, we examined the maturation of synchrony from infancy to adulthood in the three groups (FT, KC, and SC). A repeated-measures ANOVA with group as the between-subject factor revealed that synchrony increased considerably from infancy to adulthood across all participants (Fig. 2) (F(3,225) = 159.64, P = 1.86e−55, = 0.68), suggesting that while infants can only minimally share in a reciprocal, mutually adaptive interaction, adult children and their mothers can sustain a mutual exchange that is fluent, coregulated, and reciprocally attends to the social communications of both partners.

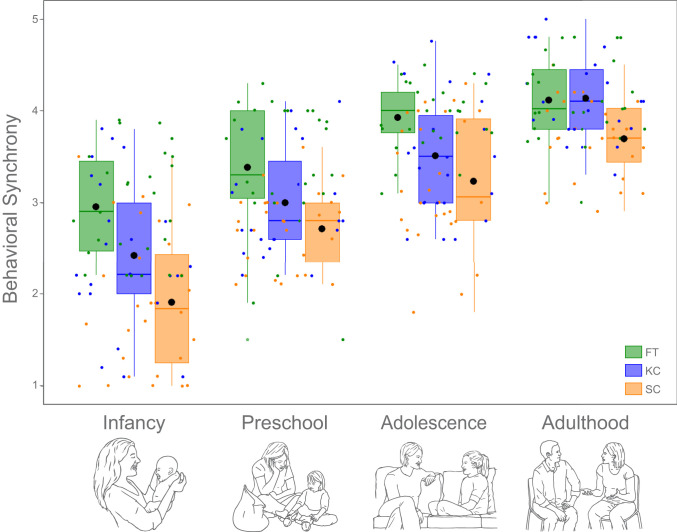
Mother–child synchrony from infancy to adulthood. Individual scores are marked by dots of respective color. Group means are marked by a black circle.
In addition, a significant group effect was found for synchrony (F(2,75) = 18.81, P = 2.38e−7,
Next, we turned to assessing the brain areas implicated in empathy to specific emotions by employing a validated paradigm (30), which exposed participants to protagonists experiencing joy, distress, or sadness in social contexts. Participants were instructed to “put themselves in the shoes” of the protagonists, to imagine how they would feel in similar conditions, and to intentionally empathize with the protagonist’s feelings, that is, to assume the vicarious position (Fig. 1B and SI Appendix, Supplementary Materials).
Independent definition of regions of interest (ROIs) was ensured by dividing the dataset into two separate experiments. In Experiment 1, data from 18 subjects (six from each group: FT, KC, and SC) were randomly selected for use as a functional localizer. Univariate whole-brain mapping of emotional empathy conditions (all emotions > scrambled image) revealed activated regions from the parietal–occipito border of the temporal lobe, across the STS to TP, as well as prefrontal and limbic regions (amygdala, parahippocampal gyrus) and visual precuneus (Fig. 3A and SI Appendix, Table S1 for full details). Based on Experiment 1, we defined ROIs including the DMPFC, VMPFC, precuneus, bilateral amygdala, bilateral (posterior) parahippocampal gyrus, bilateral inferior parietal cortex, bilateral STS, bilateral TP, and bilateral insula to be tested using RSA in the second, independent dataset (n = 78). For visual demonstration of consistency, identical maps showing similar activation patterns in the two experiments appear in Fig. 3B and SI Appendix, Fig. S1.

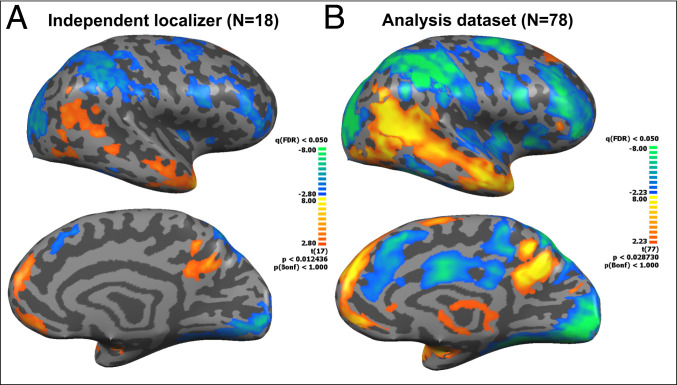
Whole-brain maps of affect-specific empathy. (A) Experiment 1: 18 subjects (six from each group, FT, KC, and SC) were randomly chosen for functional localizer. Presented contrast: emotion > scrambled image. Group means were used for ROI definition. (B) Experiment 2: Similar contrast presented for the analysis dataset (n = 78). Note resemblance of activation patterns across samples. All maps are false discovery rate corrected at q < 0.05. Note: Images present left hemisphere. For right hemisphere, see SI Appendix, Fig. S1.
Following, we assessed the neural representations of empathy to affect-specific emotions. Using RSA [CoSMoMVPA toolbox, (45)], we examined the similarity of neural representations among emotions in each ROI separately in Experiment 2 (total n = 78, FT = 27, KC = 23, SC = 28). For each ROI, we correlated the beta values of each voxel between each pair of emotions (joy–sadness, joy–distress, and sadness–distress). Dissimilarity was defined as the correlation distance (1 − Pearson correlation) between each two conditions, calculated for each subject. The dissimilarity vectors for all 78 subjects were averaged (46) resulting in 3*3 dissimilarity matrix (DSM) (see SI Appendix, Supplementary Methods for full details). High dissimilarity indicates differential patterns of activation within a given ROI, denoting sensitivity of this region to different emotions (39).
RSA analysis revealed that the neural activation patterns underpinning empathy to distinct emotions showed significant differences among our ROIs (F(5.3,390.2) = 76.16, P = 4.48e−57,

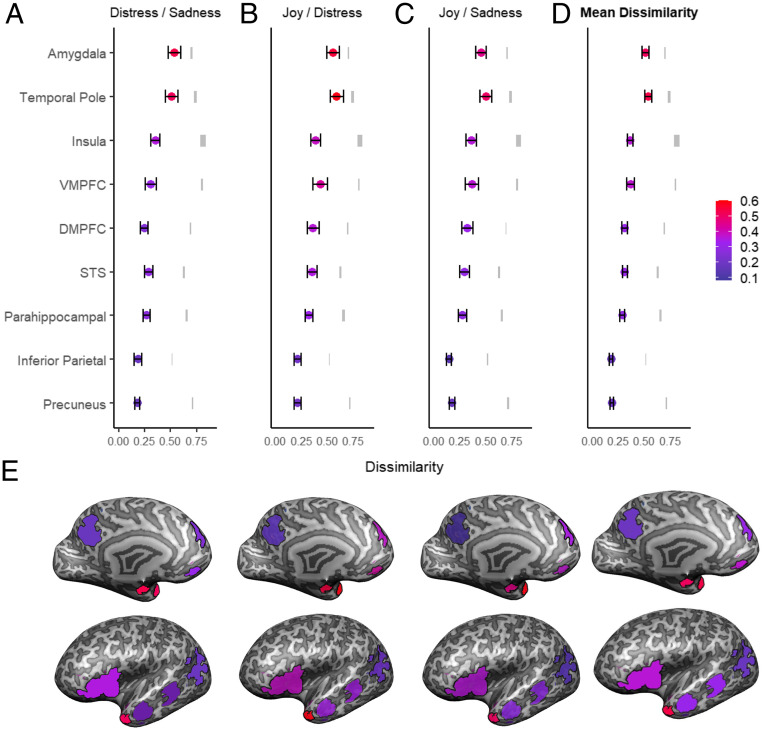
RSA across ROIs: Representational similarity was measured subject-by-subject by calculating correlation distance (1 minus Pearson correlation between two vectors of beta values of emotional pair) resulting in three dissimilarity pairs: (A) joy/distress, (B) joy/sadness, (C) and distress/sadness (D) mean dissimilarity levels across all emotional pairs A–C for each ROI. Colored dots show mean dissimilarity levels for each ROI; error bars represent 95% CIs, and gray marks are the noise ceiling bounds. A similar pattern of dissimilarity is observed across emotional pairs. TP and amygdala show highest levels of dissimilarity. (E) Actual ROIs, superimposed on an inflated brain. Color marks the dissimilarity levels according to the color code of A–D.
We next moved to test our second hypothesis; that synchrony experienced across development (averaged from four time points; see correlation SI Appendix, Table S4) would impact the brain basis of empathy to specific emotions. To this end, we selected the four ROIs which showed the highest overall sensitivity to distinct emotions: amygdala, TP, insula, and VMPFC. To reduce dimensionality, for each area we created a dissimilarity score by averaging levels of dissimilarity across the three emotional pairs, which showed intercorrelations among them (joy–sadness, joy–distress, and sadness–distress; correlation SI Appendix, Table S3).
Following, we computed four bootstrap hierarchal regression models to test the prediction from synchrony across development to dissimilarity in young adulthood for the 78 participants in the analysis dataset, controlling for group membership. Four two-step bootstrap regressions were computed, one for each of the four brain regions with highest sensitivity to different emotions: amygdala, TP, insula, and VMPFC. In each regression, group was entered in the first step and synchrony in the second step, and we examined both the overall significance of the model and the unique contribution of synchrony to the prediction of dissimilarity. The overall bootstrap regressions models were significant for three of the brain areas tested: amygdala (P < 0.01), insula (P < 0.001), and TP (P < 0.05), but the overall model for VMPFC was not significant (P > 0.05). In none of these models was group found to be a significant predictor of dissimilarity (P > 0.05). However, synchrony in the second step was found to be a unique and significant predictor of dissimilarity in two of the models: amygdala and insula (P < 0.05). Specifically, our bootstrap regression models showed that synchrony was a significant and independent predictor of dissimilarity in the amygdala (F(1,74) = 5.44, P = 0.03,

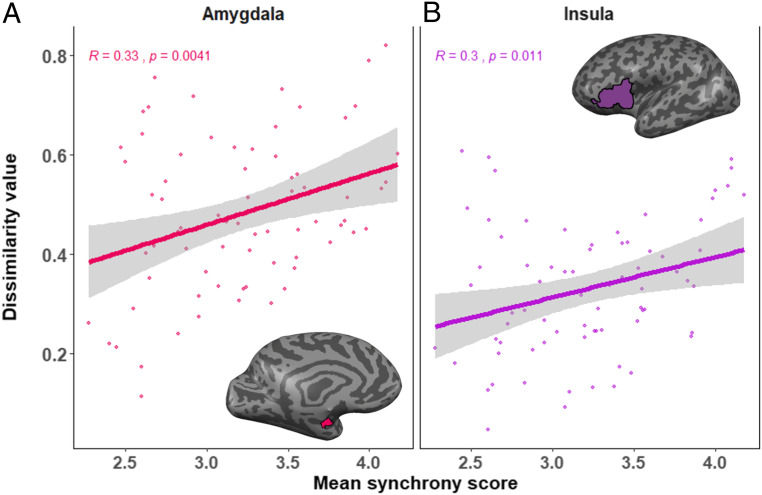
Linear regression between synchrony from infancy to young adulthood and dissimilarity. Regression and correlation values between mean synchrony and mean dissimilarity values in Amygdala (A) and Insula (B). Higher mean synchrony values were related to higher levels of neural dissimilarity.
Finally, to validate our brain findings in relation to a behavioral measure of empathy, we examined associations between empathic understanding measured in adolescence and neural dissimilarity in the four areas: amygdala, insula, TP, and VMPFC. In adolescence, we used a validated paradigm to assess prosocial moral judgement (47), which provides an index of empathic understanding (48). Participants were presented with four dilemmas in which prosocial choice is juxtaposed with a selfish choice; the choice is then challenged, and adolescents are asked to explain the reasons for their choice (e.g., returning a wallet found on the beach versus using the money to go out with friends). Score for each dilemma (range 0 to 4) is determined by whether the choice is prosocial, and the explanation is not fear based but indicates understanding of others’ distress, needs, motives, and feelings, and the scores are summed into a total empathic understanding score (see Materials and Methods).
Empathic understanding was positively associated with synchrony (r = 0.32, P = 0.004), indicating that the experience of synchrony across development links with adolescents’ empathic understanding. Empathic understanding correlated significantly with dissimilarity in the insula (r = 0.31, P = 0.005), TP (r = 0.36, P = 0.001), and VMPFC (r = 0.29, P = 0.01) but not in the amygdala (r = 0.20, P = 0.07). To test the unique contribution of empathic understanding to dissimilarity above and beyond group and synchrony, four bootstrap regressions were computed in which group was entered in the first step, synchrony in the second step, and empathic understanding score in the third. Results showed that empathic understanding was uniquely predictive of dissimilarity in the TP (F(1,73) = 8.41, P = 0.04,
While findings in animal models provide evidence for the lifelong effects of maternal presence and caregiving behavior on the developing brain (3, 4, 21), human studies that follow infants across their lengthy maturation from birth to adulthood are scarce. In this study, we capitalized on a unique “natural experiment” that involved provision of maternal–newborn skin-to-skin contact to premature infants during the period of incubation, followed by 20 y of repeated observations of the mother–child relationship in the natural ecology. This enabled us to track how initial maternal–infant contact increases mother–child synchrony first in infancy and then, with the continuity of caregiving, across development and how such enhancement impacts the brain’s capacity to empathize with others’ distinct emotions. These findings lend support to our two hypotheses. First, we found that proximity to the mother’s body in premature neonates during the period of incubation is linked with enriched caregiving in humans, similar to its role in other mammals, and that this initial improvement reverberates throughout the course of child development as suggested by "sensitive periods" perspectives (49). Second, we show that social synchrony is a mechanism by which the human brain is tuned to the social world. Overall, our study demonstrates how early caregiving patterns stabilize across lengthy developmental epochs and over time impact brain functioning, describing how human relationships are gradually represented in the brain.
Several key findings emerged from our 20-y effort. First, we describe how the mother–infant relationship matures from infancy to adult life as a consequence of initial conditions related to physical contact in the first weeks of life. Second, although we found no differences in the neural empathic response between groups, the quality of early caregiving longitudinally impacted the social brain. Finally, we pinpoint the brain regions that differentially respond to others’ distinct emotions from other brain areas that activate globally to the empathy task.
Importantly, despite differences in birth conditions, we found no group differences in the brain basis of empathy to different emotions. Several reasons may account for this lack of group difference in neural results. First, conceptual models on developmental continuity suggest that continuity over long epochs from early life to adolescence and adulthood is often indirect and proceeds through changes in aspects of the caregiving environment that gradually shape outcome (19, 50, 51). Second, our findings may suggest that neurologically intact premature infants who present normative cognitive abilities and are reared in well-functioning families can develop adequate neural empathic response in young adulthood. Still, maternal–newborn contact had a lasting effect on the development of mother–child synchrony, which, in turn, impacted the neural basis of affect-specific empathy. While our study includes infants who initially lacked full maternal bodily contact, this condition does not resemble the extreme deprivation observed in institutionally reared infants who show alterations in brain volume in adulthood despite later enrichment (52). Findings from these two longitudinal cohorts highlight the importance of a caring mother–infant relationship and underscore humans’ heightened neural plasticity in the first weeks of life and the potential for reparation when the rearing environment is normative and not extreme.
As expected, social synchrony developed over time in all children. Whereas infants can only engage in momentary stretches of give-and-receive exchanges, exhibit little adaptation to their social partners, and expansion of the interaction is limited to the infant’s nonverbal repertoire, adult-to-adult interaction can sustain long episodes of reciprocity, intimacy, and perspective-taking while reverberating the familiar rhythms of infancy and expanding the interaction through verbal and nonverbal communications that integrate the inputs of both partners into dialogue. Consistent with dynamic systems’ theory (49), synchrony was highly sensitive to initial conditions and preterm infants without initial opportunities for full maternal physical contact did not show catchup over the 20-y span and displayed consistently lower levels of synchrony compared to their peers.
Maternal–newborn contact altered these initial conditions, and our findings underscore the pervasive effects of a neonatal touch-based intervention on the adult human that resembles the effects of alterations in maternal contact on the brains of other mammalian adults (5354–55). Early skin-to-skin contact triggered a different developmental trajectory, and despite the fact that KC children showed midlevel synchrony across childhood they displayed a full catchup by early adulthood. Following the pubertal transition, these children were able to engage in a full-blown adult–adult synchrony, regardless of their high-risk beginning. Possibly, one function of the maternal–newborn bodily contact is to provide a bridge from prenatal life, when the mother’s physiological systems are tuned to the growth needs of the fetus, to postnatal social life, when moments of social synchrony externally regulate the infant’s heart rhythms, hormonal response, and brain oscillations and tune them to social life (1, 56). Because the experience of synchrony in infancy bears long-term consequences for child development (12, 16, 57), skin-to-skin contact, which is an easy-to-implement intervention that boosts the mother–infant relationship, should be advertised and widely supported in neonatal intensive care units (NICUs).
Our study employed multivariate pattern analysis in the empathy network and differentiated structures presenting distinct neural activation patterns during empathy with others’ joy, sadness, and distress from those which activated globally to the task of empathy regardless of emotional content. Brain areas presenting the most differentiated response patterns to distinct emotions were the TP, amygdala, insula, and VMPFC. These affect-sensitive regions in the empathy network integrate subcortical, paralimbic, and cortical structures which have been repeatedly shown to sustain the complex, dual-processed human empathy that integrates bottom-up rapid emotional identification with top–down mentalization and emotion regulation toward a multidimensional empathic response (58). The insula, which has shown pattern similarity between first-hand pain and pain empathy (59), integrates interoceptive and sensory signals with their affective and social values to enable participation in human social life (60). Furthermore, neural representations of empathy were found to be impacted by the mother–child relational context (61), consistent with our findings that mother–child synchrony across development is associated with affect-specific empathy representations in the insula and amygdala. As emerging research using pattern analytic methods to describe various aspects of empathy indicates that representational patterns link with relationship history and self–other similarity (57, 58), the contribution of parent–child attachment to the maturation of the neural representations of empathy may be a fruitful area for future research in both healthy development and under high-risk rearing, and our findings provide a first step in this direction.
The four regions displaying highest sensitivity to affect-specific empathy are interconnected through the uncinate fasciculus into the temporo-amygdala-orbitofrontal network (62). Through this interconnectivity, the TP receives projections from the insula and projects to the amygdala, corroborating its role as a paralimbic region (63), and the VMPFC shows extensive reciprocal interconnections with the amygdala and TP (64), modulating amygdalar response to arousal dynamics and emotional states (65). Functionally, all four regions have been shown to activate during empathy tasks [Fig. 3 and (66)] and their activity is associated with the degree of emotional intensity (67) and affective valance (68). The amygdala plays a key role in emotional and social processing (69), the insula integrates interoceptive signals and is implicated in empathy across species (70), the TP sustains theory of mind and mentalizing tasks and integrates emotionally relevant multimodal sensory information into higher-order concepts that gauge the emotional significance of social signals (71), and VMPFC-driven value signals are critical for making empathic choices based on affect-specific information (72). We suggest a putative model for insular-amygdalar-TP-VMPFC function in the empathic process; visceral bodily signals of interoceptive state originating in the insular cortex integrate with affective and exteroceptive information from the limbic system and TP region, which are regulated by value signals from the VMPFC to enable affect-specific modulation of the empathic stance in light of changing arousal dynamic, internal state, and social contexts.
Synchrony experienced from infancy to adulthood enhanced this affect-specific neural empathic response in the amygdala and insula, two interconnected areas that sustain emotional and social processing and delineate core structures of the social brain (73). The amygdala and insula are highly sensitive to rearing conditions and adults reporting early life stress show structural and functional alterations in these regions (74, 75). However, these conclusions have typically relied on adults’ retrospective accounts whereas our study prospectively followed children from birth and linked actual patterns of caregiving observed in the natural habitat with young adults’ neural empathic response, pinpointing the effects of childrearing across development on these regions. In parallel, the amygdala and insula play a critical role in parenting and its associated neurobiology. Maternal amygdala and insula response to multiaffect infant stimuli, including both crying and laughter, have been shown to predict mother–infant synchrony, oxytocin receptors abound in the amygdala and play a causal role in the initiation of mammalian mothering, and amygdala lesion disrupts maternal caregiving (76, 77). Similarly, human parents’ insular response to their infants impacts the child’s later capacity to regulate distinct emotions, including joy and distress, in preschool (29). Similar to the current findings, the link between parental insula and the child’s regulation of distinct emotion was mediated by parent–infant synchrony. It thus appears that the amygdala and insula may provide an integrative foundation for the cross-generational transmission of parenting as mediated by synchronous caregiving. Our findings may chart a developmental pathway by which a mother’s brain and bodily provisions translate into synchronous caregiving, which, via the consistency of caregiving over time, enhances the child’s amygdala and insula sensitivity to others’ distinct emotions, thereby preparing the child for his or her future role as parent.
The insula plays a key role in the brain’s capacity to process socio-temporal patterns (78). Insular projections combine interoceptive signals with social patterns within specific social contexts via temporal–social computations (78), enabling the integration of two humans into a “we mode” based on the construction of joint predictive models through shared social patterns (79). Synchrony affords infants the first context of patterned social regularities, and such experiences may register in the insula, amplified by the amygdala’s salience detection function and the amygdala–insula interlimbic connectivity (8081–82). Parental care, particularly the repetitive-rhythmic structure of synchrony, enables the infant’s brain to form predictions of mother and environment, integrating bodily, sensory, and affective information into the social moment and shaping the brain’s allostatic function via the regularity of maternal care (25). The insula defines a neural hub for allostasis, the brain’s anticipatory regulation of bodily needs that allocates timely resources to life-sustaining functions based on a hypothalamic clock that underpins rest–activity cycles in every tissue of the body (83, 84). In infancy, mother subsumes an allostatic role for the infant’s physiological systems, and studies in humans and other mammals have underscored the lifelong disruptions to clock-related functions following brief maternal separation or disrupted caregiving (8586–87). With time, the insula sustains the infant’s rudimentary “proto-self” (88), which gradually builds on the basis of bodily experiences vis-à-vis the maternal body and synchronous caregiving to undertake its own allostasis, and in adults, the insular cortex is related to the representation of interoceptive models of the self (89, 90). Our findings show that the provision of maternal–neonate bodily contact, through its enhancement of mother–child synchrony and the consistency of synchrony from infancy to adulthood, sensitizes the young adult’s insula to distinguish among affective states, improving the insula’s capacity to empathize with others to enhance collaboration as well as to differentiate among states to increase allostasis.
The TP and VMPFC, although not directly linked with synchrony, were indirectly related to the experience of synchrony through its impact on empathic understanding. Dissimilarity of these regions was predicted by empathic understanding in adolescence, providing a behavioral validation to our neural findings on the brain basis of empathy in young adulthood. The TP and VMPC have both been shown to activate during empathy tasks (72, 91) and are thought to be especially linked with the top–down mental and reflective aspect of empathy (70, 92, 93). Both areas are linked with the amygdala and insula to create a network that sustains the dual-processed human empathy (60, 94, 95). As seen, the experience of synchrony across childhood sensitized adolescents to greater prosocial behavior and cognitive empathy, assuming a position that takes into considerations others’ mental states and feelings, and such cognitive empathy, in turn, predicted greater neural sensitivity to others’ distinct emotions in the two cortical structures. Our findings, therefore, suggest that the consistent experience of parent–child synchrony may sensitize the four regions in the empathy network that respond differentially to others’ distinct emotions, either directly, as seen for the amygdala and insula, or through its impact on adolescents’ empathy, as seen for the TP and VMPFC.
Several limitations of this study should be noted. First, we did not measure behavioral empathy in young adulthood, only in adolescence, and such assessment would have been of interest. Second, while we have repeated information on mother–child synchrony, interactions with the father were not repeatedly assessed, and the child’s relationship with father and other close attachment figures could have provided valuable information. Our preterm sample is relatively homogenous; we included families of low contextual risk and infants with no major neurological damage, and participants with structural abnormalities were excluded from MRI analysis. This decision stemmed from the need to tease apart the long-term effects of maternal–newborn contact from those posed by high contextual and medical risk, which are enhanced in the context of premature birth (9697–98). However, this decision limits the generalizability of our findings, and the effects of prematurity and the KC intervention on infants born at higher medical and contextual risk should be studied. We utilized functional imaging measures, and it is possible that structural measures with our sample would have yielded group difference related to prematurity or KC. Similar to most longitudinal studies, attrition over a period of two decades may not be consistent across groups, and while families were initially case matched, by young adulthood, there were differences related to initial conditions, such as GA and birthweight, and to current household income; however, these factors were unrelated to any brain or behavioral measure in our study. Finally, ethical constraints limited full randomization and necessitated a case-matched design.
Understanding developmental continuity and change in humans—whose brain matures across three decades, implicates substantial plasticity, and affords significant recovery following initial insult—is the focus of much conceptual and empirical interest, particularly the detection of biosocial factors that tilt infants toward a resilient trajectory. Our study, which integrated an experimental design related to maternal–newborn contact and separation with a longitudinal follow-up spanning two decades, found evidence for the two mechanisms proposed to underlie developmental continuity in humans: a step-by-step progress where minor alterations gradually impact outcome through repeated iterations and a “sensitive period” perspective in which early provisions shape long-term outcomes (49). Synchrony functioned as a core mechanism underpinning social brain development both by initiating a step-by-step trajectory that incorporated gradually acquired socio-cognitive abilities into its process and through the sensitivity of the synchrony experience to touch-based intervention during the sensitive period. Our results indicate that the human social brain, like that of other mammals, is sensitive to features which are critical for human social life: rapid assessment of others’ feelings, accuracy of emotional detection, and interpretation of others’ mental states by assuming the vicarious stance. Our study, therefore, sharpens questions for future research and underscores the field of developmental social neuroscience as a potentially fruitful perspective on the human brain’s capacity for empathy, collaboration, and social living.
The study included 96 twenty-year-old young adults (M = 19.71, SD = 1.96) who were part of a longitudinal study recruited at birth and followed at our laboratory from birth until early adulthood. A total of 132 participants were scanned, of which 96 were included in the analysis (SI Appendix, Table S7 for exclusion criteria and excluded subjects breakdown). Of the study participants, 79 were born as neurologically intact preterm infants and were, at the time of brain imaging, healthy, well-functioning, had graduated from typical state-supervised high schools, and have shown normative intelligence quotient and executive function scores (see below), attesting to their age-appropriate intelligence and scholastic aptitude. Fifty-two participants were born at FT without any medical complications and with a normative developmental course. All children were reared in two-parent families to mothers who were above 21 y at childbirth and with family income above the poverty cutoff, and all families were of middle or upper-middle socioeconomic status (SES). Of the preterm group, 35 received KC for at least 14 consecutive days, at least an hour daily, during the hospitalization period in the NICUs (see below), and 44 infants, matched for demographic and medical variables, received SC. Demographic details are provided in SI Appendix, Table S6.
All participants were Israeli Jews apart from one participant who was an Israeli Arab, and this participant was of the same SES as the Israeli–Jewish participants in terms of parental education and household income.
The study utilized a birth cohort longitudinal design, and mother–child dyads in both the preterm and FT groups were recruited at birth during the same period.
The original premature cohort and their mothers was recruited in NICUs at birth from March 1996 to November 1999, prior to any available information on the KC intervention and any scientific evidence for its long-term benefits, and this enabled our case-matched protocol (see SI Appendix and ref. 17). Two mothers per week who met inclusion criteria were approached and invited to participate. Participants in the KC group were matched on key medical and demographic factors to mother–infant dyads in hospital B (for details of recruitment, see SI Appendix). Only two mothers approached to participate in the KC group, and three mothers in the control group declined participation in the follow-up.
Because the developmental benefits of the KC intervention are now readily available to the public and most western NICUs encourage mothers to engage in KC contact, the recruitment of a similar cohort is no longer possible, and our carefully matched cohort provides a rare opportunity to study the long-term effects of maternal separation and structured contact on the developing brain in a human model. See SI Appendix for a detailed description of the KC intervention.
Premature mothers and infants in both the KC and SC groups were observed nine times following the intervention; at hospital discharge, at 3, 6, 12, and 24 mo, at 5 and 10 to 12 y, and two times in young adulthood, once for a home visit and once (within the next few weeks) for an MRI scan. Details regarding attrition are in SI Appendix, Table S8B.
The original full-term cohort included 135 participants, of which 52 were scanned (38%). Families meeting inclusion criteria (healthy, dual-parent, and mothers above poverty line) were recruited from birth records in Well-Baby clinics. Families were observed four times, at 4 mo, 2 y, 5 y, and 12 y and in both a home visit and MRI in young adulthood in identical protocol to that of the preterm cohort and conducted at the same period.
We were not able to locate 33 subjects (24%), and 38 (28%) refused participation. Eleven (8%) subjects were not scanned because they did not meet safety requirements or because they refused to undergo imaging; one subject terminated the scan due to claustrophobia. A consort diagram describing the full-term cohort is in SI Appendix, Table S8A.
An additional full-term subject was recruited with no longitudinal data. This subject was part of experiment A (functional localizer).
Mother–child synchrony was observed four times: in infancy, preschool, early adolescence, and young adulthood.
1)Infancy: In infancy (M = 4.26 mo, SD = 1.14), we videotaped mother–infant interaction in the home environment. Instructions were “play with your infant as you normally do,” and 5 min of free play were videotaped.
2)Preschool: During a home visit (M = 3.21 y, SD = 1.38), mothers and children played with a set of predetermined toys for 7 min. Parents and children were given a box of toys which were selected on the basis of previous research to elicit the child’s creativity and imagination (99, 100). Toys included the following: two dolls, bottle, blanket, tea set including two cups, two plates, sugar, and milk pots, a boiler pan, wallet, colored necklace, a pair of plastic sunglasses, a sponge, three work tools, two small cars, telephone, two pet animals and two wild animals, and a small tool set.
3)Adolescence: In early adolescence (M = 12.07 y, SD = 1.62), mother and child engaged in two discussion paradigms for 7 min each, consistent with prior research (14, 17, 101, 102). In the first, a positive valance task, mother and child were asked to plan the “best day ever” to spend together, and in the second, a negative valance task, mother and child discussed a typical conflict in their relationship. In addition to mother–child interaction, in adolescence there was also an assessment of the child’s synchrony during interactions with their best friend (again in both a positive context: “plan a campaign for your school,” and negative context, “discuss the most difficult conflict in your relationship”). Synchrony with mother and friend was interrelated (r = 0.29, P = 0.008), and this finding supports the importance of the experience of mother–child synchrony across development for the child’s later attachments.
4)Young adulthood: In young adulthood (M = 19.76 y, SD = 2.01), young adults and their mothers engaged in the same positive valance task used in adolescence and in an additional 7-min support-giving task, in which each partner shared a topic of concern and the partner provided support.
The Coding Interactive Behavior (CIB) system† was used to code all interactions. The CIB is a well-validated system for coding social interactions that utilizes global rating scales (48 to 52 scales depending on ages) and includes four manuals (for infants, children, adolescents, and adults) that use similar codes which are aggregated into several theoretically based composites. The CIB is the most well-validated coding scheme for coding social behavior across the lifespan. It has been used in multiple studies across 20 countries which have yielded over 170 scientific publications to date. The system has good psychometric properties and has shown construct and predictive validity, test–retest reliability, and sensitivity to cultural contexts, interacting partner, and a variety of high-risk conditions related to psychiatric conditions, child biological conditions, and environmental stress conditions (for a review see ref. 103). Consistent with prior research, we used the parent–child synchrony CIB construct (14, 101, 104105–106), which is computed by averaging several CIB codes.
The parent–child Synchrony construct across ages comprises the same five codes: Dyadic Reciprocity, Adaptation–Regulation, Fluency–Rhythmicity, Opportunity for Joint Expansion and Elaboration of Interaction, and Joint Positive and Relaxed Mood. These global codes across development address the dyadic feature of the interaction but express behaviorally in age-appropriate ways. See SI Appendix, Supplementary Materials for a detailed description of synchrony constructs of each age group.
Coding was computed by trained coders blind to all other information. Interrater reliability computed on 15% of the sample and interrater reliability averaged, intraclass r = 0.93 (range: 0.88 to 0.99).
Parent–child synchrony showed moderate individual stability from infancy to adulthood between all four observations (SI Appendix, Table S3), attesting to the stability of the synchronous caregiving style across the entire period of child development.
During the home visit in young adulthood, the participants completed the State–Trait Anxiety Inventory for Adults [STAI (107)], comprising 20 items of state anxiety (STAI-S), evaluating current symptoms, and 20 items of trait anxiety (STAI-T), referring to feelings in the recent past as well as the Beck depression inventory (108), a questionnaire of depressive symptoms on a continuum of no depressive symptoms to low (BDI < 9, and severe (BDI > 11).
Additionally, for assessment of major life events, in young adulthood, we asked participants to rate whether they experienced a major stressful life event (e.g., death of a close person, parental divorce, car accident, major trauma, and life-threatening illness to self or family member), a moderate stressful life event (e.g., manageable medical concern and failure at school), or no such stressors.
To measure adolescents’ empathic understanding, we used a validated procedure that measures prosocial moral judgement (47), which is considered an index of empathic understanding in children and adolescents (109, 110). Adolescents were presented with four dilemmas in which self-interest conflicts with the well-being of others (e.g., child should ignore an injured person in order to attend a party versus helping and missing the party; finding money at the beach and using it for a social outing versus returning it to the station). The child is then asked what the protagonist should do and to explain the reasons for his/her choice. When the child offers a solution, the experimenter challenges the decision and points out the needs of the other (in case of a selfish choice) or the consequences for the protagonist (in case of a prosocial choice). The child can then confirm the original choice or change his/her mind.
A detailed description of empathic understanding paradigm score is in SI Appendix.
MRI data were collected using a 3 Tesla scanner (SIEMENS MAGNETOM Prisma syngo MR D13D, Erlangen, Germany) located at the Tel Aviv Sourasky Medical Center. Scanning was conducted with a 20-channel head coil for parallel imaging. Head motion was minimized by padding the head with cushions, and participants were asked to lie still during the scan. High-resolution anatomical T1 images were acquired using magnetization-prepared rapid gradient echo sequence: TR = 1860 ms, TE = 2.74 ms, FoV = 256 mm, Voxel size = 1 × 1 × 1 mm, and flip angle = 8°. Following functional images were acquired using echo planar imaging gradient echo sequence: TR = 2500 ms, TE = 30 ms, 42 slices, slice thickness = 3.5 mm, FOV = 220 mm, Voxel size = 3.2 × 2.3 × 3.5 mm, and flip angle = 82°. In total, 393 volumes were acquired over the course of the empathy paradigm. Visual stimuli were displayed to subjects inside the scanner, using a projector (Epson PowerLite 74C, resolution = 1024 × 768) and were back-projected onto a screen mounted above subjects’ heads and seen by the subjects via an angled mirror. The stimuli were delivered using “Presentation” software (http://www.neurobs.com). Before participating, participants signed an informed consent according to protocols approved by ethics committee of the Tel Aviv Sourasky Medical Center. Subjects received a gift certificate of 450 NIS (∼100USD) for their participation. The study was approved by Bar-Ilan University’s Institutional Review Board and by the Helsinki committee of the Sourasky medical center, Tel Aviv. Ethical approval no. 0084–15-TLV.
Empathy paradigm was adapted from Morelli et al. (30) During the fMRI scan, participants saw images of targets experiencing joy, sadness, or distress-eliciting emotional events embedded in a social context.
The joy context-dependent experiences included images of protagonists looking joyful along with contextual information (for instance, “this person has just won the lottery”). The sadness context-dependent experiences included images of protagonists looking sad with contextual information (“this person just lost an important sport competition”). The distress context-dependent experiences comprised images of protagonists looking stressed and anxious with contextual information of uncertainty (“this person might lose his job”). In addition to emotional stimuli, a “motor resonance” condition included targets performing mundane nonemotional actions (“this person is ironing his shirts”). In order to capture sensory visual input alone, a “scrambled image” condition was included consisting of randomly shifted pixels of the photos presented in the other conditions. The instruction for this condition (to replace context) was “look carefully at the following images.”
Subjects were explicitly instructed to “put themselves in the shoes” of the protagonists, intentionally empathize with their feelings, and imagine how they would feel in similar situations. Overall, the session included five conditions (joy, sadness, distress, motor resonance, and scrambled image), with each condition running for five blocks in a pseudorandom order ascertaining that two consecutive blocks are not of the same condition. The context (sentence) was presented for 2.5 s, followed by four photos presented for 5 s each (4*5 = 20 s). Between the blocks there was a fixation period of 10/12.5 s to avoid anticipation of the next stimulus. During fixation, participants saw a white fixation cross in the middle of the screen over a black background (see Fig. 1B for details). Prior to fMRI experiment, stimuli were validated by independent raters (see SI Appendix for full details of empathy paradigm validation).
Data preprocessing and analysis were done by BrainVoyager QX software package 20.6 (Brain Innovation, Maastricht, Netherlands) (111). The first three volumes, before signal stabilization, were discarded to allow for T1 equilibrium. Preprocessing of functional scans included three-dimensional (3D) motion correction, slice scan time correction, and spatial smoothing by a full width at half maximum 4 mm Gaussian kernel. The functional images were then superimposed on two-dimensional (2D) anatomical images and incorporated into the 3D datasets through trilinear interpolation. The complete dataset was transformed into Talairach space (112) using sinc interpolation. We used 4 mm smoothed data for whole-brain maps. RSA was performed on unsmoothed data.
To ensure independent definition of ROIs, subjects were randomly divided into two groups: in Experiment 1, a group of 18 subjects (six from each group [FT, KC, and SC] were used for ROI definition by a functional localizer. These ROIs were later on validated and examined on the rest of the subjects (analysis dataset, n = 78). The mean age of these 18 subjects was 19.44 y old (SD = 1.79), 72% males, and 94% right-handed. The subjects’ brain imaging data of the empathy paradigm was used for a multi subject general linear model, using separate subject predictors. Based on the contrast of all emotions (joy, sadness, and distress) > scrambled image, FDR-corrected whole-brain maps of affective empathy were created. Activations were used as a functional localizer for ROI definition. Insular cortex alone was defined using a rectangle bounding box, as it was not clearly activated and was a region of interest based on current literature (113). Full details on the ROIs are presented in SI Appendix, Table S3. Whole-brain maps of affective empathy for the functional localizer dataset and for the analysis dataset are presented in Fig. 3 and SI Appendix, Fig. S1.
RSA was done using MATLAB R2018a (MathWorks Inc.) with NeuroElf (J. Weber, https://neuroelf.net/) and CoSMoMVPA toolbox (45).
For each subject of the 78 “analysis dataset” group, in each ROI, beta values per voxel for each condition (= empathy for a certain emotion, sadness, joy, and distress) were extracted, resulting in three vectors (each vector is 1*number of voxels). Then, the correlation distance (= 1 minus Pearson correlation) between every two conditions was calculated, for each subject. The dissimilarity vectors for all 78 subjects were averaged, resulting in a 3*3 DSM. Following, in order to compare between different ROIs, we used a multidimensional secondary level analysis in which the correlation distances of each ROI DSM were projected into a 2D space (114).
For each ROI, the noise ceiling, the expected value of average correlation with the true model given the variability across subjects, was calculated. The upper bound was estimated by computing the correlation between each subject’s representational similarity matrix and the group-average similarity matrix. For lower bound, each subject’s representational similarity matrix was correlated with the average matrix of the other subjects (leave-one-out approach) (115). All correlation values were transformed to dissimilarity measures by subtracting them from 1.
We are grateful to the participants and their families for their kind cooperation across the years. R.F. is supported by the Simms/Mann Foundation and the Irving B. Harris Foundation. The initial study was supported by the Irving B. Harris Foundation and the Israel Science Foundation.
Group level fMRI maps are publicly available at Open Science Framework (OSF) (DOI: 10.17605/OSF.IO/QFZWU) (120). Some study data are available upon request.
1
2
3
4
7
8
9
10
11
12
13
14
15
16
17
18
20
21
22
23
24
25
27
28
29
30
31
32
33
34
35
36
37
38
39
41
42
43
44
45
46
47
48
49
51
52
53
54
55
56
57
58
59
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
114
115
116
117
118
119
120