
- Altmetric
Background
Multiple system atrophy (MSA) is a rare neurodegenerative disorder, and its parkinsonian variant can be difficult to delineate from Parkinson's disease (PD). Despite laryngeal dysfunction being associated with decreased life expectancy and quality of life, systematic assessments of laryngeal dysfunction in large cohorts are missing.
Objectives
The objective of this study was to systematically assess laryngeal dysfunction in MSA and PD and identify laryngeal symptoms that allow for differentiating MSA from PD.
Methods
Patients with probable or possible MSA underwent flexible endoscopic evaluation of swallowing performing a systematic task protocol. Findings were compared with an age‐matched PD cohort.
Results
A total of 57 patients with MSA (64 [59–71] years; 35 women) were included, and task assessments during endoscopic examination compared with 57 patients with PD (67 [60–73]; 28 women). Patients with MSA had a shorter disease duration (4 [3–5] years vs 7 [5–10]; P < 0.0001) and higher disease severity (Hoehn & Yahr stage 4 [3–4] vs 3 [2–4]; P < 0.0001). Of the patients with MSA, 43.9% showed clinically overt laryngeal dysfunction with inspiratory stridor. During endoscopic task assessment, however, 93% of patients with MSA demonstrated laryngeal dysfunction in contrast with only 1.8% of patients with PD (P < 0.0001). Irregular arytenoid cartilages movements were present in 91.2% of patients with MSA, but in no patients with PD (P < 0.0001). Further findings included vocal fold motion impairment (75.4%), paradoxical vocal fold motion (33.3%), and vocal fold fixation (19.3%). One patient with PD showed vocal fold motion impairment.
Conclusion
Laryngeal movement disorders are highly prevalent in patients with MSA when assessed by a specific task protocol despite the lack of overt clinical symptoms. Our data suggest that irregular arytenoid cartilage movements could be used as a clinical marker to delineate MSA from PD with a specificity of 1.0 and sensitivity 0.9. © 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Multiple system atrophy (MSA) is a sporadic progressive neurodegenerative disorder caused by oligodendroglial aggregation of α‐synuclein affecting predominantly the nigrostriatal, olivo‐ponto‐cerebellar, and autonomic systems, 1 , 2 resulting in a clinical presentation of dysautonomia combined with either predominantly parkinsonian (MSA‐P) or cerebellar (MSA‐C) symptoms of varying severity. Mean clinical disease onset is in the sixth decade with both sexes equally affected. 1 In contrast to idiopathic Parkinson's disease (PD), MSA progresses rapidly with a mean survival of 6 to 10 years after diagnosis. 3 In its early stage, the diagnosis of MSA according to the second consensus criteria 4 can be challenging. 2 , 5 Therefore, the Movement Disorders Society MSA study group recently addressed the importance of developing valuable diagnostic tools for securing an early diagnosis in patients with MSA not only to estimate disease prognosis but also to early initiate novel, potentially disease‐modifying treatments in clinical trials. 6
To improve diagnostic accuracy, the European MSA study group in 2008 analyzed 17 different features in patients with MSA‐P and PD 7 and showed a high prevalence of red‐flag symptoms in the MSA cohort. A factor analysis was performed to reduce the number of red flags required for a diagnosis of probable MSA by identifying 6 categories. The combination of 2 of these 6 categories was highly specific with a good sensitivity when comparing patients with MSA‐P with patients with PD, permitting an earlier diagnosis of probable MSA than when applying the first consensus criteria for the diagnosis of MSA alone. 8 With diurnal inspiratory stridor, nocturnal inspiratory stridor, inspiratory sighs, severe dysphonia, and severe dysphagia, 5 of these highly specific features include laryngopharyngeal pathology, which underlines the importance of assessing laryngopharyngeal function when suspecting MSA. 9 These red flag categories were included in the additional features of possible MSA and in the supporting features in the current second consensus criteria for the diagnosis of MSA. 4
Despite laryngopharyngeal dysfunction being associated with decreased life expectancy and quality of life, 10 systematic assessment of these functions in MSA is scarce. Previously, few studies in small cohorts addressed laryngeal motion abnormalities in MSA, including paradoxical vocal fold (VF) motion, 11 , 12 , 13 , 14 VF motion impairment, and irregular arytenoid cartilages movements, respectively (for review, see ref. 14). Various studies revealed impairment of VF motility in patients with MSA in association with inspiratory stridor, as reported in a recent consensus conference on stridor in MSA. 15 According to this consensus, standardized endoscopic evaluation of the larynx is suggested to also exclude mechanical lesions or functional VF abnormalities related to different neurologic conditions and may reveal VF motility impairment in patients with MSA with stridor. Therefore, further studies including a systematic assessment of laryngeal motion abnormalities and pharyngeal function in MSA are required.
We previously suggested a standardized, easy‐to‐implement MSA–flexible endoscopic evaluation of swallowing (FEES) task protocol to systematically assess laryngeal motion abnormalities and pharyngeal function. A pilot study on 8 patients with MSA showed that the task protocol was feasible and well tolerated. Moreover, laryngopharyngeal dysfunction could be detected when applying the MSA‐FEES task protocol before becoming clinically evident. Furthermore, we found irregular arytenoid cartilages movements (iACM) to be present in all 8 patients with MSA and suggested that iACM could serve as a biomarker to identify MSA. 14
We here present results from the Laryngopharyngeal Findings in Atypical Parkinsonian Disorders (LAPD) study, where we applied the MSA‐FEES task protocol in a large cohort of patients with MSA and compared the results with an age‐matched cohort of patients with PD.
Methods
This prospective observational study was approved by the ethics committee of the Brandenburg Medical Board (S21[a]/2017) and the ethics committee of the University of Münster (2017‐585‐b‐S) and is registered in the Fox Trial Finder of the Michael J. Fox Foundation (www.foxtrialfinder.michaeljfox.org; trial number 005066).
Participants
All participants provided written informed consent. Included participants were either diagnosed with possible or probable MSA of MSA‐P or MSA‐C variant according to the second consensus criteria 4 or met the diagnosis of idiopathic PD according to the Movement Disorders Society criteria. 16 Participants were recruited at 2 German study centers specialized in the diagnosis and treatment of movement disorders. The occurrence of inspiratory stridor was assessed by history, and the sound of inspiratory stridor was imitated to improve recognition on patient and caregiver's side, as previously suggested. 15 In addition, stridor was noted when present during the clinical examination or the course of the inpatient stay.
Endoscopic Evaluation
All patients underwent FEES. Equipment consisted of a 3.9‐mm‐diameter (ENF‐VH, Olympus, Shinjuku, Japan) or 3.5‐mm‐diameter (Storz 11101 RP2, Karl Storz, Tuttlingen, Germany) flexible fiberoptic rhinolaryngoscope with a video processor (CV‐170, Olympus, Shinjuku, Japan), and processing software (rpSzene 10.7 g on Panel‐PC‐226/227, Rehder/Partner, Hamburg, Germany), or a 2.9‐mm‐diameter flexible fiberoptic rhinolaryngoscope (CMOS, Karl Storz, Tuttlingen, Germany) with a portable video processor (CMAC, Karl Storz, Tuttlingen, Germany) linked to a 19" flat screen monitor (9519NB, Karl Storz, Tuttlingen, Germany). FEES was performed as previously described. 14
FEES‐MSA Task Protocol
During FEES, all participants underwent a MSA‐FEES task protocol, allowing for a standardized endoscopic evaluation of the laryngeal and swallowing function. 14 In brief, the protocol is divided into an examination of the laryngeal function at rest and in action followed by an evaluation of swallowing. During laryngeal assessment, the larynx is observed at rest before performing VF abductor (inhalation, sniffing) and adductor tasks (full phonation of "eee"). To perform a positioning task of the VFs and potentially provoking action‐induced motion abnormalities, the participants were asked to silently pretend as if they would phonate an "eee," thus being able to assess VF motion in an adducted position without interference of vibration attributed to phonation itself (Table 1). Taking all laryngeal movement disorders into account that have previously been described in MSA, we defined the following 4 outcome measures: (1) VF motion impairment (VFMI), a unilateral and/or bilateral reduced mobility of the VFs during abduction and adduction maneuvers; (2) VF fixation (VFF), a unilateral and/or bilateral absence of VF motion during abduction and/or adduction maneuvers; (3) paradoxical VF motion (PVFM), an adduction of VFs during abduction maneuvers and vice versa (Fig. 1); and (4) iACM, an irregular jitter and flutter of the arytenoid region (see Video). Our hypothesis was that these laryngeal movement disorders have a higher prevalence in MSA when compared with PD. The procedure was recorded, and video analysis performed separately to the FEES procedure to assess laryngeal motion abnormalities. Raters were blinded to the patients' diagnosis.

| Task | Physiological Observation | Possible Findings in MSA |
|---|---|---|
| Normal breathing |
Inspiration: mild VF abduction Expiration: VF relaxation with mild VF adduction |
VFMI (uni‐/bilaterally reduced VF motion) VFF (uni‐/bilateral lack of respiration‐linked VF motion) PVFM (uni‐/bilateral inspiratory VF adduction with glottic narrowing) iACM (uni‐/bilateral irregular movements of the arytenoid cartilages |
| Fast and deep inhalation through mouth | VF abduction |
VFMI PVFM iACM premaneuver and/or postmaneuver |
| Inspiratory sniff through nose | VF abduction |
VFMI PVFM iACM premaneuver and/or postmaneuver |
| Phonation of “eee” | VF adduction |
VFMI iACM premaneuver and/or postmaneuver |
|
Imagined nonvoiced “eee” (“Prepare to say ‘eee’”) | VF adduction | iACM premaneuver, during, and/or postmaneuver |
| Sniff–“eee”–sniff–“eee” |
VF adduction/abduction VF diadochokinesis |
VFMI PVFM iACM premaneuver, during, and/or postmaneuver |
MSA, multiple system atrophy; VF, vocal fold; VFMI, vocal fold motion impairment; VFF, vocal fold fixation; PVFM, paradoxical vocal fold motion; iACM, irregular arytenoid cartilages movements.

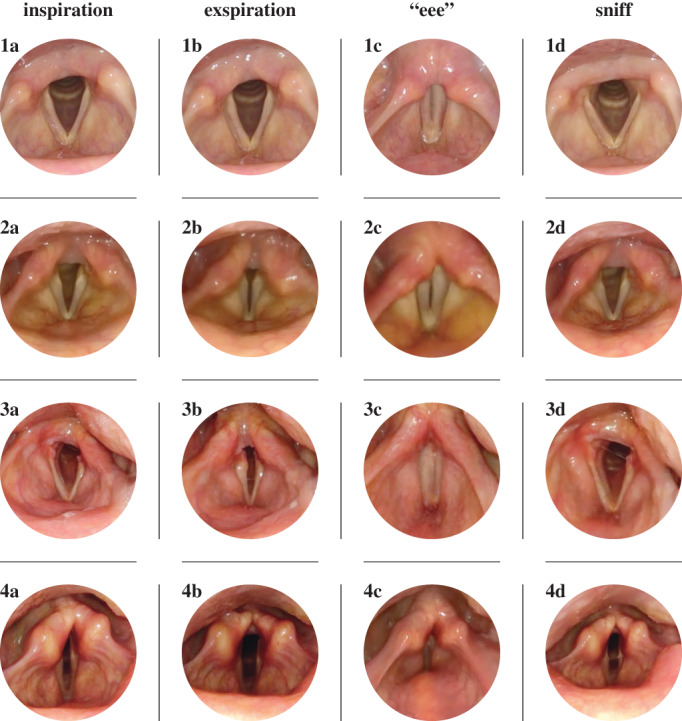
Vocal fold position during normal inspiration (a), normal expiration (b), phonation of "eee" (c), and sniffing (d) in a healthy subject (1) and patients with multiple system atrophy (MSA) (2,3,4). (1) Normal vocal fold motion with abduction during inspiration (a), slight adduction during expiration (b), near complete adduction during phonation (c), and near complete abduction during sniffing (d). (2) Example of vocal fold motion impairment (VFMI) in a patient with MSA with restricted vocal fold abduction during inspiration (a), pronounced vocal fold adduction during expiration (b), insufficient vocal fold adduction during phonation (c), and incomplete vocal fold abduction during sniffing (d). (3) Example of vocal fold fixation (VFF) in a patient with MSA with absent left vocal fold motion during inspiration (a), expiration (b), and sniffing (d) but normal vocal fold adduction during phonation (c). (4) Example of paradoxical vocal fold motion (PVFM) in a patient with MSA with paradoxical vocal fold adduction during inspiration (a), paradoxical vocal fold abduction during expiration (b), incomplete vocal fold adduction during phonation with consecutive activation of the false vocal folds (c), and paradoxical vocal fold adduction during sniffing (d).
Magnetic Resonance Imaging (MRI) Analysis
When available, cerebral MRI in the MSA cohort were visually analyzed for the occurrence of atrophy of the putamen, middle cerebellar peduncle, pons, and cerebellum according to the second consensus criteria for the diagnosis of MSA. 4
Statistical Analysis
Data were analyzed using Real Statistics Resource Pack software version 5.9.2. Distribution was calculated applying the Shapiro‐Wilk test, variance using the F test. Normally distributed values of homogeneous variance were compared using the nonpaired parametric t test, normally distributed values of inhomogeneous variance were compared using Welsh's t test. Not normally distributed values were compared applying the Mann‐Whitney U test. Point‐biserial correlation coefficient r pb was calculated to assess the relation between laryngeal findings in patients with MSA with age and disease duration. A chi‐square test was used to compare the frequency of laryngeal findings with MSA phenotypes and level of diagnostic certainty, respectively. The level of significance was set at 95% (α = 0.05).
Results
Between September 2017 and January 2020, 57 (35 women) consecutive patients with MSA (median age 64 [interquartile range, 59–71] years) were included and findings compared with an age‐matched cohort of 57 (28 women) patients with PD (age 67 [60–73] years). Patients with MSA had a shorter disease duration (4 [3–5] years in the MSA cohort vs 7 [5–10] years in the PD cohort; P < 0.0001), a higher disease severity expressed by the Hoehn and Yahr stage (4 [3–4] in the MSA cohort vs 3 [2–4] in the PD cohort; P < 0.0001) and were physically more impaired as expressed by the Unified Parkinson's Disease Rating Scale motor score (35.5 [29.8–41.8] in the MSA cohort vs 28 [19–36] in the PD cohort; P < 0.01) (Tables 2 and 3).

| Clinical Characteristics | MSA, n = 57 | PD, n = 57 | P |
|---|---|---|---|
| Women:Men | 35 : 22 | 28 : 29 | 0.19 |
| Age, y | 64 (59–71) | 67 (60–73) | 0.06 |
| Disease duration, y | 4 (3–5) | 7 (5–10) | <0.0001 |
| Disease severity, Hoehn & Yahr stage | 4 (3–4) | 3 (2–4) | <0.0001 |
| UPDRS I | 3 (2.0–4.3) | 4 (1–7) | 0.09 |
| UPDRS II | 17.5 (13.8–24) | 12 (7–17) | <0.0001 |
| UPDRS III | 35.5 (29.8–41.8) | 28 (19–36) | <0.01 |
Data are median (interquartile range).
MSA, multiple system atrophy; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale.

| n | 57 |
|---|---|
| MSA phenotype, n (%) | |
| Parkinsonian | 43 (75.4) |
| Cerebellar | 14 (24.6) |
| Diagnostic certainty, n (%) | |
| Probable | 24 (42.1) |
| Possible | 33 (57.9) |
MSA, multiple system atrophy.
To support the clinical diagnosis of MSA, we assessed cerebral MRI of the MSA cohort. In 49/57 patients with MSA, cerebral MRI was available. A total of 17 of 49 patients with MSA did not show any MSA‐associated changes on MRI; 18 had 1 characteristic MRI abnormality composed of atrophy either in the putamen, cerebellum, pons, or middle cerebellar peduncle. A total of 3 patients with MSA had 2, 10 patients with MSA had 3, and 1 patient with MSA showed 4 MSA‐characteristic MRI changes. The occurrence of pontine atrophy on MRI was correlated with longer disease duration (r pb = 0.322, P = 0.026) in contrast to all other MSA‐specific MRI changes (r pb ranging from −0.056 to 0.258, P ranging from 0.067–0.717).
Of the patients with MSA, 43.9% showed overt clinical symptoms of laryngeal dysfunction with inspiratory stridor, whereas no patient with PD had laryngeal dysfunction on clinical examination. During the MSA‐FEES laryngeal task assessment, 53/57 (93%) of patients with MSA demonstrated laryngeal dysfunction in contrast with only 1/57 (1.8%) patient with PD (P < 0.0001).
iACM were present in 52/57 (91.2%) of the patients with MSA, but were not observed in the PD cohort, resulting in a specificity of 1.0 and a sensitivity of 0.9. iACM were present at rest in 46/57 (80.7%) of the patients with MSA and could be provoked in an additional 6 patients with MSA during a positioning task (see Video). Of the patients with MSA, 43/57 (75.4%) presented with VFMI, with 38/57 (66.7%) at rest and an additional 5 during positioning maneuvers. Of the patients with MSA, 11/57 (19.3%) showed VFF (Fig. 2). A total of 10/57 (17.5%) of patients with MSA presented with only 1 of these 4 pathological findings during endoscopy, whereas 19/57 (33.3%) presented with 2, another 19/57 (33.3%) with 3, and 5/57 (8.8%) patients with MSA with 4 laryngeal movement disorders, respectively. The 1 patient with PD with laryngeal abnormalities during the MSA‐FEES task assessment showed VFMI. When analyzing the subgroup of 4 patients with MSA without laryngeal motion abnormalities on FEES, disease severity and physical impairment were similar to the rest of the MSA cohort, with a median Hoehn and Yahr of 3.5 (3–4) and a Unified Parkinson's Disease Rating Scale motor score of 32 (28.3–36.3). Also, the demographic data did not differ from the whole MSA cohort, with a median age of 62 (56.5–66) years and a disease duration of 5 (3.5–6) years.

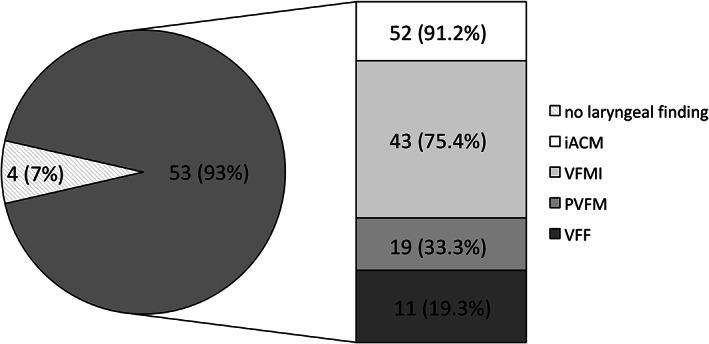
Frequency of laryngeal pathology in 57 patients with MSA. Data are n (percentage). iACM, irregular arytenoid cartilages movements; MSA, multiple system atrophy; PVFM, paradoxical vocal fold motion; VFMI, vocal fold motion impairment; VFF, vocal fold fixation.
In addition, we found no correlation of frequency of any laryngeal symptoms (iACM, VFMI, PVFM, or VFF) with disease duration (r pb ranging from −0.25 to −0.05; P from 0.06–0.92) or patients' age (r pb ranging from −0.04 to 0.12, P from 0.36–0.85). A subgroup analysis of patients with MSA with a very short disease duration revealed 4/57 patients with MSA affected for 1 year or less. All 4 exhibited laryngeal motion abnormalities; although iACM and VFMI was present in all 4, 2 had VFF, and 1 showed PVFM.
Furthermore, the frequency of laryngeal findings was independent of MSA phenotype (χ2 [4] = 3.96, P = 0.41, Cramér's V = 0.15) and a diagnostic level of certainty (χ2 [4] = 8.87, P = 0.06, Cramér's V = 0.22).
Discussion
In this prospective observational study, we applied a standardized MSA‐FEES task protocol and showed that laryngeal motion abnormalities can be detected during FEES in a relevant proportion of patients with MSA before becoming clinically evident. In our cohort, only 43.9% of patients with MSA presented overt clinical symptoms of laryngeal motion abnormality with inspiratory stridor. However, during MSA‐FEES task assessment, 93% of patients with MSA showed laryngeal motion abnormalities. In an age‐matched PD cohort, only 1 patient had laryngeal motion abnormalities on FEES despite the longer disease duration in this cohort.
With 91.2%, iACM were the most prevalent laryngeal finding in the MSA cohort. Although iACM were present in 80.7% during normal breathing, it could be provoked in another 10.5% of patients with MSA by performing a newly designed positioning task for the vocal folds. In addition to iACM, VFMI, VFF, and PVFM were present in the MSA cohort, with 43/57 of patients with MSA presenting with more than 1 laryngeal symptom on FEES.
There was a considerable discrepancy between the occurrence of overt clinical symptoms of laryngeal pathology with inspiratory stridor and actual laryngeal motion abnormalities observed during MSA‐FEES task protocol. Cortelli and colleagues 15 recently highlighted the difficulty of diagnosing the occurrence of inspiratory stridor in MSA. Although in our study we relied on the medical history for this symptom and lack video‐polysomnography confirmation, the occurrence of inspiratory stridor in our MSA cohort might be underrepresented. However, Ozawa and colleagues 17 noted that iACM predict the occurrence of severe glottic stenosis in patients with MSA and is therefore likely to antedate the clinical onset of inspiratory stridor, which could explain the occurrence of iACM without inspiratory stridor. Therefore, laryngeal function should be assessed as early as possible, even without clinical evidence of laryngeal motion abnormalities, when MSA is suspected. To further support this recommendation, we performed a subgroup analysis and showed that laryngeal motion abnormalities are present as early as 1 year after disease onset. In addition, the occurrence of laryngeal movement disorders was not correlated with disease duration or age and independent of MSA phenotype and level of diagnostic certainty.
We found MSA‐characteristic MRI changes in 32/49 patients with MSA, supporting the clinical diagnosis of MSA. However, our study lacks histopathological verification of the diagnoses, and undoubtedly, cardiovascular reflex tests and video‐polysomnography would have allowed to better characterize the MSA cohort. Nevertheless, our findings of iACM, VFMI, and VFF are in line with previous reports on single laryngeal symptoms in small MSA cohorts. 14 In contrast to single‐symptom evaluation, the MSA‐FEES task protocol allows for an identification of all MSA‐associated laryngeal movement disorders during 1 intervention. To our knowledge this is the first trial to systematically assess laryngeal dysfunction in MSA.
In addition, we performed a literature review and searched PubMed using the terms "multiple system atrophy" OR "Shy‐Drager Syndrome" OR "olivo‐ponto‐cerebellar atrophy” OR "striato‐nigral degeneration" AND “larynx" and identified 52 relevant articles between 1979 and 2020, with 44 case reports 11 , 13 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 or case series 9 , 14 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 (total number of patients 232), 10 studies 12 , 17 , 56 , 58 , 59 , 60 , 61 , 62 , 63 , 64 (total number of patients 328), and 2 reviews. 65 , 66
In summary, the case reports/series assessed the incidence of single laryngeal findings in patients with MSA either observed clinically during endoscopy or laryngeal electromyography.
Seven case reports/series 23 , 26 , 32 , 44 , 47 , 51 , 53 describe postmortem histopathological findings in laryngeal muscles, the recurrent laryngeal nerves or the nuclei ambigui, with results ranging from no motor neuron loss or laryngeal muscle denervation 23 to neuronal loss in the nucleus ambiguus, 26 , 51 , 53 small and large myelinated fiber loss in the recurrent laryngeal nerve, 32 , 44 and selective laryngeal muscle atrophy. 32 , 47
Five studies described the prevalence of single laryngeal findings with endoscopy during wakefulness and during induced sleep considering nocturnal stridor in a total of 81 patients with MSA 12 , 17 , 60 , 61 , 63 and, taken together, are in line with the variety of laryngeal movement disorders observed during systematic assessment in the MSA cohort investigated in our study.
Furthermore, VFMI was assessed in a retrospective analysis at the Mayo Clinic in Rochester. From all patients with MSA reporting voice or respiratory symptoms between 1975 and 2010 who also received otolaryngologic examination, 38 patients with MSA showed VFMI. 56 Unfortunately, Lalich and colleagues 56 omitted to provide a total number of patients with MSA who underwent laryngeal examination but did not show VFMI. Another study on 36 patients with MSA found VFMI in 17 patients with MSA. 64 The same study observed a unilateral VFF in 2 patients with MSA and bilateral VFF in the paramedian VF position in 1 patient with MSA.
PVFM were first described in a case report in 1989, 24 before other case reports followed, showing pathological coactivation of antagonizing laryngeal muscles on laryngeal electromyography. 11 , 13 , 38
iACM were first mentioned by Ward in 1981. 39 Simpson and colleagues found iACM in 3 of 6 (50%) patients with MSA during laryngoscopy. 41 Two studies observed iACM in 6 of 21 (28.6%) patients with MSA 61 and 18/28 (64.3%) patients with MSA, 17 respectively.
In comparison to previous reports the prevalence of laryngeal movement disorders observed in this cohort is higher, which might be related to the systematic assessment of laryngeal function with tasks provoking maximum VF movement and thereby revealing motion impairment.
One longitudinal observational study assessed the development of stridor in 104 patients with MSA without assessing laryngeal function. 59 Of the patients with MSA, 36/104 developed inspiratory stridor. Of the 33/36 patients with MSA with stridor that were available for follow‐up, 15 received treatment with either tracheostomy, laryngectomy, or continuous positive airway pressure (cPAP), resulting in a relative risk reduction from 2.998 to 0.147; whereas all patients in the untreated group died at the time of the survey, 12/15 who received intervention were still alive.
Six publications where identified addressing the impact of tracheostomy on survival in patients with MSA. 10 , 33 , 58 , 62 , 67 In the largest retrospective study (136 patients with MSA, 42 of whom with stridor), patients treated for stridor by either tracheostomy or cPAP had a longer median disease duration than those without treatment. Moreover, tracheotomized patients with MSA had a longer overall disease duration, longer disease duration after stridor onset, and longer disease duration after treatment compared with those treated with cPAP. 67 One study on 49 pathology‐confirmed patients with MSA showed that tracheostomy reduced the risk of death (hazard ratio, 0.21; 95% confidence interval, 0.08–0.56; P < 0.01) and sudden death (hazard ratio, 0.15; 95% confidence interval, 0.02–0.98; P < 0.05) in MSA. 68
Kurisaki and colleagues 58 reported 21 patients with MSA of whom 11 underwent tracheostomy. Although all patients without tracheostomy had deceased after a median of 6.8 years from disease onset, 4/11 patients with MSA who underwent tracheostomy were still alive at the time of the survey. Jin and colleagues 62 reported the impact of tracheostomy on sleep‐disordered breathing in MSA, performing a multivariate model analysis with independent variables including age at onset, gender, body mass index, disease duration and severity, and the presence of a tracheostoma and revealed that the occurrence of severe sleep‐disordered breathing was associated only with the presence of a tracheostoma, even after adjustment for disease duration (P < 0.05). Another study reported 2 patients with MSA with nocturnal stridor and 2 patients with MSA without nocturnal stridor who underwent tracheostomy. Although both tracheotomized patients with MSA with stridor were alive 1.9 and 7 years at the time of the publication, the other 2 patients died within 1 year of tracheostomy. 10
The underlying pathology of laryngeal symptoms in MSA still remains under debate. Initial reports discussed inspiratory stridor resulting from VF abductor paralysis, 9 , 19 , 20 , 44 , 50 , 69 , 70 supported by laryngeal electromyography data showing marked atrophy of the posterior cricoarytenoid muscles. 19 , 20 One autopsy study revealed large myelinated fiber loss of the recurrent laryngeal nerve in 1 MSA patient with abductor palsy. 44 These findings are opposed by more recent reports of dystonic innervation of laryngeal muscles with simultaneous coactivation of VF abductor and adductor muscles during inspiration. 49 , 55 , 57 , 71 These results are supported by another autopsy study that did not find focal changes of the recurrent laryngeal nerve and innervated muscles. 20
Whether laryngeal abnormalities allow for delineation of MSA from 4R‐tauopathies remains to be addressed. To date there are very few reports on laryngeal pathology in 4R‐tauopathies, indicative of a very low prevalence. Isozaki and colleagues 43 performed a postmortem investigation on laryngeal muscles in 41 patients with neurodegenerative disorders, including 4 patients with progressive supranuclear palsy (PSP). In none of the patients with PSP were myogenic changes in the investigated laryngeal muscles present. One case report describes laryngeal dystonia in progressive supranuclear palsy. 72 Reports of other laryngeal findings lack so far.
Although VFMI and VFF can be detected in a variety of underlying causes, including neurological, structural, infectious, or traumatic pathology, 73 iACM have to our knowledge not been reported in any disease other than MSA. 14 , 17 , 39 , 41 , 61 With a specificity of 1.0 and a sensitivity of 0.9, iACM could be regarded as a valuable clinical marker for MSA allowing for delineation from PD. It is therefore tempting to attribute iACM as a diagnostic biomarker. Without pathological confirmation of the diagnosis and demonstration of parallel worsening of the disease and this laryngeal finding, however, this would be precipitous. Further studies in a larger, thoroughly characterized MSA cohort with pathology confirmation of the diagnosis should investigate onset and evolvement of laryngeal movement disorders in patients with MSA. In addition, the occurrence of laryngeal movement disorders should be compared with other MSA symptoms to assess their relationship. Furthermore, a systematic assessment of laryngeal pathology in patients with 4R‐tauopathies is needed to investigate whether laryngeal findings allow for delineation of MSA also from this disease entity.
An international multicenter study under guidance of the Movement Disorders Society MSA study group is under way to collect longitudinal data on laryngopharyngeal findings in patients with MSA using the MSA‐FEES task protocol. In addition, systematic laryngeal assessment with the FEES task protocol will also be performed in patients with 4R‐tauopathies and the results compared with patients with MSA and PD.
The detection of iACM during FEES might contribute to improving the diagnostic certainty of MSA in delineation to PD, regardless of MSA phenotype, disease duration, or patient's age. FEES is becoming increasingly available in neurology departments worldwide, 74 and we therefore suggest that whenever MSA is suspected, patients should undergo a systematic and standardized endoscopic MSA‐FEES task protocol assessment of laryngeal function to improve diagnostic certainty of MSA and delineation from PD, taking other clinical signs into account. Furthermore, we suggest that endoscopic laryngeal findings, above all the occurrence of irregular arytenoid cartilages movements, should be implemented into the next revised diagnostic criteria for MSA.
Data Sharing Statement
The study protocol, statistical analysis, informed consent form, and study data, including deidentified participant data, will be made available to others with publication upon formal request and receipt of a signed material transfer agreement. Requests should be directed to the corresponding author. Data will only be shared via individual secured network connections.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
F.G.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
A.V.: 1B, 1C, 2B, 3A, 3B
I.C.: 1C, 3B
S.A.: 1C, 3B
D.G.: 1C, 3B
H.‐J.H.: 1C, 3B
R.D.: 1C, 3B
G.E.: 1C, 2C, 3B
T.W.: 1B, 1C, 2B, 2C, 3B
Full financial disclosures for the previous 12 months
F.G. reports honoraria from AbbVie Pharma, Merz Pharma, and BIAL Pharma outside the submitted work. A.V., S.A., D.G., and H.‐J.H. report no conflicts of interest. I.C. reports honoraria from AbbVie Pharma outside the submitted work. R.D. reports honoraria from Bayer AG, Boehringer Ingelheim Pharma, Daiichi‐Sankyo GmbH, Nestlé S.A., Olympus, Pfizer Pharma, and Sanofi‐Aventis GmbH outside the submitted work. G.E. reports honoraria for advisory boards from AbbVie Pharma, BIAL Pharma, Desitin Pharma, STADA Pharma, Neuroderm Inc.; honoraria from AbbVie Pharma, BIAL Pharma, Britannia Pharma, Desitin Pharma, Licher GmbH, UCB Pharma, Zambon Pharma; royalties from Kohlhammer Verlag and Thieme Verlag. T.W. reports honoraria from Biogen, BIAL Pharma, and Licher Pharma; consultancies from Abbvie Pharma and Zambon Pharma; and funding from Abbvie Pharma for the Parkinson‘s Network Muensterland+ outside the submitted work.
Acknowledgment
We thank Nadine Külzow for support with statistical data analysis.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
 Laryngeal Movement Disorders in Multiple System Atrophy: A Diagnostic Biomarker?
Laryngeal Movement Disorders in Multiple System Atrophy: A Diagnostic Biomarker?
