
- Altmetric
This guidance provides clear, concise strategies for identifying coronaviruses by transmission electron microscopy of ultrathin sections of tissues or infected tissue cultures. These include a description of virus morphology as well as cell organelles that can resemble viruses. Biochemical testing and caveats are discussed. Numerous references provide information for documentation and further study.
Numerous articles have been published on the histology and ultrastructure of pathology in patients with coronavirus disease 2019, and in an attempt to account for tissue damage and disease pathology, the hunt by electron microscopy for actual virus particles has been extensive. However, coronaviruses are complex and can resemble normal subcellular organelles. Many investigators have been misled into attributing organ damage to the presence of severe acute respiratory syndrome coronavirus 2 identified (incorrectly) by electron microscopy in the tissue, while the structures described have been simply normal subcellular organelles.
Description and discussion
The following proposed best practices for the identification of coronaviruses are offered to aid the investigator, who is not necessarily a virologist, in accurately recognizing or ruling out the existence of coronavirus. An extensive reference list is also provided to document the true appearance of coronaviruses and to demonstrate pitfalls of misidentification.
(i)Coronaviruses (Figure 1
) are enveloped viruses (as opposed to naked viruses) (i.e., they are surrounded by a covering derived from host membranes [with some viral proteins inserted]). They obtain this envelope by budding through the endoplasmic reticulum/Golgi apparatus. Thus, intracellular coronaviruses will be found inside larger vesicular/vacuolar structures and not as free single virions inside the cytoplasm. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–infected Vero cell containing abundant viral particles held within intracellular vesicular/vacuolar structures (arrows). Bar = 1 μm. Inset: Higher magnification of intracellular SARS-CoV-2 particles with cross sections through the helical nucleocapsid visible as internal black dots. Bar = 200 nm. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
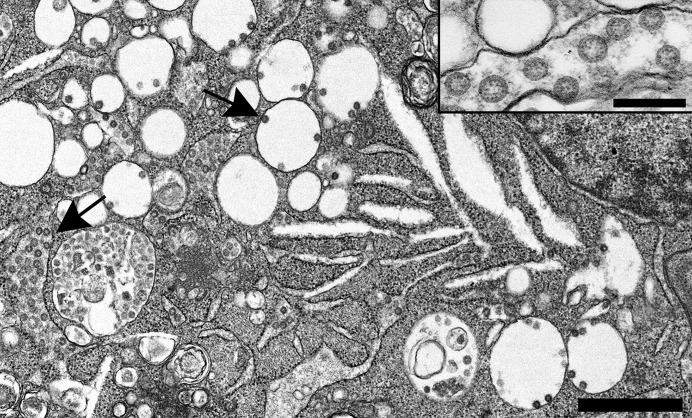
(ii)Coronaviruses do have projections on the surface; however, in thin sections, the “spikes” on the outside are not always (indeed, not usually) clearly visible, unless specially stained (e.g., with tannic acid). They may or may not appear as a very short “fuzz.”
(iii)Because intracellular coronaviruses are always grouped inside a vacuole formed by the endoplasmic reticulum/Golgi complex, if spikes are visible, they face the vacuolar contents, not the cell cytosol.
(iv)Viruses that have been released to the extracellular space by exocytosis (fusion of the vacuole with the plasma membrane) have their spikes facing the extracellular space (outside the cell).
(v)Coronaviruses have a helical nucleocapsid (like a coiled phone cord), and the helix is loosely curled up inside the viral envelope; when cut in cross section, it looks like small electron-dense dots (∼6–12 nm in diameter) inside the virus particle.
(vi)Because of the soft pliable covering, coronavirus particles are not all the same size and vary from ∼60 to 140 nm in diameter. However, they are not in the ≥200-nm range like the large paramyxoviruses—a different family of viruses.
(vii)Other vesicular and double-membrane structures are involved in virus production and can be seen in infected cells.
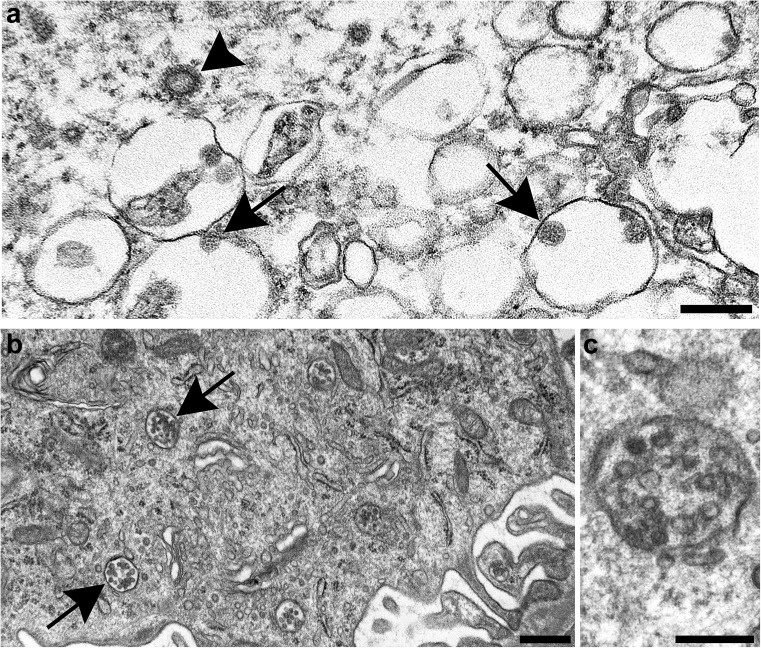
(viii)Several common subcellular organelles can mimic coronavirus, the most obvious being clathrin- or coatomer-coated vesicles (Figure 2
a) and multivesicular bodies (Figure 2b), both involved in cell protein transport. Others include vesiculating rough endoplasmic reticulum, Golgi vesicles, lysosomes, and (extracellular) glycocalyceal bodies. Normal subcellular organelles mimicking coronavirus. (a) Clathrin-coated vesicle free in the cytoplasm of a cell (arrowhead) and nearby severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) particles inside intracellular vacuoles (arrows). Cross sections through the viral nucleocapsid are visible in the SARS-CoV-2 particles (small dots inside the virus). Bar = 200 nm. (b) Membrane bound collections of vesicles (arrows) making up multivesicular bodies (MVBs) within the cytoplasm. Bar = 500 nm. (c) Higher magnification of an MVB; note the absence of dots inside the vesicles corresponding to cross sections through the viral nucleocapsid. Bar = 200 nm. Figure 2b and c are courtesy of Dr. Ricardo Vancini. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Although a few authors have demonstrated coronaviruses directly in the lung,1, 2, 3, 4 numerous investigators have misinterpreted viral imposters to be coronaviruses5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 (though Su et al. have since modified their original description of coronaviruses to “virus-like particles” [personal communication, 2020]). Not all small round-to-ovoid things are viruses or even “virus-like.” Essential skills to recognize the presence of these agents, particularly enveloped ones, include (i) expertise in virus morphology (what they look like), (ii) knowledge of virus morphogenesis (how they are constructed), and (iii) knowledge of normal subcellular organelles that can resemble them. Even then, it may be difficult, based solely on appearance, to identify unequivocally the presence of coronaviruses versus look-alikes,21 , 22 particularly in tissue that is not well fixed (e.g., autopsy material). Care should be taken to educate oneself, not only on the appearance of viruses in general23, 24, 25, 26, 27 and coronaviruses specifically,6 , 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 but also on the potential pitfalls in mistaking round structures for infectious particles.6 , 23 , 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47
Furthermore, nucleic acid and antibody testing of bulk tissue does not conclusively prove the presence of infective coronavirus, nor does it document that the detected RNA or protein is in the organ parenchyma (as opposed to in the blood in the tissue); rather, it only shows that viral components are present, but not in which cells and not whether they are actually being assembled there. If morphological identification is equivocal, only by (i) viewing actual viral morphogenesis in the tissue, (ii) using immunoelectron microscopical staining or ultrastructural in situ hybridization of virus,48 , 49 or (iii) electron microscopical visualization of particles in situ in tissue where there is biochemical evidence of viral presence (e.g., on-the-slide-embedding37 , 50 , 51 of virus-labeled paraffin-embedded tissue sections that have been labeled for coronavirus by either immunohistochemistry or in situ hybridization) can one conclusively determine that coronaviruses are growing in cells (as opposed to being simply present in circulating blood).
Summary
Intracellular coronaviruses are 60- to 140-nm round-to-ovoid particles, with dark 6- to 12-nm dots inside, and will be contained inside a vacuole; spikes (if visible) will touch the vacuolar contents, not the cell cytosol, and will not be as prominent as those on clathrin-coated vesicles. Extracellular virus particles, released through the plasma membrane by exocytosis, will cling to the cell surface and may be more likely to show surface projections, and the spikes will face the extracellular space. Thus, the ultrastructural findings in tissue described in many of the published articles do not confirm the presence of coronavirus directly in tissue and, when in doubt, investigators should consult an ultrastructural virologist or one of the virology authors listed.6 , 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 4 , 47, 48, 49
Disclosure
All the authors declared no competing interests.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
Acknowledgments
The authors received National Institutes of Health Instrumentation funding 1S10OD026776-01, “Transmission electron microscope (TEM).” We thank Natalie Thornburg, Azaibi Tamin, and Jennifer Harcourt for providing severe acute respiratory syndrome coronavirus 2–infected Vero cell cultures. Additionally, we thank Dr. Ricardo Vancini for providing Figure 2b and c and Dr. David Howell for critically reviewing this manuscript.
The findings and conclusions are those of the authors and do not necessarily represent the position of the US Centers for Disease Control and Prevention.
 Best practices for correctly identifying coronavirus by transmission electron microscopy
Best practices for correctly identifying coronavirus by transmission electron microscopy