These authors contributed equally to this work.
- Altmetric
A chemo‐, regio‐, and stereoselective mono‐hydroamidation of (un)symmetrical 1,3‐diynes is described. Key for the success of this novel transformation is the utilization of an advanced palladium catalyst system with the specific ligand Neolephos. The synthetic value of this general approach to synthetically useful α‐alkynyl‐α, β‐unsaturated amides is showcased by diversification of several structurally complex molecules and marketed drugs. Control experiments and density‐functional theory (M06L‐SMD) computations also suggest the crucial role of the substrate in controlling the regioselectivity of unsymmetrical 1,3‐diynes.
The advanced Pd catalyst system with Neolephos as a ligand enables highly selective hydroamidation of unbiased (un)symmetrical 1,3‐diynes. In this way, a wide range of synthetically useful α‐alkynyl‐α,β‐unsaturated amides are afforded in good to high yields with excellent chemo‐, regio‐, and stereoselectivities.
Introduction
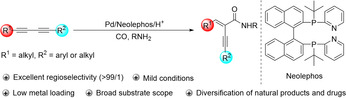
Transition metal catalyzed carbonylation reactions belong to the important examples of industrially applied homogeneous catalytic reactions. Since their discovery, they became a powerful tool for the synthesis of numerous value‐added carbonyl containing compounds. [1] Thus, carbonylation reactions are frequently applied in organic synthesis due to the versatility of the carbonyl groups and the possibility to easily expand carbon chains. [2] Within this class of reactions, hydroamidations, also called aminocarbonylations, represent a straightforward route for the conversion of olefins or alkynes, carbon monoxide and amines into the corresponding amides. Compared to the well‐explored aminocarbonylation of alkenes using catalytic systems based on cobalt,[ 3a , 3b ] nickel, [3c] iron, [3d] ruthenium, [3e] rhodium [3f] and mainly palladium,[ 3g , 3h , 3i , 3j , 3k , 3l ] the related reactions of alkynes leading to α, β‐unsaturated amides have received much less attention.
α, β‐Unsaturated amides are valuable intermediates and building blocks in organic synthesis and are used as functional molecules in material science. [4] In addition, they are occurring in natural products and bio‐active pharmaceuticals. [5] Traditionally, they are prepared by nucleophilic condensation of carboxylic acid derivatives with amines in the presence of stoichiometric amounts of activating or coupling reagents. [6] Obviously, such processes result in significant waste formation. In this context, the aminocarbonylation of alkynes can be a more atom‐economic and benign methodology. However, aminocarbonylations of alkynes (and olefins) are intrinsically challenging due to the problems associated with the acidity of the active metal hydride catalysts and the basicity of the amine reagent. Moreover, alkynes are prone to side reactions such as hydroamination and oligomerization/polymerization. Nevertheless, by selecting appropriate catalysts and optimization of reaction conditions notable progress was achieved in the past decades. [7]
So far, almost all these reactions are restricted to terminal alkynes, which can be easily controlled to afford branched[ 7c , 7d , 7e , 7f , 7g , 7h , 7i , 7j , 7k , 7l , 7m , 7n , 13 ] or linear[ 7h , 7m , 7n , 7o , 7p , 7q ] α,β‐unsaturated amides (Scheme 1 a). In fact, only few aminocarbonylations of “simple” unsymmetrical internal alkynes were reported,[ 7o , 7s ] leading to unsatisfactory regioselectivity (Scheme 1 b). Clearly, carbonylations of unsymmetrical internal alkynes are more demanding because of their low reactivity and the difficulty in achieving high regioselectivity. Notable exceptions include the recent works on hydroformylation and alkoxycarbonylation reported by the groups of Breit, [8a] You [8b] and Ma, [9] respectively. In these studies, regioselectivity could be controlled by hydrogen bonding interactions between substrates and catalysts, steric hinderance and/or chelation.

Selected catalytic hydroamidations of alkynes.
Based on our continuous interest in carbonylation reactions, recently we started to investigate the selective carbonylation of unsymmetrical 1,3‐diynes, which can be conveniently generated via alkyne coupling processes [10] and provide synthetically highly useful intermediates. Obviously, the selective hydroamidation of unsymmetrical 1,3‐diynes compared to internal monoalkynes implies further challenges: 1) due to their higher reactivity competitive hydroamination also becomes potentially easier; [11] 2) the two unbiased alkyne moieties of unsymmetrical diynes create more complex selectivity problems, including chemo‐, stereo‐, and regioselectivity. [12] Thus, it is not surprising that to the best of our knowledge no such process has been reported, yet. In fact, only in 2018 Huang [13] and co‐workers reported the first aminocarbonylations using symmetrical 1,3‐diynes. The need of a comparably high palladium catalyst loading (5 mol %), and relative harsh conditions (140 °C) offers also potential for improvements.
Herein, we present the first examples of regioselective Pd‐catalyzed hydroamidation of unsymmetrical 1,3‐diynes. Notably, the mild conditions can also be applied in various symmetrical substrates and a wide range of α‐alkynyl‐α, β‐unsaturated amides are obtained in general in good yields with excellent selectivities (Scheme 1 c).
In the past three years, inspired by the seminal work of Drent and co‐workers,[ 7d , 14 ] we prepared several novel bidentate pyridyl‐substituted phosphine ligands. [15] Some of these ligands showed significantly improved performance for Pd‐catalyzed alkoxycarbonylations of olefins or alkynes. Mechanistic studies revealed that the nitrogen atoms of the pyridyl groups act as proton shuttle to accelerate the nucleophilic attack on the intermediate Pd acyl complex and thereby increasing the rate of the overall transformation. [16] We envisioned that these ligands might also improve the reactivity in Pd‐catalyzed aminocarbonylations. Hence, in our initial studies we compared the influence of these and related ligands for the hydroamidation of 1‐(5‐(trimethylsilyl)penta‐2,4‐diyn‐1‐yl)pyrrolidine‐2,5‐dione (1 a) with aniline at room temperature.
Results and Discussion
As shown in Scheme 2, applying 1,1′‐ferrocenediyl‐bis(tert‐butyl(pyridin‐2‐yl)phosphine) L1 a highly selective monocarbo‐nylation (regioselectivity: >99/1) occurred leading to product 3 aa. Unfortunately, the reactivity of this system is too low (15 % yield). In the presence of other pyridyl‐substituted ligands such as 1,3‐bis(tert‐butyl(pyridin‐2‐yl)phosphanyl)‐propane L2, 1,4‐bis(tert‐butyl(pyridin‐2‐yl)phosphanyl)butane L3, 1,2‐bis((tert‐butyl‐(pyridin‐2‐yl)phosphanyl)methyl)benzene L4 and 2,2′‐((2,7‐di‐tert‐butyl‐9,9‐dimethyl‐9H‐xanthene‐4,5‐di‐yl)bis‐(tert‐butylp‐hosphanediyl))dipyridine L5, under these mild conditions no conversion is observed, although most of these ligands have been proved to be active in Pd‐catalyzed alkoxycarbonylations. Surprisingly, using 2,2′‐bis(tert‐butyl(pyridin‐2‐yl)phosphaneyl)‐1,1′‐binaphthalene L6 (Neolephos), the desired product 3 aa was afforded in 84 % yield and with excellent regioselectivity (>99/1). In addition, the corresponding acid, which is generated via hydroxycarbonylation, was isolated as a side product in 16 %. It should be noted that although ligand L6 showed high chemoselectivity for mono‐alkoxycarbonylation of 1,3‐diynes, it was not the most efficient ligand in Pd‐catalyzed alkoxycarbony‐lation of dienes [15d] or diynes. [15e] Commercially available ligands L7–L16, including mono and bidentate phosphine ligands, which are commonly used in other carbonylations, [17] were examined, too; however, in all cases no product was detected under the standard conditions. Comparison of L6 with L1–L5 demonstrated that the backbones of ligand, which may lead to different bite angles in Pd complexes, are also crucial for this transformation. To improve the benchmark reaction further, we evaluated the influence of critical reaction parameters in the presence of L6 (for more details, see Table S1–S6 in SI). It is worth pointing out that there was no reaction without acid or in the presence of weak acid (HOAc or PhCO2H, Table S2), indicating the importance of the strong acid for the generation of catalytically active Pd hydride species (Figure 1). As a result, the formation of the acid by‐product could be prevented using triflic acid instead of PTSA⋅H2O.

Relative energy of the active catalysts as well as corresponding alkyne complexes of substrates 9 and 10.

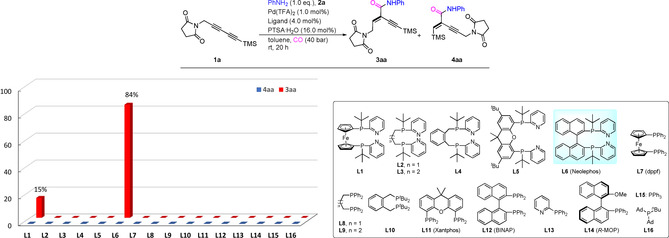
Pd‐catalyzed aminocarbonylation of the unsymmetrical 1,3‐diyne 1 a: Influence of phosphine ligands. Reaction conditions: 1 a (0.25 mmol), Pd(TFA)2 (1.0 mol %), ligand (4.0 mol % for L1‐L12, and 8.0 mol % for L13‐L16), PTSA⋅H2O (16.0 mol %), PhNH2 (2 a, 0.25 mmol), CO (40 atm), 23 °C and toluene (1.0 mL), 20 h. The ratio of 3 aa/4 aa and yield were determined by GC and GC‐MS analysis.
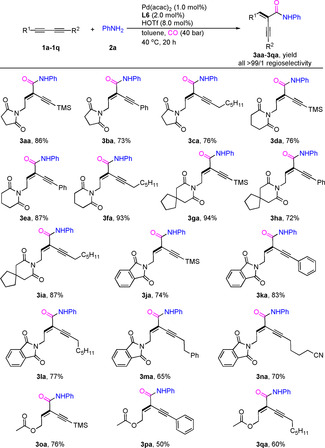
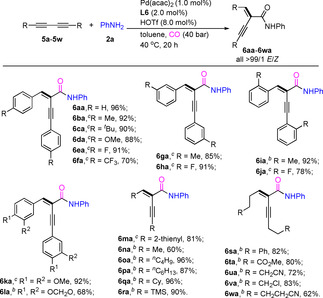
With optimized reaction conditions established, we set up to explore the scope of different unsymmetrical 1,3‐diynes using 2 a as the nucleophile. As shown in Table 1, the catalytic system can be conveniently applied to various unsymmetrical 1,3‐diynes derivatized from propargylamine or alcohol. In general, good to high yields (50–94 %) were obtained with substrates based on succinimide, glutarimide or phthalimide derivatives (3 aa–3 na). While the corresponding free propargylic alcohol derivative gave no clean conversion, the acetylated substrates 1 o–1 q reacted smoothly and gave the corresponding amides in 50–76 % yields. Notably, in all these cases excellent regioselectivity (>99/1) was observed and exclusive formation of the E‐regioisomers took place. In addition, the reaction of 1,3‐diyne bearing two different aromatic substituents also proceeded well to afford the corresponding product in 86 % yield with low regioselectivity (60/40) (Scheme S1). Apart from unsymmetrical 1,3‐diynes, a variety of symmetrical substrates provided the corresponding α‐alkynyl‐α, β‐unsaturated amides under very mild conditions, too (Table 2). In fact, without further optimization products 6 aa–6 ma were obtained in 68–96 % with excellent stereoselectivity. More specifically, aromatic 1,3‐diynes 5 a–5 f with either electron‐donating (OMe, Me, tBu) or electron‐withdrawing (F, CF3) substituents on the phenyl ring provided 6 aa–6 fa in high yields (70–96 %) and excellent selectivity. Substituents in the ortho‐position of the phenyl ring have no significant influence on both reactivity and selectivity of this reaction; thus, 6 ia–6 ja were produced in 78–92 % yield. In addition, the thiofuryl‐substituted substrate was well tolerated by the catalyst to afford 6 ma. Next, the reactivity of aliphatic 1,3‐diynes was investigated. Gratifyingly, also in these cases the palladium‐catalyzed hydroamidation proceeded selectively, affording carbonylative products 6 na–6 wa in 60–96 % yields. It should be noted that the obtained products may undergo isomerization processes in the presence of palladium hydride species; but no side‐products were observed in all cases. Furthermore, functional groups such as cyano, ester and trimethylsilyl survived in products 6 ra and 6 ta–6 wa.

|
|
Reaction conditions: 1 (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %) HOTf (8.0 mol %), aniline (2 a, 0.25 mmol), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown.

|
|
[a] Reaction conditions: 5 (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %) HOTf (8.0 mol %), aniline (2 a, 0.25 mmol), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown. [b] 60 °C. [c] PTSA⋅H2O (16.0 mol %) was used instead of HOTf.
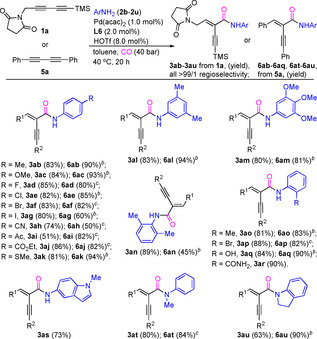
Next, we evaluated the scope of this novel hydroamidation process with respect to the reactivity of amines using unsymmetrical (1 a) and symmetrical (5 a) 1,3‐diyne as a standard coupling partner, respectively (Table 3). A variety of arylamines with electron‐neutral, electron‐deficient, and electron‐rich substituents led to the corresponding carbonylative products in good yields (51–90 %) and again with excellent regioselectivities (>99/1). Apparently, the substituents on the arylamines have no real impact on the catalysis. Specifically, the reactions of arylamines bearing Me, F, Cl, Ac, CN, CO2Et, MeO and MeS substituents proceeded smoothly, providing the corresponding products 3 ab–3 ae, 3 ah–3 ak in good isolated yields. [19] Notably, bromine‐ and even iodine‐substituted arylamines, which are known to be sensitive to palladium catalysis, also worked well and afforded the corresponding products (3 af–3 ag). The position of substituents on the phenyl ring has no influence on the reaction outcome. Hence, arylamines 2 n–2 p afforded 3 an–3 ap in 81–89 % yields. Interestingly, the 2‐aminophenol (2 q) and 2‐aminobenzamide (2 r), which contain two nucleophilic positions, also reacted selectively to give the amides 3 aq and 3 ar in 84 % and 90 % yield, respectively. Furthermore, the heterocycle‐substituted amine 2 s proved to be a viable coupling partner and gave 3 as in 73 % yield. While secondary aromatic amines (2 t and 2 u) gave the desired products in good yields (3 at and 3 au), in case of benzyl amine or n‐butylamine no product was detected under these conditions. It should be noted that almost all these tested amines have similar behavior in the hydroamidation of symmetrical 1,3‐diynes 5 a, providing products 6 ab–6 aq and 6 at–6 au in 45–94 % yield. Unfortunately, there was no conversion at all when aliphatic amines such as benzylic amine or n‐pentylamine was used instead of aniline (Scheme S2), and this is probably due to the higher basicity of aliphatic amines than aniline. [20]

|
|
[a] Reaction conditions: 1 a or 5 a (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %) HOTf (8.0 mol %), arylamines (2, 0.25 mmol), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown. [b] 60 °C. [c] PTSA⋅H2O (16.0 mol %) was used instead of HOTf.
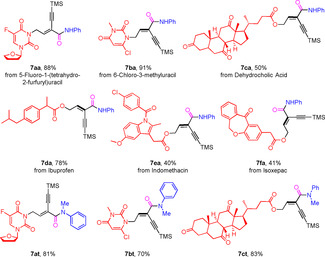
In general, our methodology vide supra allows to functionalize aromatic amines to the corresponding enyne amides in a straightforward manner under very mild conditions. We thought that this unusual transformation, which also increases the molecular complexity, can be of interest for many life science applications including the diversification of natural products and synthetic drugs. Notably, the resulting 1,3‐enyne motif can be additionally modified in several ways. [18] In this respect, the hydroamidation of several structurally more complex molecules was studied to prove functional group tolerance and compatibility with bio‐relevant derivatives (Table 4). We were particularly delighted to find that this Pd‐catalyzed regioselective hydroamidation of 1,3‐diynes progressed well with substrates 7 a and 7 b derivatized from uracil, one of the nucleobases of RNA, and furnished products 7 aa and 7 ba in high yields and excellent regioselectivity. Compound 7 c, containing dehydrocholic acid, which is a drug for stimulation of biliary lipid secretion, could also be applied to afford the amide product 7 ca in decent yield. Moreover, nonsteroidal anti‐inflammatory drugs such as ibuprofen, indomethacin and isoxepac are viable components for 1,3‐diynes substrates 7 d–7 f in this transformation. Finally, the products 7 at–7 ct were prepared from the corresponding secondary aniline.

|
|
[a] Reaction conditions: diynes (0.25 mmol), arylamines (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %), HOTf (8.0 mol %), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown and in all cases the regioselectivities are >99/1.
To understand the observed regioselectivity and to gain more mechanistic insights, several control experiments with unsymmetrical 1,3‐diynes were conducted. As shown in Scheme 3, the different substituents on the diyne unit control the regioselectivity of the carbonylation reaction to a significant extent. In general, the following selectivity order is observed: TMS ≤ prim. alkyl < aryl ≪ CH2OAc ≈ CH2NR2. Hence, testing 8 with our catalyst under standard conditions, aminocarbonylation proceeded preferentially at the phenyl‐substituted alkyne group (regioselectivity: 70/30) [Eq. (1)]. However, applying propargylamine/alcohol derivatives, hydroamidations regioselectively took place at the triple bond substituted with these functional groups. As an example, the protected propargyl alcohol derivative 9 was synthesized and gave the amide product 9 b in 56 % yield with >99/1 regioselectivity [Eq. (2)], which demonstrates the crucial role of the oxygen atom for determining the regioselectivity.

![Pd‐catalyzed hydroamidation of 1,3‐diynes: Control experiments. Standard conditions: diynes (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %) HOTf (8.0 mol %), aniline (0.25 mmol), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown and the ratios of regioisomers were determined by GC and GC‐MS analysis as well as 1H NMR analysis. [a] 70 °C.](/dataresources/secured/content-1765820637387-7e810198-a558-4dfe-a55b-fed34b378329/assets/ANIE-60-371-g006.jpg)
Pd‐catalyzed hydroamidation of 1,3‐diynes: Control experiments. Standard conditions: diynes (0.25 mmol), Pd(acac)2 (1.0 mol %), L6 (2.0 mol %) HOTf (8.0 mol %), aniline (0.25 mmol), CO (40 atm), 40 °C in toluene (1.0 mL), 20 h. Yields of isolated products are shown and the ratios of regioisomers were determined by GC and GC‐MS analysis as well as 1H NMR analysis. [a] 70 °C.
Interestingly, the steric nature of the substituents on the alkyne also plays a decisive role. Hence, the regioselectivity is increased to 98/2 when trimethyl(phenylbuta‐1,3‐diyn‐1‐yl)silane 10 was used [Eq. (3)] instead of 8. In order to figure out, whether the carbonyl group plays a decisive role in the control of regioselectivity, the unsymmetrical 1,3‐diyne 11 without nitrogen atom but with carbonyl group was prepared and tested. Here, product 11 a was obtained with a completely different regioselectivity [Eq. (4)], which disproved the involvement of the carbonyl group in the determination of the regioselectivity.
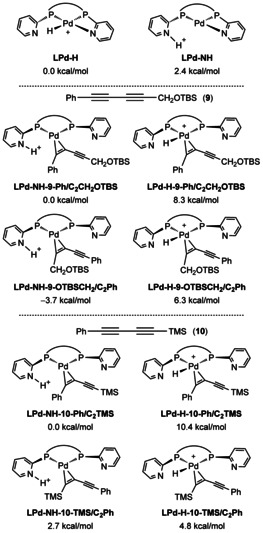
To understand the experimentally observed regioselectivity further, density functional theory computations using different methods in gas phase as well as in solution with and without dispersion corrections were carried (see the SI for more details). If not otherwise mentioned, we used the M06L/TZVP computed Gibbs free energies under the consideration of solvation effect (toluene) on the basis of the B3PW91/TZVP gas phase optimized geometries for discussion and other results are given in the SI for comparison. In our study we chose substrates 9 and 10 using the complex of palladium and ligand L6 as active catalyst resulting in totally opposite regioselectivity. These results are further established with those of substrate 1 a. Due to the chirality of the ligand and the possible diastereomers of the formed complex, we used the C 2‐symmetrical (R,R)‐ligand for our computation with the nitrogen atoms of the pyridyl groups in the Pd complex pointing towards the Pd center. This enables a hemilabile coordination of the ligand; and such complexation modes have been found in the molecular structures of similar complexes. [16a]
Since the regioselectivity of metal‐catalyzed carbonylation reactions of non‐symmetrical C=C or C≡C bonds comes from the M‐H insertion resulting in the formation of alkyl or alkenyl complex, we computed this elementary step to elucidate the origin of the regioselectivity. For the protonated active catalyst (Figure 1), there are two stable isomers, and the one with proton on the Pd center (LPd‐H) is more stable than the one with proton on the N atom of one 2‐pyridyl ring (LPd‐NH) by 2.4 kcal mol−1. Such small energy difference reveals the possibility of their mutual exchange upon the change of the coordination sphere. It is noted that LPd‐NH is not stable und has been optimized directly to LPd‐H at the MN15/TZVP level. In both complexes, one 2‐pyridyl nitrogen is coordinated to the Pd center. With the coordination of substrate 9, it shows that complexes with N‐H functionality are more stable than those with Pd‐H group; and the most stable complex has the C≡C coordination terminated with CH2OTBS substituent, and that with Ph substituent is less stable by 3.7 kcal mol−1. For the coordination of substrate 10, the same energetic patterns have been found and the most stable complex has the C≡C coordination terminated with Ph substituent and that with TMS substituent is less stable by 2.7 kcal mol−1. This energetic order reveals the increasing steric effect of CH2OTBS, Ph and TMS substitutions.
Based on these complexes, we computed this elementary step and the simplified potential energy surface for the regioselective formation of the alkenyl complexes is shown in Figure 2. For substrate 9, two transition states for the N‐H insertion into the C≡C bond have been located, which differs from the traditional Pd‐H insertion. The transition state with the sterically less hindered CH2OTBS group at the C≡C bond (LPd‐NH‐9‐OTBSCH2/C2Ph‐TS) is more stable than that one with Ph group (LPd‐NH‐9‐Ph/C2CH2OTBS‐TS) at the C≡C bond by 3.0 kcal mol−1, and this reveals a kinetic preference.

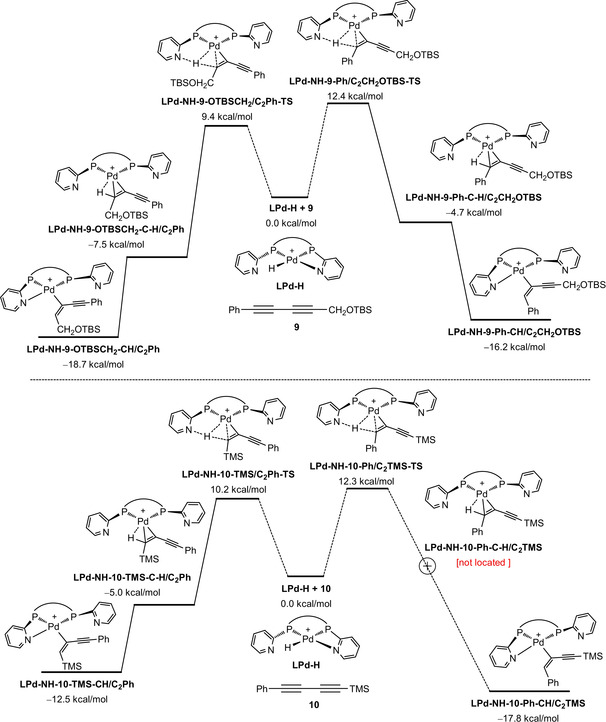
Potential energy surface for the regioselective formation of alkenyl complexes of 9 (top) and 10 (bottom).
Following these transition states, the corresponding intermediates with C−H agostic interaction have been located (LPd‐9‐OTBSCH2‐C−H/C2Ph, −7.5 kcal mol−1 and LPd‐9‐Ph‐C−H/C2CH2OTBS, −4.7 kcal mol−1). It is interesting to note that the presence of the hemilabile 2‐pyridyl ligand results in the more stable alkenyl intermediates ((LPd‐9‐OTBSCH2‐CH/C2Ph and LPd‐9‐R1‐CH/C2R2); and the former is also more stable than the latter by 2.5 kcal mol−1, which indicates a thermodynamic performance. These energy differences rationalize the observed regioselectivity of substrate 9, which is favored both kinetically and thermodynamically. For substrate 10, different results have been found (Figure 2). For example, the transition state with sterically less hindered Ph group at the coordinated C−C bond is higher in energy than that with the sterically more hindered TMS group by 2.1 kcal mol−1 (LPd‐NH‐10‐Ph/C2TMS‐TS vs. (LPd‐NH‐10‐TMS/C2Ph‐TS). Such reversed energetic difference is indeed understandable, since the C atom bonded to Ph is less negatively charged than that bonded to TMS (−0.241 vs. −0.725) in the transition states due to the difference in electronegativity of carbon and silicon atoms. Consequently, the former will have weaker attractive interaction than the latter. Next, it is not possible to locate the C−H agostic intermediate following LPd‐NH‐10‐Ph/C2TMS‐TS, and all attempts resulted in the direct formation of the corresponding alkenyl complex LPd‐10‐PhCH/C2TMS. In contrast, the corresponding C−H agostic intermediate (LPd‐10‐TMS‐C−H/C2Ph) following LPd‐NH‐10‐TMS/C2Ph‐TS was located (−5.0 kcal mol−1). Subsequently, the more stable alkenyl intermediate (LPd‐10‐TMSCH/C2Ph) was located. On the potential energy surface, the alkenyl intermediate LPd‐10‐PhCH/C2TMS is more stable than LPd‐10‐TMSCH/C2Ph (−17.8 vs.‐12.5 kcal mol−1) due to the enhanced steric difference between Ph and TMS, although the formation of the former is less favored kinetically than the latter formation. Consequently, the observed regioselectivity of substrate 10 should be governed thermodynamically by considering the reversibility between (LPd‐NH‐10‐TMS/C2Ph‐TS) and (LPd‐10‐TMS‐C−H/C2Ph).
A similar potential energy surface for substrate 1 a has been found (Figure S3). For the CH2R terminated C≡C bond, the reaction has an insertion free energy barrier of 9.9 kcal mol−1 and goes directly to the corresponding alkenyl intermediate with exergonic reaction free energy of 20.4 kcal mol−1. For the TMS terminated C≡C bond, the reaction has an insertion free energy barrier of 9.6 kcal mol−1 followed by an C−H agostic intermediate (−4.6 kcal mol−1) and the reaction goes to the corresponding alkenyl intermediate with exergonic reaction free energy of 13.5 kcal mol−1. This indicates once again the thermodynamic origin of the observed regioselectivity.
To analyze the steric effect of different substituents, we computed the relative energies of the products (Figure 3). For example, 9 a is more stable than 9 b by 1.7 kcal mol−1, while 10 a is more stable than 10 b by 10.6 kcal mol−1. Furthermore, 3 aa is more stable than 4 aa by 10.0 kcal mol−1 (Figure S3). To confirm this thermodynamic trend, we computed the relative energy of isomers with H substitution as reference and the substitution of CH2OTBS, Ph and SiMe3 is less favored by 1.7, 2,4 and 11.1 kcal mol−1, respectively, indicating the increasing steric interaction. This energetic order is in line with the observed regioselectivity.

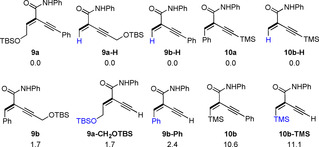
Relative energy (kcal mol−1) of the regioisomers as well as the simplified reference molecules.
Conclusion
In summary, we have developed a general and convenient Pd‐catalyzed hydroamidation of (un)symmetrical 1,3‐diynes. For the first time differently substituted 1,3‐diynes undergo highly chemo‐, regio‐, and stereoselective transformation to the corresponding α,β‐unsaturated amides. This novel catalytic transformation was enabled by the “built‐in‐base” ligand L6 (Neolephos) under mild conditions and provided a general approach to a variety of interesting functionalized synthetic building blocks in good to high yields. The utility of the catalytic system is showcased in versatile modifications of several structurally complex molecules and marketed drugs. Mechanistic studies and M06L‐SMD density functional theory computations revealed the key role of the intrinsic substituents of substrates and the ligand in determining the selectivity. We believe these findings will provide new impetus for other selectivity‐controlled carbonylation reactions using unsymmetrical substrates.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work is supported by Evonik Performance Materials GmbH, the BMBF (Bundesministerium für Bildung und Forschung), and the State of Mecklenburg‐Vorpommern. We thank the analytical team of LIKAT for their kind support. J.Y. thanks the Chinese Scholarship Council (CSC) for financial support. Open access funding enabled and organized by Projekt DEAL.
References
1
1a
1b
1c
1d
1e
2
2b
2c
3
3a
3b
3c
3d
3e
3f
3g
3h
3i
3j
3l
4
4b
4d
5
6
6c
6d
6e
7
7a
7b
7c
7d
7e
7f
7g
7h
7k
7m
7n
7o
7q
7r
8
8a
8b
8c
8d
8e
10
10a
10b
10c
10e
11
11b
12
12a
12b
12c
12d
14
15
15a
15b
15c
15d
15e
16
16a
16c
17
17a
17b
17d
17e
17g
17h
17i
18
18c
18d
18e
19
20
 A General and Highly Selective Palladium‐Catalyzed Hydroamidation of 1,3‐Diynes
A General and Highly Selective Palladium‐Catalyzed Hydroamidation of 1,3‐Diynes