
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Despite the high prevalence of potential drug–drug interactions in pediatric intensive care units, their clinical relevance and significance are unclear. We assessed the characteristics and risk factors of clinically relevant potential drug–drug interactions to facilitate their efficient monitoring in pediatric intensive care units. This retrospective cohort study reviewed the medical records of 159 patients aged <19 years who were hospitalized in the pediatric intensive care unit at Seoul National University Hospital (Seoul, Korea) for ≥3 days between August 2019 and February 2020. Potential drug–drug interactions were screened using the Micromedex Drug-Reax® system. Clinical relevance of each potential drug–drug interaction was reported with official terminology, magnitude of severity, and causality, and the association with the patient’s clinical characteristics was assessed. In total, 115 patients (72.3%) were exposed to 592 potential interactions of 258 drug pairs. In 16 patients (10.1%), 22 clinically relevant potential drug–drug interactions were identified for 19 drug pairs. Approximately 70% of the clinically relevant potential drug–drug interactions had a severity grade of ≥3. Exposure to potential drug–drug interactions was significantly associated with an increase in the number of administrated medications (6–7 medications, p = 0.006; ≥8, p<0.001) and prolonged hospital stays (1–2 weeks, p = 0.035; ≥2, p = 0.049). Moreover, clinically relevant potential drug–drug interactions were significantly associated with ≥8 prescribed drugs (p = 0.019), hospitalization for ≥2 weeks (p = 0.048), and ≥4 complex chronic conditions (p = 0.015). Most potential drug–drug interactions do not cause clinically relevant adverse outcomes in pediatric intensive care units. However, because the reactions that patients experience from clinically relevant potential drug–drug interactions are often very severe, there is a medical need to implement an appropriate monitoring system for potential drug–drug interactions according to the pediatric intensive care unit characteristics.
Introduction
Coadministration of two or more drugs is associated with a potential drug–drug interaction (PDDI), which is the possibility that the drugs alter each other’s effect [1]. Critically ill patients are at a higher risk of drug-drug interactions (DDIs), not only due to multiple medications but also because of disease complexity, accompanying organ dysfunction, and pharmacotherapy complexity [2]. Moreover, adverse drug reactions (ADRs) including DDIs in ICU are highly associated with prolonged hospitalization and higher morbidity and mortality [3]. With increasing concerns regarding medication safety in intensive care units (ICUs), numerous studies have reported PDDIs over the past decades. According to a recent meta-analysis, the proportion of adult patients in ICUs with exposure to at least one PDDI is 58%, which is higher than that in general wards [4]. In pediatric intensive care units (PICUs), the overall prevalence of PDDIs is 59.4%–75.2%, similar to that in adult ICUs [5,6].
PDDIs do not always result in adverse events or actual harm because some drugs are coadministered intentionally with favorable effects in ICUs. For example, opioids and benzodiazepine are concomitantly administered for analgosedation as a component of basic pain management in ICUs [7]. In addition, some medications must be coadministered despite well-known interactions. Therefore, the assessment of DDI-related negative effects or ADR intensity can help clinicians identify drug combinations that should be avoided [8]. Nevertheless, there are only a few studies that assessed the clinical relevance of PDDIs and PDDI-related ADRs without mentioning causality and severity [6,9].
In this study, we aimed to assess clinically relevant (CR)-PDDIs in PICUs, excluding common PDDIs without clinical significance. Specifically, we focused on providing guidance for efficient PDDI monitoring in PICUs by identifying the characteristics and risk factors of CR-PDDIs in this critical care setting.
Methods
Data sources and eligibility criteria
This retrospective study was conducted in the PICU at the Seoul National University Hospital (Seoul, Republic of Korea), which is a mixed unit for medical and surgical pediatric patients. The unit has 24 beds and is staffed 24 h a day, 7 days a week, by four pediatric intensivists. Clinical pharmacy services are provided 5 days a week by a clinical pharmacist, who reviews the medication records, including drug dosing, administration route, drug concentration, reported ADRs, and any drug-related queries, of all PICU patients [10]. A clinical pharmacist was assigned to stay in the PICU for 4 h each day for dedicated jobs. PDDIs in PICU patients were monitored from March 2019. The assigned pharmacists recorded clinical interventions and the associated outcomes in a pharmaceutical care database.
The data of all patients aged <19 years who were admitted to and discharged from the PICU between August 2019 and February 2020 were analyzed. We excluded patients who were admitted for end-of-life care and those who stayed in the PICU for <3 days (Fig 1). This minimum length of PICU stay was adopted to ensure sufficient observation time for identifying CR-PDDIs. The study was approved by the Institutional Review Board (IRB No. C-1908-058-1054) at the Seoul National University Hospital. The requirement to obtain informed consent from patients was waived.

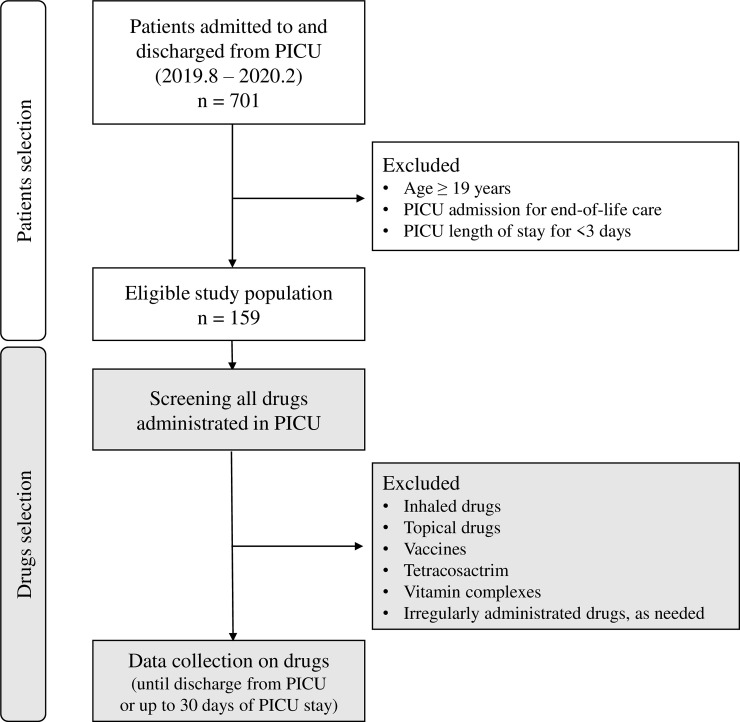
Flow chart of the study protocol.
Data were retrospectively compiled from electronic medical records into a structured data collection form as follows: patients’ demographics (including age, sex, and major diagnosis), department at admission, cause of PICU admission, predicted mortality rate using the prediction algorithm of Pediatric Risk of Mortality IV to assess severity at admission, length of PICU stay, and PICU outcome. Additionally, the accompanying complex chronic conditions (CCCs) that each patient presented during PICU admission were identified according to the updated pediatric CCC classification system, version 2 [11]. We established the age groups according to the criteria suggested by the Ministry of Food and Drug Safety at the International Conference on Harmonisation, and the definition of pediatric sepsis [12,13].
We obtained data regarding all drugs administered to the eligible patients until discharge from PICU or 30 days after PICU admission, whichever occurred first (Fig 1). The drug data included generic name, precise administration timing, and administration route. The following drugs were excluded because they are associated with less systemic drug interactions than other drugs: 1) inhaled drugs, such as salbutamol, ipratropium, budesonide, acetylcysteine, and epinephrine; 2) topical drugs; 3) vaccines; 4) tetracosactrin for adrenal function test; 5) vitamin complexes; and 6) any drug administered irregularly and intermittently as needed, such as diuretics, antipyretics, and analgesics (drugs that were not administered according to a regular dosing interval or on consecutive days). Administration of drugs within a 24-h period were regarded as concurrent exposure. However, if one drug was changed to another, it was not regarded as coadministration.
Identification of PDDIs
PDDIs were reviewed using the Micromedex Drug-Reax® system (Truven Health Analytics, Greenwood Village, CO, USA). Several software packages are available for screening PDDIs, but this software has been validated and found to be superior to others. In the present study, we used it for screening PDDIs in the PICU [5,6,14,15]. During the study period, there were 14 drugs that could not be searched in the Micromedex Drug-Reax® system; therefore, these drugs were excluded (S1 Appendix). The PDDI classifications were based on severity (contraindicated, major, moderate, and minor) and documentation (excellent, good, and fair). A “contraindicated” drug combination should never be used concurrently. “Major” interactions could be life-threatening and/or require medical intervention to minimize or prevent severe adverse effects. “Moderate” interactions could result in exacerbation of the patient’s condition and/or require treatment alteration. “Minor” interactions had limited clinical effects that could be included in the frequency or severity of adverse effects, but generally, they do not require a major treatment alteration. At the documentation level, “excellent” indicated that controlled studies had clearly established the existence of a drug interaction. “Good” indicated that documentation strongly suggested an interaction, but well-controlled studies were lacking. “Fair” indicated that available documentation was poor, although pharmacologic considerations led clinicians to suspect an interaction.
Assessment of CR-PDDIs
The clinical relevance of each PDDI was thoroughly reviewed by two authors (YHC and JDP), based on the changes in vital signs, laboratory test results, electrocardiogram, and judgment of the assigned doctor. To clarify clinical relevance, only DDIs causing ADRs that altered the treatment course and interventions of any form were regarded as CR-PDDIs. The development of CR-PDDIs was observed until discharge from the PICU or 30 days after PICU admission, whichever occurred first.
CR-PDDIs were reported according to the causality and magnitude of ADRs resulting from DDIs. The causality of events was classified into certain, probable/likely, possible, unlikely, conditional/unclassified, and unassessable/unclassifiable, according to the system proposed by the World Health Organization Collaborating Center for International Drug Monitoring, the Uppsala Monitoring Center [16]. DDIs causing ADRs with a high causality, such as certain, probable/likely, and possible, were regarded as CR-PDDIs. The terminology and severity of CR-PDDIs were evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [17].
Statistical analysis
Continuous nonparametric data are presented as median with interquartile range and categorical variables as number with percentage. Logistic regression was performed to identify variables that were significantly associated with any PDDI and CR-PDDI. Patient characteristics such as sex, age, department, CCC number, PICU admission reasons, predicted mortality rate at admission, average prescribed medication number, and PICU stay duration were independent variables in the model. All variables were included in analyzing the predictors of CR-PDDIs and PDDIs. However, when identifying associations of three variables (CCC number, prescribed medication number, and length of PICU stay) with a CR-PDDI, the top two of three divided groups were merged because of small populations in each subgroup. For each variable, the odds ratio (OR) and 95% confidence interval (CI) were determined. Variables with a significant univariate result (p<0.05) were included in the multivariate logistic regression analysis. Results with p≤0.05 were considered statistically significant. Statistical analyses were performed using SPSS software (version 25; SPSS Inc., IBM, Armonk, NY, USA).
Results
General characteristics
Data of 159 pediatric patients admitted to the PICU were analyzed (Table 1). The median age of the children was 0.92 (0.33–5.25) years, and 96 patients (60.4%) were aged <2 years. Approximately 40% of the patients belonged to the general pediatric department, and the median CCC number was 2 (1–3). The most common reason for PICU admission was postoperative/procedure care (90, 56.6%), followed by respiratory failure (33, 20.8%). The patients in the PICU received an average of 8.0 (5.3–10.5) medications and were exposed to an average of 1.2 (0.0–2.5) PDDIs. The median length of PICU stay was 6.1 (4.0–11.5) days per patient.

| Characteristic | n (%) or median (IQR) |
|---|---|
| Sex | |
| Male | 91 (57.2) |
| Female | 68 (42.8) |
| Age (years) | 0.9 (0.3–5.3) |
| 0–27 days | 23 (14.5) |
| 28 days–23 months | 73 (45.9) |
| 2–5 years | 27 (17.0) |
| 6–11 years | 14 (8.8) |
| 12–18 years | 22 (13.8) |
| Department | |
| General pediatrics | 63 (39.6) |
| Pediatric cardiology | 11 (6.9) |
| Thoracic surgery | 45 (28.3) |
| General and neurosurgery | 40 (25.2) |
| Diagnosis | |
| Cardiovascular disease | 55 (34.6) |
| Haemato-oncology disease | 24 (15.1) |
| Neurologic disease | 23 (14.5) |
| Gastrointestinal disease | 23 (14.5) |
| Respiratory disease | 15 (9.4) |
| Other diseasesa | 19 (11.9) |
| Number of complex chronic conditions | 2 (1–3) |
| Reason for PICU admission | |
| Post-operative/procedure care | 90 (56.6) |
| Respiratory failure | 33 (20.8) |
| Shock | 10 (6.3) |
| Mental change | 7 (4.4) |
| For continuous renal replacement therapy | 6 (3.8) |
| Other causes | 13 (8.2) |
| Predicted mortality rate at admission (%) | 1.9 (0.9–7.3) |
| Average number of prescribed medications per patientb | 8.0 (5.3–10.5) |
| Average number of PDDIs exposed per patientb | 1.2 (0–2.5) |
| Length of stay in PICU (days) | 6.1 (4.0–11.5) |
| Survival to PICU discharge | 150 (94.3) |
IQR, interquartile range; PICU, pediatric intensive care unit; PDDI, potential drug–drug interaction.
aIncluded other congenital/genetic defects, metabolic diseases, and renal/urologic diseases.
bObserved during admission in pediatric intensive care unit (maximum 30 days).
PDDI exposure
In total, 115 patients (72.3%) were exposed to 592 PDDIs of 258 drug pairs. According to severity, 2.6%, 56.2%, and 39.0% of the 592 PDDIs were classified as contraindicated, major, and moderate, respectively, but based on documentation, 7.0%, 41.9%, and 52.3% were classified as having excellent, good, and fair scientific evidence, respectively. The frequency of the 258 drug pairs that caused PDDIs according to severity was as follows: contraindicated (6, 2.3%), major (141, 54.7%), and moderate (98, 38.0%). All patients exposed to PDDIs experienced one or more PDDI(s) with moderate or greater severity.
The most frequently identified PDDIs classified based on severity are shown in Table 2 (only those with approximately 1% prevalence). Midazolam + remifentanil, enalapril + spironolactone, and enalapril + furosemide were the most common combinations causing PDDIs. Furthermore, most of the common drug pairs resulting in PDDIs were thoroughly monitored by routine or intensive care in the PICU.

| Severity | Drug pair | Evidence | Frequency | Potential adverse events | Monitoring methods |
|---|---|---|---|---|---|
| Contraindicated | Nitroglycerin + sildenafil | Excellent | 5 | Potentiation of hypotensive effects | Blood pressure |
| Major | Midazolam + remifentanil | Fair | 40 | Increased risk of hypoventilation | Respiratory rate, SpO2 |
| Enalapril + spironolactone | Good | 33 | Hyperkalemia | Electrolyte | |
| Piperacillin/tazobactam + vecuronium | Good | 17 | Enhanced and/or prolonged neuromuscular blockade | N/A | |
| Piperacillin/tazobactam + vancomycin | Good | 10 | Increased risk of acute kidney injury | Vancomycin trough level | |
| Midazolam + phenobarbital | Fair | 8 | Increased risk of hypoventilation | Respiratory rate, SpO2 | |
| Famotidine + tacrolimus | Fair | 7 | Increased tacrolimus toxicity | Tacrolimus level, ECG | |
| Potassium chloride + spironolactone | Fair | 7 | Hyperkalemia | Electrolyte | |
| Aspirin + furosemide | Good | 7 | Reduced diuretic effectiveness, nephrotoxicity | Urine output, Cr | |
| Aspirin + spironolactone | Good | 7 | Reduced diuretic effectiveness, hyperkalemia, nephrotoxicity | Urine output, Electrolyte, Cr | |
| Vecuronium + nicardipine | Good | 6 | Enhanced neuromuscular blockade | N/A | |
| Phenobarbital + remifentanil | Fair | 6 | Increased risk of CNS depression | Assessment of sedation levela | |
| Famotidine + fluconazole | Fair | 5 | Increased fluconazole toxicity | ECG | |
| Moderate | Enalapril + furosemide | Good | 33 | Postural hypotension (first dose) | Blood pressure |
| Furosemide + vecuronium | Good | 13 | Increased or decreased neuromuscular blockade | N/A | |
| Furosemide + sildenafil | Fair | 12 | Increased risk of hearing loss | N/A | |
| Esomeprazole + levothyroxine | Good | 6 | Decreased levothyroxine effectiveness | Thyroid hormone test | |
| Aspirin + enalapril | Excellent | 6 | Decreased effectiveness of enalapril | Blood pressure | |
| Esomeprazole + fluconazole | Fair | 5 | Increased esomeprazole plasma concentrations | N/A | |
| Esomeprazole + iron | Fair | 5 | Reduced iron bioavailability | Hemoglobin, iron level | |
| Lansoprazole + iron | Fair | 5 | Reduced iron bioavailability | Hemoglobin, iron level | |
| Bosentan + sildenafil | Excellent | 5 | Increased bosentan plasma concentrations and decreased sildenafil plasma concentrations | Liver function test, Echocardiography |
CNS, central nervous system; ECG, electrocardiogram; SpO2, percutaneous arterial oxygen saturation; Cr, creatinine; N/A, not available.
aSedation level was assessed by State Behavioral Scale based on protocol in our PICU.
The PDDI-causing drug combinations frequently documented by the different departments are shown in Table 3. In pediatric cardiology and thoracic surgery, interactions of enalapril + spironolactone and enalapril + furosemide were observed in more than half of the patients (58.9%). In general pediatrics, midazolam + remifentanil was the most common PDDI-causing combination used in approximately 30% of the patients, whereas each of the other drug combinations associated with PDDIs was coadministered to a small proportion of patients.

| Drug–drug combination | Number of patients exposed (%) |
|---|---|
| General pediatrics (n = 63) | |
| Midazolam + remifentanil | 19 (30.2) |
| Piperacillin/tazobactam + vecuronium | 6 (9.5) |
| Furosemide + sildenafil | 6 (9.5) |
| Midazolam + phenobarbital | 6 (9.5) |
| Furosemide + vecuronium | 5 (7.9) |
| Piperacillin/tazobactam + vancomycin | 5 (7.9) |
| Potassium chloride + spironolactone | 5 (7.9) |
| Vecuronium + nicardipine | 5 (7.9) |
| Bosentan + sildenafil | 5 (7.9) |
| Pediatric cardiology and thoracic surgery (n = 56) | |
| Enalapril + spironolactone | 33 (58.9) |
| Enalapril + furosemide | 33 (58.9) |
| Midazolam + remifentanil | 15 (26.8) |
| Aspirin + furosemide | 7 (12.5) |
| Aspirin + spironolactone | 7 (12.5) |
| Piperacillin/tazobactam + vecuronium | 6 (10.7) |
| Furosemide + sildenafil | 6 (10.7) |
| Aspirin + enalapril | 6 (10.7) |
| Furosemide + vecuronium | 5 (8.9) |
| General and neurosurgery (n = 40) | |
| Midazolam + remifentanil | 6 (15.0) |
| Piperacillin/tazobactam + vecuronium | 5 (12.5) |
| Famotidine + tacrolimus | 4 (10.0) |
| Esomeprazole + levothyroxine | 4 (10.0) |
| Furosemide + vecuronium | 3 (7.5) |
Causality and severity of CR-PDDIs
Twenty-two clinically relevant adverse events related to PDDIs were identified in 16 patients (10.1%). There were 19 drug pairs associated with CR-PDDIs (Table 4), and 5 of those were combinations related to frequent PDDIs. The causality was classified as probable for 54.5% of the total CR-PDDIs and as possible for the remaining CR-PDDIs. There was no case of mortality, but a severity of grade ≥3 was confirmed for 15 (68.1%) CR-PDDIs. Two drug pairs associated with CR-PDDIs could not be assessed for causality and severity according to the CTCAE because of a lack of data.

| Drug pair | Observed adverse events | Causality | Severity grade (frequency) | Incidence (%)c | Management |
|---|---|---|---|---|---|
| Enalapril + spironolactonea | Hyperkalemia | Probable | Grade 2 (1) Grade 3 (2) | 9.1 | Spironolactone discontinuation |
| Levofloxacin + pentamidine | Electrocardiogram QT corrected interval prolonged | Probable | Grade 4 (1) | 100 | Levofloxacin discontinuation |
| Defibrotide + nadroparin | Bronchopulmonary hemorrhage | Probable | Grade 3 (1) | 25 | Nadroparin discontinuation, transfusion |
| Esomeprazole + propranolol | Sinus bradycardia, hypotension | Probable | Grade 1, 3 (1) | 50 | Propranolol discontinuation, hydration, inotropic drugs |
| Bosentan + sildenafila | Alanine aminotransferase increased | Probable | Grade 3 (1) | 20 | Bosentan discontinuation |
| Ciprofloxacin + propranolol | Sinus bradycardia, hypotension | Probable | Grade 1, 3 (1) | 100 | Propranolol discontinuation, hydration, inotropic drugs |
| Pentamidine + sulfamethoxazole/trimethoprim | Electrocardiogram QT corrected interval prolonged | Probable | Grade 3 (1) | 50 | Sulfamethoxazole/trimethoprim discontinuation |
| Desmopressin + dexamethasone | Hyponatremia | Probable | Grade 4 (1) | 100 | Desmopressin discontinuation, dexamethasone dosage reduction |
| Furosemide + propranolol | Sinus bradycardia, hypotension | Probable | Grade 1, 3 (1) | 33.3 | Propranolol discontinuation, hydration, inotropic drugs |
| Lansoprazole + warfarin | INR increased | Probable | Grade 2 (1) | 100 | Warfarin dosage adjustment after lansoprazole discontinuation |
| Dexamethasone + vecuronium | Decreased vecuronium effectivenessb | Possible | N/A (1) | 25 | Vecuronium dosage increase |
| Esomeprazole + levothyroxinea | Hypothyroidism | Possible | Grade 2 (1) | 16.7 | Levothyroxine dosage increase |
| Midazolam + voriconazole | Depressed level of consciousness | Possible | Grade 3 (1) | 100 | Midazolam dosage reduction |
| Furosemide + vecuroniuma | Decreased vecuronium effectivenessb | Possible | N/A (2) | 15.4 | Vecuronium dosage increase |
| Phenobarbital + remifentanila | Depressed level of consciousness | Possible | Grade 3 (1) | 16.7 | Remifentanil dosage reduction |
| Baclofen + remifentanil | Depressed level of consciousness | Possible | Grade 3 (1) | 100 | Remifentanil dosage reduction |
| Digoxin + norepinephrine | Atrial flutter, atrial fibrillation | Possible | Grade 3 (1) | 100 | Norepinephrine dosage reduction, cardioversion |
| Dopamine + digoxin | Atrial flutter, atrial fibrillation | Possible | Grade 3 (1) | 33.3 | Dopamine dosage reduction, cardioversion |
| Iron + lansoprazole | Anemia | Possible | Grade 2 (1) | 20 | Change lansoprazole to famotidine |
aListed in most frequently identified potential drug–drug interactions.
bNot available in Common Terminology Criteria for Adverse Events v5.0.
cRatio of number of clinically relevant potential drug–drug interactions compared to the number of exposures for each drug pair.
Factors associated with PDDIs and CR-PDDIs
The association between patient characteristics and the risk of PDDI exposure is presented in Table 5. In the univariate logistic regression analysis, sex, age (2–5 years), pediatric cardiology and thoracic surgery department, the average number of prescribed medications per person, and length of PICU stay were significantly associated with PDDIs. However, in the multivariate logistic regression analysis, only the number of prescribed medications and length of PICU stay showed a statistically significant association with PDDI exposure. Compared with patients administered an average of ≤5 medications per day, Patients receiving 6–7 (OR 5.6, 95% CI 1.6–19.1, p = 0.006) and >8 medications per day (OR 36.9, 95% CI 9.8–138.9, p<0.001) were more likely to be exposed to PDDIs than patients treated with an average of ≤5 medications per day. Moreover, compared with patients who stayed in the PICU for <1 week, those who stayed for 1–2 weeks had a five-fold higher likelihood of PDDI exposure (p = 0.035), and those who stayed for >2 weeks had an eight-fold higher likelihood of PDDI exposure (p = 0.049).

| All PDDIsa | Clinically relevant PDDIsb | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Sex | ||||||||
| Female | Reference | Reference | Reference | |||||
| Male | 2.2 (1.1–4.5) | 0.028 | 2.3 (0.8–6.4) | 0.105 | 0.9 (0.3–2.7) | 0.933 | ||
| Age (years) | ||||||||
| <2 | Reference | Reference | Reference | |||||
| 2–5 | 3.8 (1.1–13.6) | 0.039 | 3.8 (0.7–19.9) | 0.111 | 1.4 (0.3–5.6) | 0.656 | ||
| 6–11 | 2.9 (0.6–13.6) | 0.186 | 3.2 (0.4–25.2) | 0.268 | 1.8 (0.4–9.7) | 0.475 | ||
| 12–18 | 0.8 (0.3–2.1) | 0.714 | 0.9 (0.2–4.2) | 0.963 | 1.7 (0.4–7.1) | 0.445 | ||
| Department | ||||||||
| General/Neurosurgery | Reference | Reference | Reference | |||||
| General pediatrics | 2.1 (0.9–4.7) | 0.091 | 0.6 (0.2–2.0) | 0.357 | 3.2 (0.7–15.5) | 0.155 | ||
| Cardiothoracic departmentc | 4.9 (1.9–13.0) | 0.001 | 1.3 (0.3–5.0) | 0.708 | 1.9 (0.3–10.1) | 0.471 | ||
| Complex chronic condition | ||||||||
| 0–1 | Reference | Referenced | ||||||
| 2–3 | 1.9 (0.9–4.1) | 0.103 | ||||||
| ≥4 | 1.8 (0.7–4.9) | 0.243 | 5.5 (1.9–16.2) | 0.002 | 4.4 (1.3–14.2) | 0.015 | ||
| Reason for PICU admission | ||||||||
| Post-operation/procedure | Reference | Reference | ||||||
| Respiratory failure | 0.8 (0.3–1.8) | 0.549 | 1.9 (0.5–7.3) | 0.334 | 1.0 (0.2–4.7) | 0.928 | ||
| Mental change | 0.5 (0.1–2.5) | 0.406 | 2.3 (0.2–22.7) | 0.465 | 1.3 (0.1–15.5) | 0.843 | ||
| Shock | 1.5 (0.3–7.8) | 0.601 | 1.6 (0.2–14.4) | 0.697 | 0.7 (0.1–8.5) | 0.74 | ||
| CRRT | 1.9 (0.2–17.3) | 0.559 | 7.0 (1.1–46.3) | 0.043 | 1.8 (0.2–17.4) | 0.626 | ||
| Other causes | 2.1 (0.4–10.2) | 0.351 | 2.6 (0.5–14.2) | 0.287 | 1.3 (0.2–10.8) | 0.809 | ||
| Predicted mortality ratee | 1.0 (0.9–1.1) | 0.214 | 1.0 (0.9–1.0) | 0.512 | ||||
| Prescribed medicationsf | ||||||||
| ≤5 | Reference | Reference | Referenced | Referenced | ||||
| 6–7 | 7.7 (2.7–22.1) | <0.001 | 5.6 (1.6–19.1) | 0.006 | ||||
| ≥8 | 52.8 (16.4–170.7) | <0.001 | 36.9 (9.8–138.9) | <0.001 | 14.8 (1.9–115.0) | 0.01 | 12.3 (1.5–99.1) | 0.019 |
| Length of stay in PICU | ||||||||
| <1 week | Reference | Reference | Referenced | Referenced | ||||
| 1–2 weeks | 3.8 (1.7–10.7) | 0.011 | 4.5 (1.1–17.9) | 0.035 | ||||
| ≥2 weeks | 6.3 (1.4–28.3) | 0.017 | 7.7 (1.0–59.1) | 0.049 | 6.2 (2.0–18.9) | 0.001 | 3.4 (1.1–11.7) | 0.048 |
CRRT, continuous renal replacement therapy; PDDI, potential drug–drug interaction; PICU, pediatric intensive care unit.
aHosmer-Lemeshow goodness-of-fit test: p = 0.619, bHosmer-Lemeshow goodness-of-fit test: p = 0.692.
cIncluded departments of pediatric cardiology and thoracic surgery.
dTwo consecutive top groups were merged owing to the small number of patients in each group.
eEstimated based on the Pediatric Risk of Mortality Score IV.
fAverage number of prescribed medications by the PICU (maximum 30 days) per person.
The factors related to CR-PDDIs are presented in Table 5. In the univariate logistic regression analysis, the number of CCCs, reasons for PICU admission (for continuous renal replacement therapy), the average number of prescribed medications per patient, and length of PICU stay were significantly correlated with CR-PDDIs. However, the only independent variables that significantly affected the CR-PDDI occurrence in the final model based on the multivariate analysis were ≥4 CCCs (OR 4.4, 95% CI 1.3–14.2, p = 0.015), ≥8 prescribed medications (OR 12.3, 95% CI 1.5–99.1, p = 0.019), and a PICU stay of ≥2 weeks (OR 3.4, 95% CI 1.1–11.7, p = 0.048).
Discussion
In this study, we characterized CR-PDDIs according to their severity, causality, and standard terminology by focusing on the clinical significance of identified PDDIs. Furthermore, we determined the predictors of CR-PDDIs and PDDIs. To date, there are only a few studies on the prevalence, common drug combinations, and risk factors of PDDIs in critically ill children [5,6,18,19]. However, studies on the negative effect or intensity of expected PDDIs are limited. To our knowledge, this study is the first to assess the clinical significance of PDDIs from the perspective of pediatric intensivists.
In the present study, although the prevalence of PDDIs was 72.3% in our patient population, CR-PDDIs were observed in only 10.1% of the patients. The prevalence of PDDIs was considerably higher in our study than in previous studies because we included only patients hospitalized for ≥3 days. Therefore, the prevalence of CR-PDDIs is expected to decrease further when all patients are included. This result is consistent with studies in adult ICUs, where only a few CR-PDDIs were observed among numerous PDDIs [4,20]. Despite the low prevalence in the present study, more than 50% of the CR-PDDIs exhibited a severity of grade ≥3 and required immediate management. Moreover, CR-PDDIs occurred between drugs used less frequently than between drugs involved in common PDDIs. According to a report by Dai et al., exposure to the most common PDDIs may not pose the greatest risk for patients because clinicians may be familiar with and prepared to manage the DDIs [5]. Furthermore, it was found that most of the potential ADRs could be detected early or prevented by routine intensive care in PICU. For example, most of the common PDDIs can be actively monitored by frequently checking vital signs, performing blood tests that include drug level analysis, controlling the hourly urine output, and regularly assessing the sedation depth, which will ensure the detection of actual ADRs [21]. As seen in the drug combinations involved in PDDIs in our study, most of the drugs were anti-infective, cardiovascular, and central nervous system agents, which are high-risk medications known to develop DDIs according to previous studies [22–25]. Nevertheless, intensivists cannot avoid prescribing these high-risk medications as they are essential for managing and treating diseases requiring intensive care. Therefore, in this special environment, the basic management in ICUs can play an important role in monitoring PDDIs. Furthermore, it is presumed that the level of care in PICUs can ultimately affect CR-PDDIs.
Remarkably, the prevalence of PDDIs and CR-PDDIs did not differ according to the department to which the patients belonged. However, drug combinations of frequently identified PDDIs varied with the department. Similar to our results, differences in the pattern of PDDIs have been reported, especially in adult ICU patients, including cardiothoracic patients [24,25]. The common drug combinations associated with PDDIs also differ between PICUs and adult ICUs. For example, despite the heterogeneity of patients included in the studies, aspirin, insulin, and clopidogrel are the most common drugs implicated in DDIs in adult ICUs [4], whereas these drugs are not commonly used in pediatric patients. Furthermore, even among pediatric patients, frequent drug combinations related to PDDIs differ between hospitals [5,6,18,19]. This is presumed to be due to differences in the composition of critically ill patients, drug preferences of intensivists, and drug permits in each country. In South Korea, remifentanil is permitted for off-label use in PICU patients requiring mechanical ventilation. Consequently, remifentanil with midazolam is a common drug pair causing PDDIs. Therefore, we suggest that high-risk drugs requiring monitoring of PDDIs should be selected depending on the patients admitted to the PICU.
Polypharmacy and prolonged hospitalization in ICU are well-known risk factors of PDDIs in ICUs, [5,6,24,25]. The results of this study are consistent with those of previous studies; however, we discovered that these risk factors eventually increase the likelihood of “actual” DDIs and not just “potential” DDIs. These two risk factors are not considered independent. Because the daily exposure to drugs increases with increase in the length of PICU stay [5], prolonged hospitalization is estimated to indirectly increase the risk of DDIs through polypharmacy. Consequently, the number of medications is the most important predictive factor of whether PDDIs will lead to actual ADRs. In addition, comorbidities were also revealed to increase the risk of PDDIs not only in adult ICUs but also in PICUs [5,22]. However, in the present study, the CCCs were significantly associated only with a higher prevalence of CR-PDDIs and not with PDDIs. Here, because we had a smaller sample size than the previous studies, it was not possible to identify the relationship between CCCs and PDDIs. Despite this limitation, we demonstrated that patients with multiple chronic conditions were at a higher risk of CR-PDDIs, and thus, special attention is required.
Finally, to limit the actual manifestation of PDDIs, clinicians should be aware of the probability of DDI occurrence, based on the characteristics and risk factors of CR-PDDIs. In our hospital, clinical pharmacists were recently introduced in the PICU, and it has been demonstrated that they can contribute to efficient and safe pharmacotherapy, including DDI monitoring, in the ICU setting [10,20,26]. However, due to the limited number of clinical pharmacists, not all drugs administered in PICUs can be manually monitored. To overcome this problem, a clinical decision support system integrated with the electronic health records was developed. This system is expected to prevent adverse events including DDIs through automatic monitoring and alerts in hospital. Nevertheless, concerns about frequent alert fatigue with clinically insignificant DDIs have been raised, since the monitoring system are based on large-scale common data [27]. Consequently, electrical and automatic monitoring systems are not enough to prevent significant DDIs, especially in ICUs where drugs with a high probability of DDIs are frequently prescribed and DDIs actually occur between infrequently used drugs. Therefore, without relying only on monitoring systems, clinicians, ‘themselves’, should pay attention to CR-PDDIs whether they prescribe commonly or rarely used drugs. Furthermore, we recommend preemptive investigation of patient characteristics commonly encountered in their respective PICUs, drugs used frequently in each hospital, and the status of CR-PDDIs as well as PDDIs. Subsequently, an appropriate monitoring plan for comprehensive and effective surveillance of ADRs caused by PDDIs could be developed according to the results and modified depending on each PICU environment.
This study has some limitations. First, although the chosen research institute is one of the major tertiary hospitals in South Korea with a large-scale PICU with 24 beds, the study was conducted in a single institution and restricted to a short period of 6 months, limiting the representation and generalization of the findings. Thus, a multicenter study with a longer study period is required to obtain more concrete information. Second, because we conducted a retrospective study, there might be an underreporting of some ADRs. Nevertheless, since clinical pharmacists assigned to our PICU had to screen PDDIs, the data about drug use and DDIs could be considered to be collected prospectively. Third, because all the drugs administered in the PICU were included in this study regardless of where the drug was first initiated, the timing of CR-PDDI detected could not considered. To provide efficient guidance for monitoring PDDI, it is necessary to conduct further research regarding when CR-PDDI occurs following PDDI identification. Fourth, although the high accuracy of the Micromedex Drug-Reax® system has been proven, the information about the pediatric population is limited because there is a lack of studies in pediatric settings. Additionally, some drug types could not be searched in this system due to a lack of information; thus, the associated PDDIs have to be confirmed later.
Conclusion
In conclusion, a large proportion of patients in PICUs are exposed to PDDIs, but most of those do not appear as actual DDIs under routine intensive care. However, once CR-PDDIs occur, they are of high severity. Hence, there is an urgent need to develop a monitoring system for preventing CR-PDDIs via PDDI identification, especially based on the characteristics of the PICU.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
 Clinical significance of potential drug–drug interactions in a pediatric intensive care unit: A single-center retrospective study
Clinical significance of potential drug–drug interactions in a pediatric intensive care unit: A single-center retrospective study
