
- Altmetric
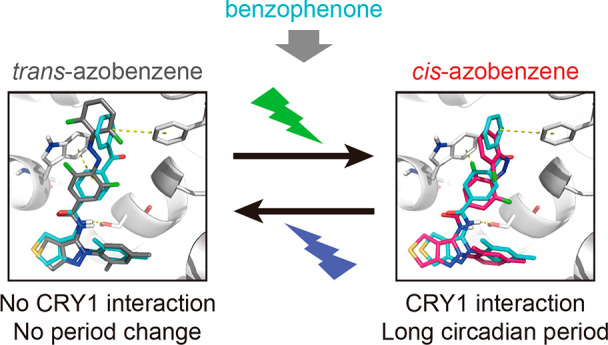
CRY1 and CRY2 proteins are highly conserved components of the circadian clock that controls daily physiological rhythms. Disruption of CRY functions are related to many diseases, including circadian sleep phase disorder. Development of isoform-selective and spatiotemporally controllable tools will facilitate the understanding of shared and distinct functions of CRY1 and CRY2. Here, we developed CRY1-selective compounds that enable light-dependent manipulation of the circadian clock. From phenotypic chemical screening in human cells, we identified benzophenone derivatives that lengthened the circadian period. These compounds selectively interacted with the CRY1 photolyase homology region, resulting in activation of CRY1 but not CRY2. The benzophenone moiety rearranged a CRY1 region called the “lid loop” located outside of the compound-binding pocket and formed a unique interaction with Phe409 in the lid loop. Manipulation of this key interaction was achieved by rationally designed replacement of the benzophenone with a switchable azobenzene moiety whose cis–trans isomerization can be controlled by light. The metastable cis form exhibited sufficiently high half-life in aqueous solutions and structurally mimicked the benzophenone unit, enabling reversible period regulation over days by cellular irradiation with visible light. This study revealed an unprecedented role of the lid loop in CRY-compound interaction and paves the way for spatiotemporal regulation of CRY1 activity by photopharmacology for molecular understanding of CRY1-dependent functions in health and disease.
Introduction
CRY proteins belong to the photolyase/cryptochrome family and consist of a highly conserved photolyase homology region (PHR) that binds to flavin adenine dinucleotide (FAD) and a diversified CRY C-terminal domain (CCT).1 In plants and insects, CRY acts as a blue light photoreceptor by using FAD as a cofactor. In contrast, mammalian CRYs, CRY1 and CRY2, are light-independent transcriptional repressors2 that regulate circadian rhythms and blood glucose levels in partially overlapping ways.3−5 Mutation of CRY1 and CRY2 genes results in familial delayed and advanced sleep phase in humans, respectively, due to the altered period length of the circadian clock.6,7 Furthermore, increasing evidence indicates distinct roles of CRY1 and CRY2, as shown, for example, by CRY2-dependent degradation of a proto-oncogene product, c-MYC.8 Development of small molecule modulators, especially isoform-selective tools, will accelerate the understanding of shared and distinct functions of CRY1 and CRY2 as attractive therapeutic targets of circadian clock-related diseases. However, high similarity of the FAD-binding pocket (16 out of 17 residues are identical in CRY1 and CRY2) in the PHR9,10 has rendered the design of isoform-selective compounds challenging, ever since the discovery of a compound targeting both CRY1 and CRY2.11 Recently identified CRY1- or CRY2-selective compounds also interact with the FAD-binding pocket but require the CCT, which is diversified between CRY1 and CRY2, for their selective effects.12,13 To date, no compound has been reported to target the conserved PHR in an isoform-selective manner.
In mammals, Per, Cry, Clock, and Bmal1 genes form a transcription-translation feedback loop to generate circadian rhythms at a cellular level. CLOCK-BMAL1 heterodimer activates transcription of Per and Cry genes, and their protein products, PER and CRY, inhibit CLOCK-BMAL1 function. Degradation of PER and CRY results in reactivation of CLOCK-BMAL1 to start the cycle over again.14 Because these clock components reside ubiquitously in almost all cells of the body, spatiotemporal control over compound activity, in addition to isoform selectivity, is required to precisely regulate the target protein for a deeper understanding of its functions and local control of the circadian clock system. Photopharmacology is an emerging approach that utilizes photoresponsive molecular moieties to control drug activity with high spatiotemporal resolution using light stimulus.15,16 Azobenzenes are the most commonly used photoswitches that enable light-dependent reversible isomerization of the compound structure between the thermally stable trans-form and thermally unstable cis-form and have been applied to noninvasively control biological processes ranging from milliseconds to hours.17−19 Because of the short half-life of the cis-isomer in most cases, its application to long-term biological processes, such as circadian rhythms, has been particularly challenging.
Here, we discovered isoform-selective compounds against CRY1 PHR, and by meticulous design of their azobenzene derivatives, achieved light-dependent reversible manipulation of mammalian CRY1 function to control cellular circadian rhythms. This study provides benzophenone as a useful platform to develop azobenzene-based photoswitchable molecules, and forms the basis of precise control of the circadian system.
Results
Identification of CRY1-Selective Compounds
We conducted cell-based phenotypic screening of circadian clock modulators in human U2OS reporter cells20 and identified hit compounds that affected the circadian period. Here, we focus on a benzophenone derivative TH303 (Figure 1A, left). TH303 caused dose-dependent period lengthening in two clock gene reporter cells Bmal1-dLuc and Per2-dLuc (Figures 1B,C) and repressed Per2-dLuc activity stronger than Bmal1-dLuc (Figures 1B,D). These biological effects and the chemical structure of TH303 were similar to a phenylpyrazole derivative KL101 that we recently identified as a CRY1-selective compound12 (Figure 1A, right, and B–D). We therefore tested a hybrid molecule TH129 (a benzophenone derivative of KL101; Figure 1A, middle) and observed similar circadian effects with higher potency than TH303 (Figures 1B–D). Neither TH303 nor TH129 affected cell viability (Figure S1A). Substitution of the benzoyl group of TH129 severely reduced the period-lengthening activity (TH124–TH128; Figure 1E), indicating its essential role in circadian clock regulation.

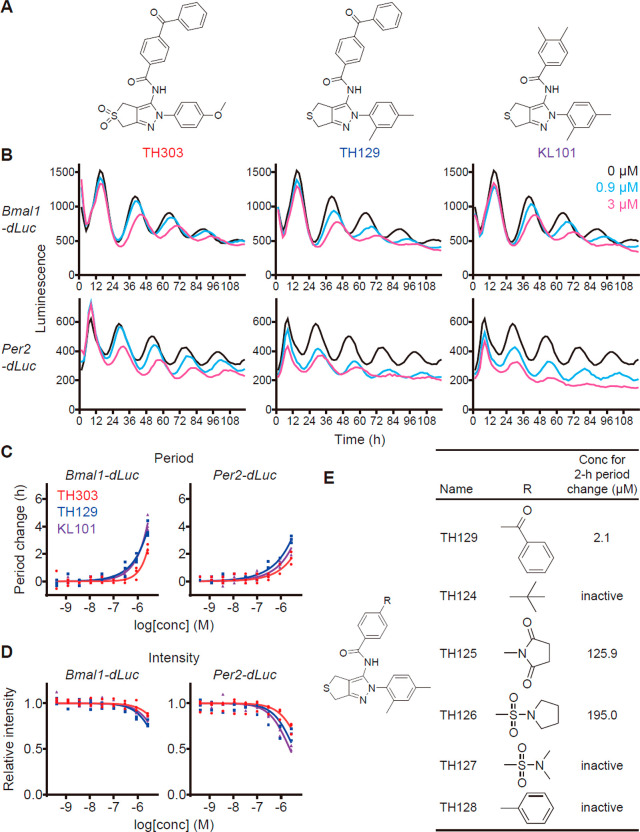
Benzophenone derivatives lengthen the circadian period. (A) Chemical structures of TH303, TH129, and KL101. (B–D) Effects on circadian rhythms in Bmal1-dLuc and Per2-dLuc U2OS cells. Luminescence rhythms in the presence of various concentrations of compounds (B, mean of n = 3) and changes in period (C) and luminescence intensity (D) compared to a DMSO control are shown (n = 3 biologically independent samples). (E) Period-lengthening activity of TH129 derivatives in Bmal1-dLuc cells. The concentrations for 2-h period-lengthening of the derivatives are shown (n ≥ 2 biologically independent samples).
Among the target protein candidates for period lengthening,11,21,22 TH303 and TH129 stabilized CRY1 but not CRY2 in HEK293 cell degradation assays (Figure 2A), without affecting the activities of CKIδ, CKIα, or CK2 in in vitro kinase assays (Figure S1B). Consistent with the repressive effect of CRY1 on Per2 transcription,14 stabilization of CRY1 by TH303 and TH129 resulted in the reduction of Per2-dLuc reporter activity in human U2OS cells (Figure 1D) and Per2::Luc knock-in reporter activity in mouse fibroblasts (Figure 2B, wild type). Per2::Luc repression by TH303 and TH129 was abolished in Cry1 knockout and Cry1/Cry2 double knockout cells, while it was enhanced in Cry2 knockout cells (Figure 2B). Consistently, the period lengthening effects of TH303 and TH129 were blunted by Cry1 knockout but not Cry2 knockout (Figures 2C and S1C). These results together indicated CRY1-selectivity of TH303 and TH129.

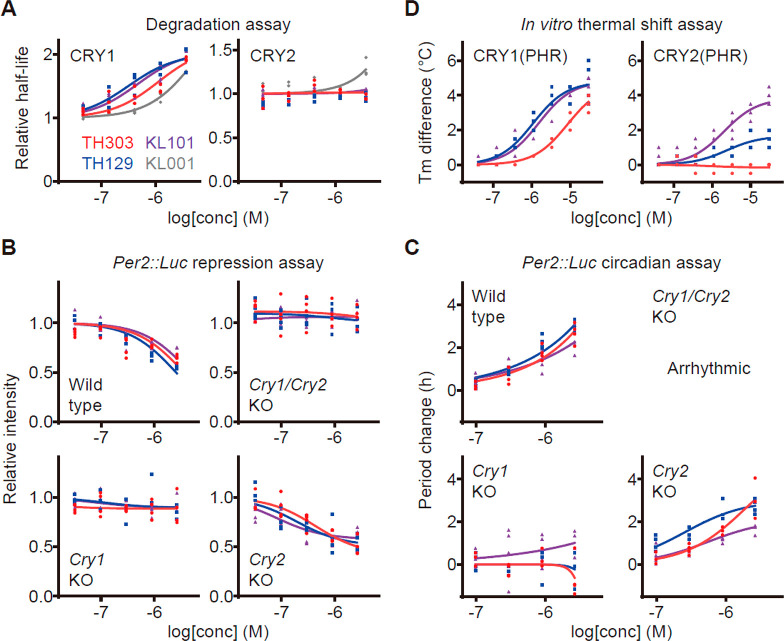
TH303 and TH129 are selective against CRY1. (A) Effects of TH303, TH129, and KL101 on CRY degradation in HEK293 cells. The half-lives of CRY1-luciferase fusion protein CRY1-LUC and CRY2-LUC relative to LUC are plotted by setting a DMSO control to 1 (n = 3 biologically independent samples). Effects of KL001, which stabilizes both CRY1 and CRY2, are also shown (gray). (B) Effects on Per2::Luc knock-in reporter activity in wild type, Cry1/Cry2 double knockout, Cry1 knockout, and Cry2 knockout fibroblasts. Changes in luminescence intensity compared to a DMSO control are shown (n = 4 biologically independent samples from two experiments). (C) Effects on circadian period in Per2::Luc knock-in fibroblasts. Changes in period compared to a DMSO control are shown (n = 4 biologically independent samples from two experiments). (D) Interaction with CRY1(PHR) and CRY2(PHR) in vitro. Changes in denaturing temperatures of recombinant CRY proteins in the presence of various concentrations of compounds compared to a DMSO control are shown (n = 4 biologically independent samples from two experiments). Compound interaction induced thermal stabilization.
CRY1 and CRY2 proteins consist of a highly conserved PHR and a diversified CCT. KL101 requires the CCT for its CRY1-selective effect and, therefore, interacts with CRY1(PHR) and CRY2(PHR) similarly in in vitro thermal shift assays12 (Figure 2D). To our surprise, TH303 and TH129 showed strongly reduced interaction with CRY2(PHR) compared with CRY1(PHR) (Figure 2D). Therefore, TH303 and TH129 are the first-in-class compounds selectively targeting CRY1(PHR).
Compound-Induced Rearrangement of CRY1
To reveal the molecular basis of CRY1-compound interaction, we determined the X-ray crystal structures of CRY1(PHR) in complex with TH303 and TH129 (Table S1). The overall structures of CRY1-TH303 (PDB ID: 7D1C) and CRY1-TH129 (7D19) were similar to CRY1-KL101 (6KX6) and CRY1-apo (6KX4) (Figure 3A). The methoxyphenyl substituent of TH303 and the m-xylene substituent of TH129 located chiefly at hydrophobic region 2 of the FAD-binding pocket by interacting with R358, A362, F381, and W397. They also interacted with L400 in hydrophobic region 1 and H355 and H359 in the affinity region (Figure 3B). The dihydrothienopyrazole moiety in TH303 and TH129 formed a stacking interaction with H355 in the affinity region, a hydrogen bond to R358, and hydrophobic interactions with A388 and I392 in hydrophobic region 2. The amide group of both compounds formed a canonical H-bond with S396 and an H-bond with an induced “in” conformation of Q289 in the affinity region. The benzophenone moiety formed hydrophobic and stacking interactions with W292, F296, and L400 in hydrophobic region 1 and F409 in the lid loop outside the FAD-binding pocket (gray in Figure 3B).

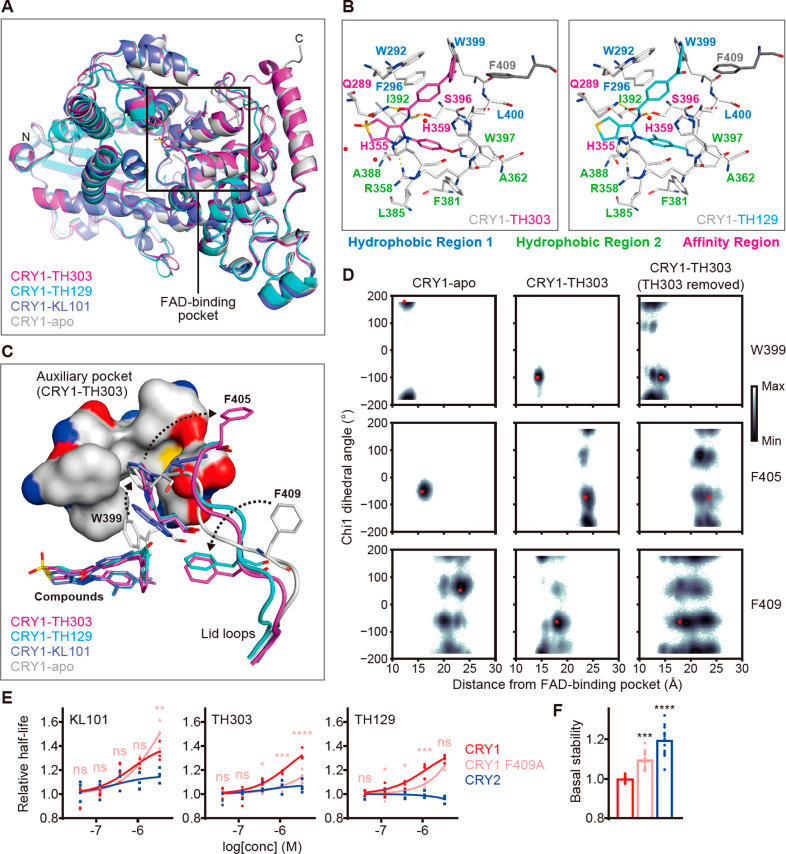
TH303 and TH129 interact with the FAD-binding pocket and rearrange the lid loop of CRY1. (A) Overall X-ray crystal structures of CRY1 in apo form and in complex with TH303, TH129, and KL101. The FAD-binding pocket (box) is shown in B. (B) Interactions of TH303 and TH129 with CRY1. Residues in hydrophobic region 1, hydrophobic region 2, and the affinity region are shown in blue, green, and magenta characters, respectively. Red nonbounded spheres and yellow dashed lines represent water molecules and hydrogen bonds, respectively. F409 (gray) in the lid loop that interacted with the benzophenone moiety of the compounds is also shown. (C) Effects of TH303 and TH129 on W399 and the lid loop. Conformational changes in W399, F405, and F409 are visualized by dashed arrows. The auxiliary pocket of CRY1-TH303 is shown by the surfaces of residues F295, F296, A299, F306, I314, M398 and S404. Negative, positive, and sulfur-containing regions are colored red, blue, and yellow, respectively. (D) Gibbs free energy landscape corresponding to W399, F405, and F409 Chi1 dihedral angle and distance from center of mass of the FAD-binding pocket, calculated using combined trajectory of three independent MD simulation runs of CRY1-apo, CRY1-TH303, and CRY1-TH303 (TH303 removed). Red dots represent poses in the corresponding crystal structure. (E, F) Effects of KL101, TH303, and TH129 on CRY degradation in HEK293T cells. The half-lives of CRY1-LUC, CRY1 F409A-LUC mutant, and CRY2-LUC relative to LUC are plotted by setting a DMSO control to 1 (E, n = 4 biologically independent samples from two experiments; ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05 for CRY1 F409A relative to CRY1; ns, not significant). Basal stability is shown in F (n = 12; ****P < 0.0001 and ***P < 0.001, relative to CRY1).
Compared to CRY1-KL101,12 the significantly increased steric bulk of the benzophenone caused a large conformational change in the side chain of W399, which was ejected from the FAD-binding pocket and inserted into an “auxiliary pocket” composed of F295, F296, A299, F306, I314, M398, and S404, leading to a positional shift of ∼6 Å at the ζ3-carbon (Figures 3C and S2). The auxiliary pocket is typically occupied by F405 in the lid loop (in CRY1-apo and CRY1-KL101). Insertion of W399 into this pocket in CRY1-TH303 and CRY1-TH129 resulted in the ejection of F405 and conformational rearrangement of the lid loop inducing an interaction of F409 with the benzoyl group (Figure 3C). The flexibility of these residues was evaluated by molecular dynamics (MD) simulations (Figures 3D and S3). The movement of W399 and F405, but not F409, was very limited in CRY1-apo. Interaction with the compound in CRY1-TH303 restricted the motion of F409 and increased F405 mobility. All three residues became more flexible after removal of the compound (TH303 removed), and W399 exited the auxiliary pocket to re-enter the FAD-binding pocket. These static and dynamic structures revealed that TH303 induced unique conformations of W399 and F409. Because the replacement of the benzoyl group in TH129 severely reduced period-lengthening activity (Figure 1E), we further analyzed the role of F409 in the effects of the compounds. A CRY1 F409A mutant showed reduced responses to TH303 and TH129 compared with wild type CRY1 in cell-based degradation assays while retaining a KL101 response (Figure 3E). Together, the results suggest that the interaction of F409 in the lid loop with the benzophenone moiety plays a key role in the effect of TH303 and TH129 on CRY1.
Rational Design for Replacement of Benzophenone with Azobenzene
Incorporation of a photoswitchable azobenzene moiety into a compound allows reversible photoisomerization between the two isomers, cis and trans. Typically, UV light is used for the trans-to-cis isomerization, and visible light drives the reverse process. Furthermore, usually the cis isomer is thermally unstable, and will isomerize back to the trans isomer in time. The structural difference between the isomers of the photoswitch-modified compound is expected to cause distinct affinity toward the target protein and, therefore, enable reversible modulation of its activity.15,16 The essential role of the benzophenone moiety for CRY1 regulation (Figure 3) prompted us to photopharmacologically manipulate its interaction with F409 for controlling the circadian clock, under the hypothesis that benzophenone moiety is structurally and electronically similar to the azobenzene photoswitch (Figure 4A). Screening of the Cambridge Structural Database (CSD) showed clear similarity of benzophenone and cis-azobenzene substructures in both ring angles and distances between the two aromatic rings (Figure 4B). Through a comparative analysis of protein–ligand complexes from the Protein Data Bank (PDB), the geometry distributions in the CSD and the PDB were found to be in very good agreement (Figure S4A). Moreover, the experimental dipole moments of benzophenone (3.0 D23) and cis-azobenzene (3.0 D) are identical, while trans-azobenzene has a dipole moment of 0 D.24 This striking analogy was confirmed by density functional theory (DFT) calculations (Table S2).

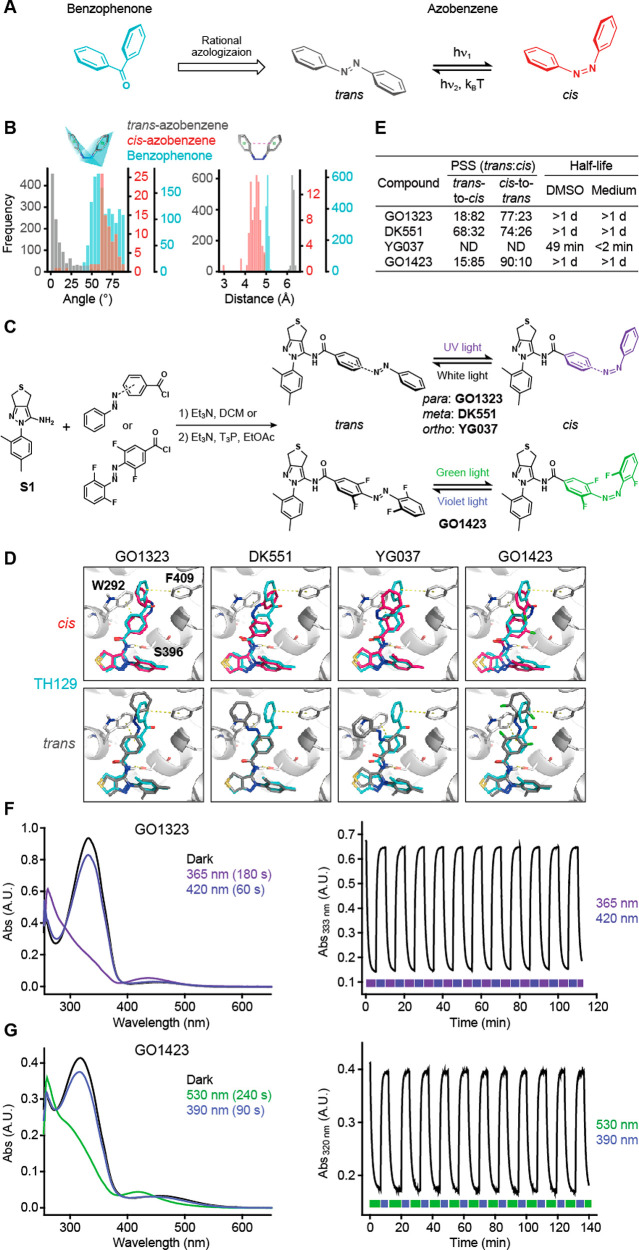
Rational design and characterization of azobenzene derivatives of TH129. (A) Rational azologization of benzophenone. (B) Distributions of ring angles and distances of benzophenone and cis- and trans-azobenzene structures in the CSD. (C) Synthesis and photoisomerization scheme of GO1323, DK551, YG037, and GO1423. (D) Docking poses of cis- (red) and trans- (gray) isomers of the compounds superposed with CRY1-TH129 (white-cyan). (E) Photophysical properties of the compounds. (F, G) Photoisomerization spectra and reversible photochromism of GO1323 (F) and GO1423 (G) in DMSO solution (∼30 μM, 25 °C). The photoisomerization UV–vis spectra (left) show thermally adapted photoswitches (dark), and PSSs reached at 365 nm (180 s), 420 nm (60 s), 530 nm (240 s), and 390 nm (90 s). The reversible photochromism graphs (right) show cycling between two PSSs of each compound, starting from the thermally adapted state. For GO1323, 365 nm light is used for trans-to-cis and 420 nm light for cis-to-trans isomerization, while for GO1423, cycles were performed using 530 and 390 nm light.
We therefore designed photoswitchable TH129 analogues by replacing the benzophenone with the azobenzene moiety. With the aim of determining the most favorable azobenzene regioisomer for light modulation, we considered all three possible structures (GO1323, DK551, and YG037 for para, meta, and ortho positions, respectively) (Figure 4C). Docking simulations of the azobenzene derivatives into the CRY1-TH129 crystal structure showed that the cis-isomers of GO1323 and DK551 mimicked the bent geometry of the benzophenone moiety to engage in a π–π interaction with F409, which was not formed by the largely different conformations of the other binding poses (Figures 4D and S4B), suggesting a possibility of light-dependent regulation of CRY1. Furthermore, the calculated dipole moment of the entire molecule supported the bioisosteric replacement of benzophenone with cis-azobenzene, especially attached at the para position (Table S3).
Based on rational design, we synthesized azobenzene derivatives of TH129 by acylation of amine S1 with acyl chloride derivatives of the corresponding azobenzenes (Figure 4C). In addition to the structural change, photophysical properties of the photoswitchable compounds play an important role in enabling reversible regulation of the target protein function. The photostationary state (PSS) determines the trans/cis ratio under light irradiation, and a high PSS is necessary to obtain large light-induced changes of the effect of the compound. Furthermore, in order to control the circadian period in cellular assays, which requires several days to evaluate the biological effect, the cis-isomer needs to be highly stable (i.e., display slow thermal cis-to-trans isomerization) in aqueous solutions. Achieving both high PSS and high thermal stability of the metastable cis-isomer presents a major challenge in photopharmacology.19,25 Interestingly, GO1323 showed high trans-to-cis PSS after UV light irradiation (82% cis isomer) and subsequent cis-to-trans PSS after irradiation with white light (77% trans isomer). Moreover, we observed a long half-life (>1 d) of the cis-isomer in both DMSO and cell culture medium (Figures 4E and S4C). In contrast, DK551 showed low PSS after UV light irradiation (32% cis isomer), while the half-life of cis-YG037 was short, preventing reliable determination of the PSS. Performed reversible photochromism of GO1323 indicated high photostability of this photoswitch (Figure 4F).
Photopharmacological Regulation of the Clock
We then analyzed the effects of GO1323, DK551, and YG037 on the circadian period using Bmal1-dLuc reporter U2OS cells. All the compounds were first thermally adapted to obtain their trans isomers (>98%). Dark-kept GO1323 showed only a minor effect on the period, while UV-irradiated (cis-enriched) GO1323 caused significant period lengthening (Figure 5A). Subsequent white light irradiation in vitro (trans-enriched GO1323) reduced the period-lengthening activity equivalent to the dark sample. DK551 exhibited light-dependent changes with lower period effects compared with GO1323, and the effects of neither YG037 nor TH129 were changed by light. These results were consistent with CRY1 docking simulations (Figure 4D) and photophysical properties (Figure 4E). Furthermore, irradiation with white light to cell culture also resulted in the deactivation of UV-irradiated GO1323 (Figure 5B). In vitro thermal shift assays revealed UV light-dependent and CRY1-selective interaction of GO1323 with CRY(PHR) (Figure 5C), supporting the modulation of CRY1 activity by light for the circadian period control.

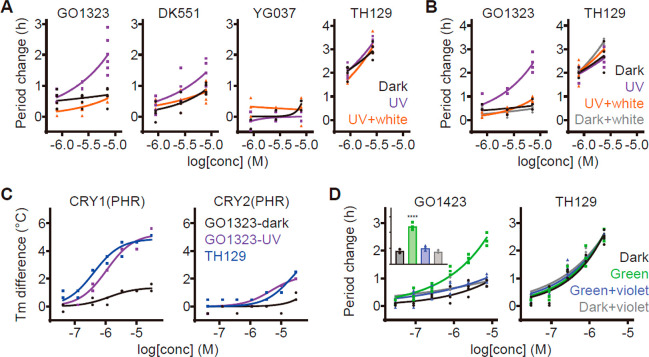
Azobenzene derivatives of TH129 regulate the circadian period in a light-dependent manner. (A) Effects of GO1323, DK551, and YG037 on circadian rhythms in Bmal1-dLuc U2OS cells. Changes in period compared to a DMSO control are shown (n = 3–6 biologically independent samples). Diluted compounds were irradiated in vitro with UV light (60 min; purple) followed by white light (10 min; orange) and then applied to the cells. (B) Effect of GO1323 and TH129 on circadian rhythms. Changes in period compared to a DMSO control are shown (n = 4 biologically independent samples). UV light-irradiated compounds were applied to the cells (purple), then irradiated in cellulo with white light (10 min; orange). (C) Interaction with CRY1(PHR) and CRY2(PHR) in vitro. Changes in denaturing temperatures of recombinant CRY proteins in the presence of various concentrations of compounds compared to a DMSO control are shown (n = 2 biologically independent samples). (D) Effect of GO1423 and TH129 on circadian rhythms. Changes in period compared to a DMSO control are shown (n = 5 biologically independent samples). Compounds were applied to the cells and then irradiated in cellulo with green light (30 min; green) and with violet light for back-isomerization (10 min; violet). Inset of the left panel represents the period changes at 7 μM GO1423 (****P < 0.0001, relative to dark control).
GO1323 provided the basis of light-dependent reversible control of the circadian period. However, trans-to-cis photoisomerization in cell culture was hampered because of the cytotoxicity of UV light as well as a high concentration of luciferin in cell culture medium for circadian luciferase reporter assays, resulting in strong absorption of UV light. We therefore synthesized GO1423 (Figure 4C) by introducing the tetra-o-fluoro azobenzene moiety whose trans-to-cis and cis-to-trans isomerization can be achieved by visible light: green and violet, respectively.26 Similar to GO1323, the docking pose of cis-GO1423 resembled TH129 (Figure 4D), and GO1423 showed high PSS as well as a very long half-life of the cis-isomer (Figures 4E and S4C). Moreover, repeated photoisomerization cycles indicate high photostability (Figure 4G). In cellular circadian assays, thermally adapted and dark-kept GO1423 (>98% trans) was almost inactive, while green light-irradiated (cis-enriched) GO1423 caused period lengthening (Figure S5). Furthermore, cellular irradiation with green light activated GO1423 and subsequent irradiation with violet light resulted in its deactivation, without affecting TH129 (Figure 5D). Altogether, we developed isoform-selective photoswitchable modulators of CRY1 and enabled reversible control of the circadian period by visible light.
Discussion
We discovered previously unknown benzophenone derivatives that selectively target CRY1 for circadian clock regulation. This finding brought about two breakthroughs: (1) isoform selectivity against a highly conserved region, and (2) reversible and noninvasive long-term regulation of biological processes, specifically the circadian clock, utilizing photopharmacology. Development of isoform-selective compounds against highly homologous proteins has been the major challenge in drug discovery. In contrast to the difficulty of molecular design due to high (91%) sequence similarity of the entire PHR (∼500 residues) between CRY1 and CRY2, unbiased phenotypic screening of circadian clock modulators resulted in the identification of unique tools, TH303 and TH129. CRY(PHR) contains two functional pockets: the FAD-binding pocket, which is recognized by the C-terminal region of a ubiquitin ligase FBXL3 for degradation,10 and the secondary pocket, which interacts with CLOCK-BMAL1 for transcriptional repression.27,28 TH303 and TH129 occupied the FAD-binding pocket, resulting in stabilization of CRY1 and consequently causing the period lengthening. These compounds also rearranged the lid loop structure through a unique interaction of the benzophenone moiety with CRY1 F409. The lid loop is located at an interaction interface with FBXL3 and PER210,29 and has functional importance: CRY1 F409A mutation resulted in increased basal stability of CRY1 (Figure 3F) and hyper repression of Per2 reporter activity.30 Therefore, the lid loop provides a new target to control CRY function by compounds.
Application of a cutting-edge photopharmacological approach opens the possibility of spatiotemporal manipulation of circadian rhythms. We previously introduced photoremovable protecting groups (PPGs) to the CKI inhibitor longdaysin and demonstrated inducible regulation of the circadian period by light.31 This method, however, is limited by the irreversible nature of PPG activation. In contrast, an azobenzene photoswitch enables light-induced reversible regulation, but its successful introduction into the compound of interest is not always straightforward. One of the greatest challenges in photopharmacology is to obtain and maximize the difference of binding affinity between the cis- and trans-isomer of the photoswitchable drug.32,33 Here, we have shown that benzophenone is a suitable moiety for rational azologization of biologically active compounds. The bent geometry and dipole moment of benzophenone were critical in successfully obtaining “turn-on” type photoswitches as a result of higher similarity with the metastable cis-isomer. Detailed structure–activity relationship and photochemical analyses of all possible azobenzene regioisomers led to discovery of the ideal substitution for light-dependent regulation of CRY1. Long half-life of the cis-isomer, high PSS and stability, and photoreversibility in cell culture medium were crucial photochemical parameters for successful demonstration of reversible manipulation of circadian rhythms in long-term cellular assays. Although the majority of biologically applied photoswitching molecules to date utilize UV light for trans-to-cis isomerization,15,16,32,33 UV is poorly biocompatible because of cytotoxicity and negligible tissue penetration. Due to the deeper penetration and lower toxicity of visible light, GO1423 comprising a tetra-o-fluoroazobenzene moiety has an advantage for future application to circadian clock regulation at tissue levels using green and violet light for isomerization. Given that CRY1 is related to many diseases such as sleep phase disorder,6 diabetes,4,5 and cancer,34 isoform-specific spatiotemporal regulation of CRY1 by photopharmacology represents a promising basis of targeted chronotherapy in the future.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c12280.
Author Contributions
† D.K., S.M., and T.O. contributed equally.
Notes
The authors declare no competing financial interest.
Acknowledgments
We thank Natsuko Ono, Daniel Bader, Dr. Yoshiki Aikawa, Ayano Shiba, Naoya Kadofusa, Dr. Shinya Oishi, Dr. Kazuhiro Abe, Dr. Kunio Hirata, and Dr. Toshiya Senda for technical assistance. This work was supported in part by JST PRESTO Grant JPMJPR14LA (T.H.); JSPS Grants 18H02402 and 20K21269 (T.H.), and JP1905463 (K.I.); Takeda Science Foundation (T.H.); Uehara Memorial Foundation (T.H.); The Netherlands Organization for Scientific Research NWO–CW Top grant (B.L.F.) and VIDI Grant 723.014.001 (W.S.); the Royal Netherlands Academy of Arts and Sciences KNAW (B.L.F.); the Ministry of Education, Culture and Science Gravitation program 024.001.035 (B.L.F.); and the European Research Council Advanced Investigator Grant 227897 (B.L.F.). X-ray diffraction data collection and preliminary experiments were carried out at beamlines BL44XU of SPring-8 (Proposal Nos. 2017A6743, 2017B6743, 2018B6843, and 2019A6942), BL41XU of SPring-8 (Proposal No. 2018B1011), and BL-17A of Photon Factory (Proposal Nos. 2016R-63, 2017G563, and 2019G024). Recombinant CRY expression and beamline experiments were supported in part by BINDS from AMED (Support Nos. JP19am0101074-0055 and JP19am0101071-0529).
 Photopharmacological
Manipulation of Mammalian CRY1
for Regulation of the Circadian Clock
Photopharmacological
Manipulation of Mammalian CRY1
for Regulation of the Circadian Clock