
- Altmetric
Replying to H. Rzepa. Nature Communications 10.1038/s41467-021-21433-8 (2021)
We are writing in response to Rzepa’s theoretical analysis of the generation of C2, which cites our recent paper describing the first chemical synthesis of C2 (ref. 1). Rzepa suggests on the basis of several in silico approaches that the formation of free C2 from alkynyl-λ3-iodane and fluoride ion would be prohibitively endo-energetic. He proposes three possible explanations of the apparent discrepancy between these theoretical calculations and our experimental findings, of which one is that some species other than C2 is actually formed.
As our original paper was primarily experimental, describing room-temperature chemical synthesis of C2 and the first bottom-up chemical synthesis of nanocarbons from C2, we should like to respond to the latter point. We believe that the evidence presented in our paper for the generation of C2 itself is compelling1. In particular, the connected-flask, solvent-free experiment clearly supports the generation of free C2 gas, for the following reasons:
When 13C-labeled 1b-13Cβ was used, a 1:1 mixture of O- ethynyl galvinoxyl 15-13Cα and 15-13Cβ was obtained (Fig. 1a). Connected-flask, solvent-free experiment. a
13C-labeling experiment. b APCI mass spectrum (positive ion mode) obtained from the contents in Flask B.
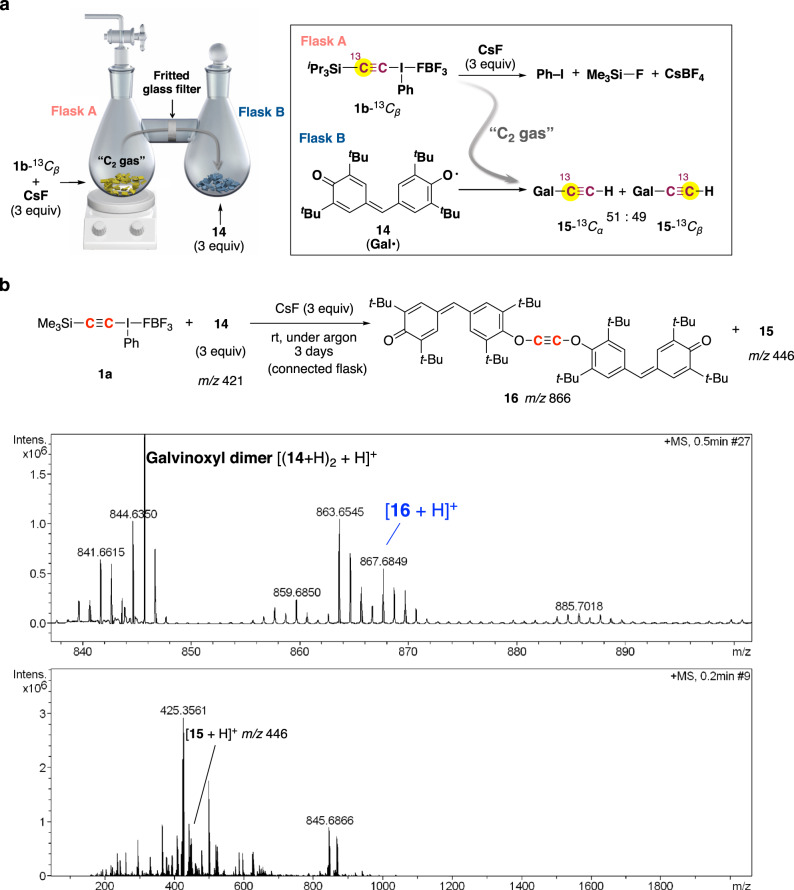
APCI mass spectrum of the contents in Flask B included the peak assignable to acetylene digalvinoxyl ether 16 (Fig. 1b).
It is more difficult to establish conclusively whether free C2 is generated in solution, but the following experimental facts are relevant:
3.The relative rate of hydrogen abstraction between CH2Cl2 and 9,10-dihydroanthracene (12) is calculated to be ca. 1:20 (per 1H).
4.Galvinoxyl radical 14 solely produced O-ethynyl galvinoxyl 15 (not 16).
5.The amount of the mixture of 15-13Cα and 15-13Cβ obtained by the reaction of 1b-13Cβ with 14 differed in solvents of different viscosities (η).
These results indicated that the radical character of the intermediate is much milder than that of the common unstabilized alkynyl radical, which is consistent with the presence of an interaction between the radicals in C2, in other words, a singlet biradical (charge-shift bonding) character, as suggested in a recent theoretical study2.
At present, we cannot explain the discrepancy between Rzepa’s theoretical evaluation and our experimental results. However, as discussed in our original paper, we believe that all our experimental observations can only be rationalized in terms of the generation of gaseous C2. Our findings have attracted great interest, and we anticipate that independent experimental findings will emerge in the near future. We ourselves are working on the direct observation of chemically generated C2 by means of Raman spectroscopy, ESR spectroscopy, and other methods, and we hope that this work will provide definitive experimental evidence for the bond length and electronic state of C2 (ref. 3 and references therein).
Author contributions
K.M., M.O., and M.U. conceived and designed the experiments. S.N., Y.M., T.H., T.O., and K.M. conducted the experiments. K.M. and M.U. wrote the manuscript. All authors participated in data analyses and discussions. K.M., M.K., and M.U. directed the project.
Data availability
The data that support the findings of this study are available from the authors on request.
Competing interests
The authors declare no competing interests.
References
1.
2.
3.
 Reply to “A Thermodynamic assessment of the reported room-temperature chemical synthesis of C2”
Reply to “A Thermodynamic assessment of the reported room-temperature chemical synthesis of C2”
