
- Altmetric
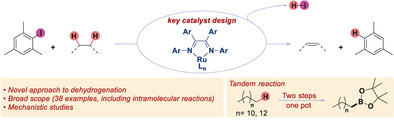
The direct dehydrogenation of alkanes is among the most efficient ways to access valuable alkene products. Although several catalysts have been designed to promote this transformation, they have unfortunately found limited applications in fine chemical synthesis. Here, we report a conceptually novel strategy for the catalytic, intermolecular dehydrogenation of alkanes using a ruthenium catalyst. The combination of a redox‐active ligand and a sterically hindered aryl radical intermediate has unleashed this novel strategy. Importantly, mechanistic investigations have been performed to provide a conceptual framework for the further development of this new catalytic dehydrogenation system.
An approach to the catalytic dehydrogenation of alkanes and heterocycles uses Ru and redox‐active ligands. A wide range of functionalized substrates afforded dehydrogenated products in good yields. Preliminary mechanistic studies suggest that a redox‐active ligand‐assisted intermolecular hydrogen atom transfer is crucial to this process.
Introduction
Alkenes are important building blocks for a variety of applications spanning petrochemistry to natural product synthesis. [1] The direct, catalytic dehydrogenation of alkanes offers the most versatile and efficient strategy to access alkenes. [2] Indeed, industrially, alkenes are generally accessed through a dehydrogenation process. However, the extremely harsh reaction conditions have limited the possibility to harness this reaction in fine chemical synthesis. [3] A seminal report of dehydrogenation by Crabtree in 1979, in which tert‐butylethylene (TBE; 3,3‐dimethyl‐1‐butene) served as a hydrogen acceptor to produce the corresponding alkene‐IrIII‐complex from an IrI species, has spurred the development of milder, Ir‐catalyzed protocols for alkane dehydrogenation (Scheme 1 a). [4] The proposed mechanism of this reaction features an oxidative addition of the inert C−H bond, mediated by noble metal complexes, followed by a β‐hydride elimination. [5] The same strategy has also been demonstrated with Ru‐ and Os‐pincer complexes, [6] however, with limited applicability. A novel approach has been inspired by the exquisite reactivity achieved with desaturase enzymes, which can dehydrogenate alkanes with high site‐selectivity through a stepwise process under mild reaction conditions. [7] To mimic this reactivity with non‐enzymatic catalytic systems, White and co‐workers have managed to divert a non‐heme iron hydroxylation catalyst toward dehydrogenation. [8] However, further oxidation of the double bond under the reaction conditions could not be prevented, precluding the use of this method for alkene synthesis from alkanes. Subsequently, the Baran and Gevorgyan groups have developed alternative strategies for the dehydrogenation reaction employing small molecule‐based reagents to prevent overoxidation. [9] In these processes, highly reactive aryl radical intermediates are used as controlling elements to mediate the desaturation of alkanes by an intramolecular hydrogen atom transfer (HAT) process (Scheme 1 b). [9] While these methods offer practical solutions for intramolecular dehydrogenation, they have not yet demonstrated their applicability in more challenging intermolecular dehydrogenations.

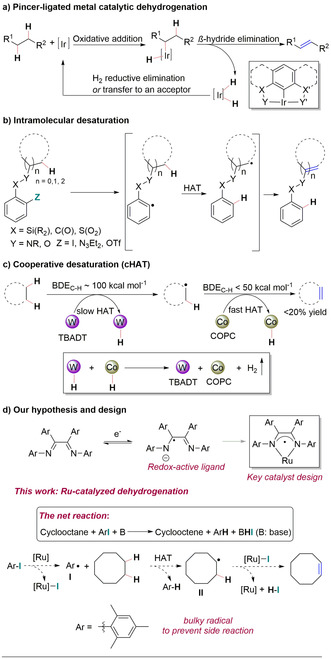
Previous works: a) Pincer‐ligated metal catalytic dehydrogenation, b) Intramolecular desaturation, c) Cooperative desaturation (cHAT), and d) Our hypothesis and design.
Recently, Sorensen and co‐workers reported the most successful attempt so far to mimic enzymatic reactivity in an intermolecular process, which generates alkenes from alkanes through a radical pathway. [10] In this work (Scheme 1 c), a dual catalytic system composed of a tungsten photocatalyst, which can initially abstract a hydrogen atom from a C−H bond, and a cobalt cocatalyst, which can generate alkenes from the resulting carbon‐centered radical, is used in a tandem fashion to perform dehydrogenation of simple alkane substrates upon release of hydrogen gas. Despite the low yield and limited substrate scope reported, this reaction remains the state‐of‐the‐art in the area of catalytic intermolecular dehydrogenation reactions based on a HAT process, clearly highlighting the need for the development of new strategies.[ 10 , 11 ]
Herein, we report a conceptually new strategy for the HAT‐mediated intermolecular dehydrogenation reaction of alkanes. We have used a redox‐active ligand to facilitate the Ru‐catalyzed generation of highly reactive yet sterically hindered aryl radicals, which can mediate a facile intermolecular alkane dehydrogenation reaction (Scheme 1 d).
Results and Discussion
Our key hypothesis to develop this new reaction relies on the use of redox‐active ligands [12] to enable an otherwise challenging combination of one‐ and two‐electron processes at a Ru‐center.[ 12d , 12e , 12f , 13 ] In particular, aromatic diimine ligands have often been shown to formally adopt a monoanionic, monoradical character which could possibly help us to divert the reactivity of Ru species toward radical pathways.[ 12d , 12e , 12f , 13 , 14 ] In theory, this could allow the generation, from a reaction between a Ru‐center and an aryl iodide, [15] of a highly reactive aryl radical intermediate I, which could then participate in an intermolecular HAT process; the newly generated carbon‐centered radical II can then react with the partially oxidized Ru‐intermediate to release the desired alkene and HI which can subsequently be trapped by a base (Scheme 1 d). In this process, the aryl iodide formally plays the role of a mild oxidant for the dehydrogenation process.
We started our investigation by selecting cyclooctane and iodobenzene as benchmark substrates and Ru3(CO)12 as precatalyst. We first focused our attention on conjugated diimine ligands as their ability to adopt a monoanionic radical character had been previously shown by Chirik and co‐workers in hydrofunctionalization reactions. [14] Interestingly, the same set of ligands did not lead to any product formation for our target dehydrogenation reaction (Supporting Information, Tables S1, S2). We envisaged that adding phenyl rings on the ligand backbone may further enhance the propensity of this ligand to exhibit a non‐innocent behavior owing to a possible additional delocalization of the radical into a more extended π‐system. Gratifyingly, this strategy led to almost full conversion of the starting material, especially when bidentate diimine ligands were employed. Unfortunately, the yield of cyclooctene was relatively low, and the coupling product between the phenyl radical and the aromatic solvent was observed (Supporting Information, Figure S1). Notably, we reasoned that the lifetime of the aryl radical and potentially the chemoselectivity of the HAT process could be readily influenced by tuning the steric and electronic properties of the aryl radical to favor an intermolecular HAT process. [16] We decided to investigate the effect of introducing substituents in the ortho position of the starting aryl iodide (Supporting Information, Figure S1). This resulted in a significant increase in the yield of cyclooctene when a mixture of alkane (5 equiv), mesityl iodide (1 equiv), diimine ligand (L4, 18 mol %), and Ru3(CO)12 (3 mol %) were allowed to react at 150 °C in chlorobenzene for 24 hours (see the Supporting Information: optimization of the model reaction).
Next, we sought to explore the substrate scope of this dehydrogenation protocol (Scheme 2). Generally, cycloalkanes with a larger ring size gave the corresponding alkenes in a higher yield. Cyclooctane, cyclododecane, and cyclopentadecane afforded 3 a in 84 % yield, 3 d as a mixture of E/Z (2/3) isomers [17] in 82 % yield, and 3 e as an unidentified mixture of E/Z isomers in 91 % yield, respectively. By contrast, the smaller ring size cycloalkanes gave the corresponding alkenes in 45 % (3 b) and 72 % (3 c) yields. It should be noted that the yields of these cycloalkenes seem to correlate with the lower boiling points of the corresponding alkane substrates, possibly hinting a material loss through evaporation. Unfunctionalized linear alkanes also successfully underwent a dehydrogenation reaction, giving the corresponding alkenes (3 f–i) as a mixture of isomers in 52 % to 67 % yield. Furthermore, a mixture of substituted cyclohexanes gave alkene regio‐isomers (3 j and 3 j′) in 34 % (17 % of 3 j + 17 % of 3 j′) yield. When the less crowded 1‐iodo‐2‐methylbenzene was used as an aryl radical precursor, the alkene regio‐isomers (3 j and 3 j′) were obtained in 49 % (17 % of 3 j + 32 % of 3 j′) yield. Interestingly, the isomeric ratio of 3 j/3 j′ remained constant throughout the reaction, ruling out the occurence of isomerization as a side reaction when using the substituted cyclohexane substrates (see the Supporting Information, control experiments (2) and (3)). These results also indicate that the selectivity obtained might reflect the kinetic selectivity of the process. Next, we explored the reactivity toward ethers and aliphatic amines. They all underwent successful dehydrogenation to the corresponding alkenes (3 k–q) with good chemoselectivity. In the case of tertiary aliphatic amines, good stereoselectivity was observed providing the corresponding enamines (E) in moderate yield (3 o–q). The chemo‐ and stereoselectivity of ether and aliphatic amine dehydrogenations are similar to previous studies employing Ir‐pincer catalysts. [18]

![Intermolecular dehydrogenation scope of alkanes, ethers and aliphatic amines. Reaction conditions: Ru3(CO)12 (3 mol %), L4 (18 mol %), 1 (0.5 mmol), 2 n (2.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 24 h, 1H‐NMR yields using CH2Br2 as the internal standard. [a] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (10 mmol). [b] Ru3(CO)12 (5 mol %), L4 (30 mol %). [c] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (5 mmol). [d] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (5 mmol), 1‐iodo‐2‐methylbenzene (1 b, 0.5 mmol).](/dataresources/secured/content-1766030345473-f42da7e5-9c18-48ca-a569-db1abdf9b80a/assets/ANIE-60-7290-g004.jpg)
Intermolecular dehydrogenation scope of alkanes, ethers and aliphatic amines. Reaction conditions: Ru3(CO)12 (3 mol %), L4 (18 mol %), 1 (0.5 mmol), 2 n (2.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 24 h, 1H‐NMR yields using CH2Br2 as the internal standard. [a] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (10 mmol). [b] Ru3(CO)12 (5 mol %), L4 (30 mol %). [c] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (5 mmol). [d] Ru3(CO)12 (6 mol %), L4 (36 mol %), 2 n (5 mmol), 1‐iodo‐2‐methylbenzene (1 b, 0.5 mmol).
This method also proved to be efficient toward aryl‐containing heterocycles (Scheme 3). Dehydrogenated products, such as indoles (6 a–d), quinolines (6 e and 6 f), isoquinolines (6 g and 6 h), lactone (6 i), and arylimine (6 j), were isolated in moderate to good yield. In contrast to previously reported methods, [19] a range of functional groups (F, Br, methyl, methoxyl, and ester) (6 b–d, 6 f, 6 h, and 6 i) were tolerated. Harmine (6 k), a potent alkaloid used in several medical applications, [20] could also be obtained, showcasing the synthetic potential of the new protocol. Next, we evaluated the scope of intramolecular reactions using this procedure with slightly modified conditions (using 0.1 mL of PhCl as solvent). The corresponding unsaturated products (7 a–c) were isolated in good yield, similar to the previous protocol. [9c]

![Intermolecular dehydrogenation scope of alkanes, ethers and aliphatic amines. Reaction conditions: Ru3(CO)12 (3 mol %), L4 (18 mol %), 1 (0.6 mmol), 5 n (0.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 16 h, isolated yields. [a] Ru3(CO)12 (5 mol %), L4 (30 mol %), 1 (1.1 mmol), 5 n (0.5 mmol). [b] PhCl (0.1 mL), 18 h.](/dataresources/secured/content-1766030345473-f42da7e5-9c18-48ca-a569-db1abdf9b80a/assets/ANIE-60-7290-g001.jpg)
Intermolecular dehydrogenation scope of alkanes, ethers and aliphatic amines. Reaction conditions: Ru3(CO)12 (3 mol %), L4 (18 mol %), 1 (0.6 mmol), 5 n (0.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 16 h, isolated yields. [a] Ru3(CO)12 (5 mol %), L4 (30 mol %), 1 (1.1 mmol), 5 n (0.5 mmol). [b] PhCl (0.1 mL), 18 h.
Functionalized aryl alkanes are rarely used as substrates [21] in intermolecular dehydrogenation reactions, because of the high propensity of conjugated aryl alkenes to participate in polymerization and other side reactions. Interestingly, using our reaction conditions from Scheme 2, we could obtain the desired product 9 a in a low GC‐yield (11 %) and the radical dimerization product 9 b as a mixture of diastereoisomers [22] (meso/DL=1/1) in 18 % GC‐yield, when propyl benzene was employed as a substrate. Then, we started to optimize the conditions for propyl benzene dehydrogenation using a selection of different redox‐active ligands[ 12 , 23 ] and phosphine ligands (Supporting Information, Table S3). Finally, using a slightly modified version of our original protocol (Scheme 4), the aryl alkanes bearing different substituents (Cl, Br, phenyl, and methoxyl) gave the desirable conjugated aryl alkenes in moderate to good yields (9 a–f, 9 h, and 9 h′). Compared to the previously reported dehydrogenation of tetrahydronaphthalenes,[ 11e , 24 ] we obtained the aromatic product 9 h′′ in a low yield (12 %) and the conjugated aryl alkene as a mixture of regioisomers [25] (9 h/9 h′=1/1.2) in 47 % (21 % of 9 h + 26 % of 9 h′) using 6‐methoxy‐1,2,3,4‐tetrahydronaphthalene as a substrate. Additionally, an aromatic heterocycle also gave the corresponding dehydrogenated product (9 g) in 32 % yield.

![Intermolecular dehydrogenation scope of aryl alkanes. Reaction conditions: Ru3(CO)12 (3 mol %), dppp (10 mol %), L13 (30 mol %), 1 (0.5 mmol), 8 n (7.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 24 h, 1H‐NMR yields using CH2Br2 as the internal standard. [a] reaction conditions from Scheme 2. [b] 8 n (2.5 mmol).](/dataresources/secured/content-1766030345473-f42da7e5-9c18-48ca-a569-db1abdf9b80a/assets/ANIE-60-7290-g007.jpg)
Intermolecular dehydrogenation scope of aryl alkanes. Reaction conditions: Ru3(CO)12 (3 mol %), dppp (10 mol %), L13 (30 mol %), 1 (0.5 mmol), 8 n (7.5 mmol), Cs2CO3 (1 mmol), PhCl (1 mL), 150 °C, 24 h, 1H‐NMR yields using CH2Br2 as the internal standard. [a] reaction conditions from Scheme 2. [b] 8 n (2.5 mmol).
To demonstrate the synthetic applicability of this protocol, we used dodecane, tetradecane, and cyclooctane as substrates to perform gram‐scale tandem reactions (Scheme 5). Two terminal borylation products (10 a and 10 b) were isolated in 52 % and 54 % yields from one‐pot tandem reactions (Scheme 5 a). [26] An epoxide product (10 c) was isolated in 67 % yield proceeding through two separate steps (Scheme 5 b). [27] Overall, the results using this catalytic system are synthetically relevant and are comparable to the state‐of‐the‐art in radical‐based intermolecular dehydrogenation reactions.

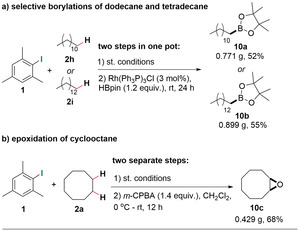
Applicability and scalability of our dehydrogenation protocol.
We were particularly interested to confirm both the involvement of radical species (see the Supporting Information, control experiments (1)) and the implication of redox non‐innocence in enabling this novel reactivity. First, we conducted radical coupling and trapping experiments under optimum conditions (Scheme 6 a). While the preceding, highly reactive aryl radical (Scheme 1 d, intermediate I) could not be trapped directly by BHT/TEMPO, we were nevertheless able to detect it through reaction with a more nucleophilic aromatic solvent (34 % of coupling product 10 d), thus indicating that this species is likely generated under our reaction conditions [Scheme 6 a, Eq. (1)]. Furthermore, we observed the radical dimerization product 9 b (18 %) and the radical alkyl‐BHT trap product 10 e (46 %) during additional control reactions [Scheme 6 a, Eqs. (2) and (3)]. Additionally, the dimer 9 b (meso) [22] was isolated and structurally characterized by 1H/13C‐NMR and X‐ray analysis (Supporting Information, page 15, isolation of the dimer 9 b). Moreover, the radical trap product 10 e was also isolated and structurally characterized by 1H/13C‐NMR (see the Supporting Information, radical trap experiments). Alkyl‐BHT adducts similar to 10 e have been reported previously in the literature.[ 28 , 29 ] Using TEMPO as another radical trap, 12 % of 3 a was obtained while 84 % of 1 was recovered from this experiment [Scheme 6 a, Eq. (4)]. Overall, these observations strongly support the presence of both an aryl and alkyl radical in accordance with our initial hypothesis (Scheme 1 d). Finally, we performed an intermolecular competition experiment and observed a large primary kinetic isotope effect (Scheme 6 b, KIE: 6.5), a result comparable to literature values (KIE: 4.8–11) for C−H amination involving an intermolecular HAT mechanism enabled by Ru‐bis‐imido complexes. [30]

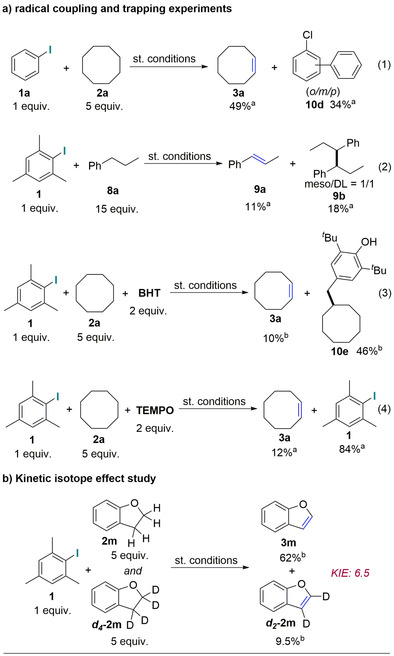
Radical coupling and trap reactions and kinetic isotope effect study. Reactions were performed under optimum conditions (0.5 mmol scales). GC yield using dodecane as the internal standard. 1H NMR yields using CH2Br2 as the internal standard.
Next, we performed mechanistic and organometallic studies (Figure 1). First, we conducted preliminary stoichiometric experiments to isolate any potential reaction intermediates. Stirring Ru3(CO)12 at 110 °C in toluene for 24 hours in the presence of two variations of the ligand gave both complexes I and II in 61 % and 42 % yield, respectively (Figure 1 a) along with small amounts of diligated and triligated species (see SI, X‐ray analysis). In contrast to previously reported Ru‐diimine complexes, which were often reported as dinuclear species, [31] the products of our reactions were isolated as mononuclear ruthenium complexes. Single crystal X‐ray analysis of complex II showed the ruthenium atom set in a square pyramidal geometry with the diimine ligand placed in the same plane with a bite angle of 76.59(6)°. The mononuclear nature and the vacant coordination site resulted in a high air‐ and moisture‐sensitive behavior, with the compound decomposing within seconds when exposed to air. Interestingly, the influence of the redox‐active ligand scaffold resulted in unusual bond lengths for the carbon–nitrogen and the amino–ruthenium bonds, a result consistent with our initial hypothesis. The Ru–N1 and Ru–N2 distances are within the range of values reported for anionic nitrogen‐based ligands (2.03 Å complex II vs. 1.97 Å literature). [32] Accordingly, we observe elongation of the C−N bonds (1.28 Å literature vs. 1.36 Å complex II) when compared to imine bond lengths, as well as the shortening of the C−C bond (1.51 Å literature vs. 1.40 Å complex II) towards a range more typical for an alkene bond. The solid‐state structure of complex I and II clearly supports an ambiguous oxidation state at the Ru‐center (Supporting Information, Figures S2, S3). By analogy with similar species previously reported, [33] it could be regarded as a RuI species supported by a monoanionic, radical ligand. [34]

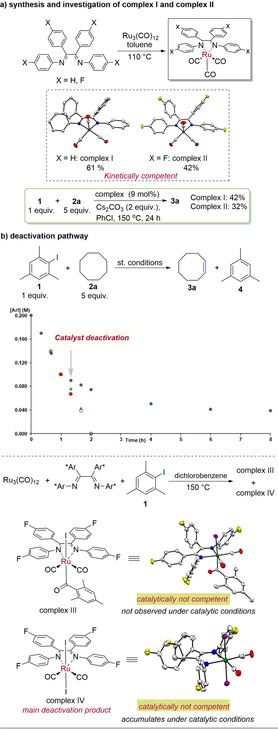
Mechanistic and organometallic studies. For more details, see the Supporting Information.
Gratifyingly, performing the reaction using isolated complex I and II as catalyst led to 42 % and 32 % yields (Figure 1 a, below) of product formation, respectively, and showed a similar initial rate and kinetic profile when compared with the normal reaction conditions involving the in situ generated catalyst, supporting the kinetic competency of these complexes. Unfortunately, catalysts I and II seem to be completely deactivated after reaching the fourth turnover, a result which explains the lower yield observed in this case (Supporting Information, Table S4). Recently, Bures [35] and Blackmond [36] described a new method to determine catalyst deactivation and product inhibition by carrying out a set of three simple experiments, where the concentration of the reagents is varied while keeping the concentration of the catalyst constant (Figure 1 b, top). It was evident from these experiments that after the fifth turnover, the rate of the reaction clearly decreased because of a possible catalyst deactivation, which involved no product inhibition. Unfortunately, even using the approach of the initial rate, we could not gather further information as the low homogeneity of the reaction hampered the collection of reproducible data.
In an attempt to shed light on the deactivation pathway, we next performed another stoichiometric experiment involving aryl iodide reagents. Interestingly, in the absence of the diimine ligand, no reaction was observed even at elevated temperatures, supporting the role of the ligand in promoting the activation of the iodide oxidant. Instead, the addition of mesityl iodide to pre‐formed complex I or II led to a full conversion to a new complex. In contrast to what has been reported with other classes of diimine ligands bearing alkyl substituents on the backbone instead of aryl groups, it was not possible to observe or isolate any of the oxidative addition products. [37] Instead, the trans carbonyl insertion complex III was obtained (Figure 1 b, bottom) along with the diiodo ruthenium complex IV. In contrast to complex I, these two complexes both exhibit an octahedral geometry, with Ru−N and C−N bond lengths and angles within the range of a RuII complex with dative nitrogen coordination, indicating a more classical behavior of the neutral diimine ligand in this oxidized state. We then set out to evaluate the catalytic and kinetic competence of all the isolated ruthenium complexes for the dehydrogenation of cyclooctane. Complexes III and IV only gave trace amounts of the product (Supporting Information, Table S4), suggesting that these species are possible deactivation products. More importantly, the di‐iodo species IV could be observed by 19F NMR spectroscopy under catalytic conditions and its concentration steadily increased over the course of the reaction (Supporting Information, Figure S4). This observation, when combined with the catalytic incompetence observed above, clearly suggests that the formation of the di‐iodo species is the major deactivation pathway under the reaction conditions. This result provides critical information for the design of second‐generation catalysts for this transformation.
While the detailed mechanism remains unclear at this stage, the observed unusual bond lengths in the solid state, the indirect detection of the two proposed carbon‐centered radical intermediates and the large primary KIE strongly support a redox‐active ligand assisted HAT pathway [30] for this intermolecular dehydrogenation reaction, similar to the one postulated in Scheme 1 d.
Conclusion
The use of Ru3(CO)12 and a diimine ligand (L4)/diketone ligand (L13) has unlocked the challenging intermolecular dehydrogenation of alkanes. The combination of a redox‐active ligand and a sterically hindered aryl radical intermediate has enabled this novel strategy, which can be used to synthesize a wide variety of alkene products. Mechanistic studies have shed light on crucial aspects of this conceptually novel catalytic system. We thus believe that the results reported herein will serve as a platform to develop a completely new family of dehydrogenation catalysts.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank the Max‐Planck‐Society and ETH Zürich (ETH Research Grant ETH‐45 19‐2) for generous funding. We thank Prof. B. List for sharing analytical equipment, and the NMR, MS, and X‐ray departments of the MPI für Kohlenforschung and ETH Zürich for technical assistance. We also thank Dr. Shin for the analysis of oligomers/polymers by GPC. L.H. thanks the China Scholarship Council for a scholarship. Open access funding enabled and organized by Projekt DEAL.
References
1
1a
1b
2
3
3a
3b
4
4a
4e
5
5b
5c
5d
6
6a
6b
6c
6d
7
7a
9
9a
9d
9d
9e
9e
10
10b
11
11a
11b
11c
11d
11e
11e
12
12b
12c
13
13a
13b
14
14a
14b
14c
15
15a
15a
16
16a
16c
16c
17
18
18a
18b
19
19a
19a
19b
19e
19e
19g
19g
19h
20
21
21a
21b
21c
22
22a
22b
23
23a
23a
24
24a
25
26
26b
26c
27
27
29
30
30a
30b
31
31a
31b
33
33a
33c
34
34a
35
35a
35a
37
 Ruthenium‐Catalyzed Dehydrogenation Through an Intermolecular Hydrogen Atom Transfer Mechanism
Ruthenium‐Catalyzed Dehydrogenation Through an Intermolecular Hydrogen Atom Transfer Mechanism
