
Competing Interests: This work was supported in part through Battelle Memorial Institute’s former prime contract with the US National Institute of Allergy and Infectious Diseases (NIAID) under Contract No. HHSN272200700016I and Laulima Government Solutions, LLC current prime contract with NIAID under Contract No. HHSN272201800013C (S.Y.). Y.C. and J.H.K. performed this work as former employees of Battelle Memorial Institute and current employees of Tunnel Government Services (TGS), a subcontractor of Laulima Government Solutions, LLC under Contract No. HHSN272201800013C. Battelle Memorial Institute/Laulima Government Solutions/Tunnell Government Services serve(d) as employers for government contractors only and had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials
- Altmetric
Ebola virus (EBOV), a member of the mononegaviral family Filoviridae, causes severe disease associated with high lethality in humans. Despite enormous progress in development of EBOV medical countermeasures, no anti-EBOV treatment has been approved. We designed an immunotoxin in which a single-chain variable region fragment of the EBOV glycoprotein-specific monoclonal antibody 6D8 was fused to the effector domains of Pseudomonas aeruginosa exotoxin A (PE38). This immunotoxin, 6D8-PE38, bound specifically to cells expressing EBOV glycoproteins. Importantly, 6D8-PE38 targeted EBOV-infected cells, as evidenced by inhibition of infectious EBOV production from infected cells, including primary human macrophages. The data presented here provide a proof of concept for immunotoxin-based targeted killing of infected cells as a potential antiviral intervention for Ebola virus disease.
Introduction
Ebola virus (EBOV; Mononegavirales: Filoviridae: Ebolavirus) causes Ebola virus disease (EVD), a severe human disease associated with lethality. There have been at least 19 EVD outbreaks since the discovery of EBOV in 1976 [1]. From 2013 to 2016, the largest EVD outbreak occurred in Western Africa (mainly Guinea, Liberia, and Sierra Leone), with 28,652 cases and 11, 325 deaths [2, 3]. The second-largest EVD outbreak occurred in Democratic Republic of the Congo from 2018 to 2020, with 3,481 reported cases and 2,299 deaths [4]. The high lethality associated with EVD (average ≈44% since 1976) underscores the need for effective medical countermeasures, such as vaccines and therapeutics, against EBOV. During and after 2013–2016 EVD outbreak, enormous progress was made in medical countermeasure development, resulting in United States (U.S.) Food and Drug Administration approval of the first vaccine, Ervebo, in December 2019 [5].
EBOV has a non-segmented, negative-sense, RNA genome and produces enveloped, filamentous virions. The EBOV genome is approximately 19 kb in length with seven genes in the following order: 3'-leader-NP-VP35-VP40-GP-VP30-VP24-L-trailer-5'. These seven genes encode seven structural proteins: nucleoprotein (NP) encapsidates the EBOV genomes and antigenomes; polymerase cofactor (VP35) mediates transcription/replication and immune evasion; matrix protein (VP40) drives virion assembly/egress, regulates transcription/replication, and mediates immune evasion; glycoprotein (GP1,2) mediates virion entry and inhibits the intrinsic immune response; transcriptional activator (VP30) mediates transcription initiation, re-initiation, enhancement, anti-termination, and GP mRNA editing and acts as an RNAi silencing suppressor; ribonucleoprotein complex-associated protein (VP24) regulates transcription/replication and virion assembly/egress, and large protein (L), which contains an RNA-directed RNA polymerase domain and mediates transcription/replication, mRNA maturation, and GP mRNA editing [6]. EBOV GP1,2 is synthesized as a preproprotein that is first cleaved by signalase during translocation into the endoplasmic reticulum to remove the signal peptide and then post-translationally cleaved by furin-like protease into GP1 and GP2 subunits that remain connected by a disulfide bond. The GP1-GP2 heterodimers trimerize to form mature GP1,2 peplomers that are incorporated into cellular membranes, including the plasma membrane, and ultimately into the virion envelope during virion budding [6]. Finally, co-transcription mRNA editing of the GP produces several secreted proteins (e.g., sGP, ssGP, Δ-peptide) with largely undetermined function [6].
Antibodies targeting EBOV GP1,2 are of great interest in various strategies for vaccine and therapeutic development. Although monoclonal antibody 114 (mAb114) and mAb cocktail REGN-EB3 were proven to be effective against EVD under certain conditions in the 2019 Pamoja Tulinde Maisha (PALM) randomized controlled clinical trial [7], lethality remains high, even in treated populations. The EBOV GP1,2-specific human mAb KZ52 demonstrated potent EBOV-neutralization activity in vitro, protected guinea pigs from disease caused by guinea-pig-adapted EBOV [8] but failed to protect EBOV-exposed nonhuman primates from developing lethal disease [9, 10]. On the other hand, EBOV non-neutralizing mAbs, such as 6D8, completely protected laboratory mice and rhesus monkeys when used in combination in a cocktail [11]. These and additional data indicate that the neutralization ability of an mAb does not solely predict its protective efficacy in vivo and other immune functions of an antibody, such as antibody-dependent cell-mediated cytotoxicity (ADCC), also play a role in protection [12].
We investigated an alternative application of mAb technology for direct targeted killing of EBOV-infected cells. Recombinant immunotoxins (RITs) are engineered chimeric proteins consisting of a cytotoxic protein moiety linked to a targeting protein moiety, such as an antibody variable domain (Fv) or a ligand that binds to a surface antigen selectively displayed on the target cell of interest. Most RITs in clinical trials or approved by the U.S. Food and Drug Administration contain a diphtheria toxin (DT), a Pseudomonas exotoxin A (PE), or a ricin cytotoxic moiety [13–15]. Wild-type PE consists of three domains: domain I is the cell-binding domain that targets low-density lipoprotein receptor-related protein 1 (LRP-1); domain II facilitates toxin translocation into the cytoplasm; and domain III is the catalytic domain that catalyzes the inactivation of eukaryotic translocation elongation factor 2 (EEF2) by ADP-ribosylation, thereby inhibiting protein synthesis and ultimately leading to cell death. A PE-based RIT typically contains the N-terminal-targeting moiety fused to a 38-kDa-truncated portion of PE (PE38), containing only domains II and III [16]. Therefore, in this study, we developed an RIT directly targeting EBOV GP1,2. We showed that this RIT selectively inhibits infectious EBOV production from infected cells, demonstrating the feasibility of RIT use as a novel antiviral EVD intervention.
Materials and methods
Cells
Human hepatocarcinoma Huh-7 cells were provided by Hideki Ebihara (Laboratory of Virology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, Montana, United States of America [USA]). Grivet (Chlorocebus aethiops) kidney epithelial Vero E6 cells (#CRL-1568) were obtained from the American Type Culture Collection (Manassas, Virginia, USA). All cells were grown in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific, Waltham, Massachusetts [MA], USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Millipore Sigma, St. Louis, Missouri, USA). Human monocyte-derived macrophages (MDMs) were generated from human whole blood (Biological Specificity Corporation, Colmar, Pennsylvania, USA), as described previously [17]. All cells were cultured at 37°C in a humidified 5% carbon dioxide atmosphere.
Virus
Ebola virus/H.sapiens-tc/GIN/2014/Makona-C05 (GenBank #KP096420; hereafter: EBOV) was provided by the Public Health Agency of Canada (PHAC), Winnipeg, Canada. EBOV was propagated in Vero E6 cells at a multiplicity of infection (MOI) of 0.01 in DMEM supplemented with 2% FBS. Viral titers were quantified by plaque assay on Vero E6 cells, as described previously [18]. All experiments with EBOV were performed in a biosafety level 4 (BSL-4) laboratory of the Integrated Research Facility at Fort Detrick (IRF-Frederick) following approved standard operating procedures.
Recombinant immunotoxin expression plasmid construction
The heavy-chain and light-chain sequences of mAb 6D8 were provided by John Dye (U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Maryland, USA). A single-chain variable fragment (scFv) of mAb 6D8—which contains the variable regions of the heavy (VH) and the light chains (VL) of mAb 6D8, connected by a 15-amino-acid linker (Gly4Ser)3 (Fig 1)—was generated by de novo synthesis and cloned into a pCR2.1 vector (ATUM, Newark, California [CA], USA) to generate pCR2.1-6D8scFv. pCR2.1-6D8scFv was digested with enzymes NdeI and HindIII. The resulting 6D8scFv fragment was used to replace the NdeI-HindIII fragment from PE-toxin expression plasmids pYC15-PE38 encoding YC15-PE38 [19]. The resulting 6D8-PE38 RIT expression plasmid was designated as p6D8-PE38.


Schematic diagram and the amino acid sequence of the recombinant immunotoxin 6D8-PE38.
The heavy chain of monoclonal antibody (mAb) 6D8 (6D8-VH, yellow) is connected to the light chain of mAb 6D8 (6D8-VL, blue) by a (Gly4Ser) linker (grey), which forms a single-chain variable fragment (scFV) of mAb 6D8. This scFv is fused to the effector domains II and III of Pseudomonas exotoxin A 38 (PE38, light and dark green, respectively).
Recombinant immunotoxin expression and purification
6D8-PE38 RIT was expressed and purified, as described previously [19, 20]. Briefly, the RIT expression plasmid p6D8-PE38 was transformed into Max Efficiency DH5α Escherichia coli BL21(DE3) (New England Biolabs, Ipswich, MA, USA). Then, isopropyl-β-D-thiogalactopyranoside (IPTG, Millipore Sigma) was used to induce RIT expression. The inclusion body fraction was isolated from the bacterial pellets by lysozyme treatment and high-speed centrifugation (27,000 xg for 50 min at 4°C). The RIT was denatured and solubilized in denaturing buffer (6 M guanidine HCl, 2 mM EDTA, 100 mM Tris-HCl, pH 8), followed by reduction of disulfide bonds by addition of dithioerythritol powder (Millipore Sigma) to achieve a 10-mg/ml concentration, and incubation overnight at room temperature. The solubilized reduced RIT (MW = 66 kDa) was then refolded in refolding buffer (0.5 M arginine, 1 mM EDTA, 100 mM Tris-HCl, pH 9.5, 551 mg/ oxidized glutathione). The refolded proteins were dialyzed, and then purified by anion exchange chromatography using Q Sepharose and Mono Q anion exchangers (GE Healthcare Life Sciences, Pittsburgh, Pennsylvania, USA) and size-exclusion chromatography on a TSK3000 column (Millipore Sigma). The purified RIT concentration was determined using a bicinchoninic acid (BCA) protein assay, according to the manufacturer’s instructions (Thermo Fisher Scientific). The protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue solution (SimplyBlue Safe Stain, Thermo Fisher Scientific).
Surface staining by flow cytometry
Vero E6 cells (4 x 105 cells/well, 6-well plate format) were transiently transfected with 2.5 μg of empty or EBOV (variant Yambuku, isolate Mayinga) GP1,2 or Marburg virus (MARV, variant Mt. Elgon, isolate Musoke) GP1,2 pCAGGS expression plasmids [21, 22] using Roche X-tremeGENE HP DNA transfection reagent (Millipore Sigma). Cells were dissociated with enzyme-free cell-dissociation buffer (Thermo Fisher Scientific) 48 h post-transfection (p.t.) and stained with 2 μg/ml of mAb 6D8, followed by Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Thermo Fisher Scientific) on ice. As a control, all cells were stained with the same concentration of mouse IgG, followed by the same secondary antibody. These samples were analyzed with an LSRFortessa flow cytometer (Becton, Dickinson and Company, San Jose, CA, USA), and the data were analyzed with FlowJo software (Tree Star, Ashland, Oregon, USA). All experiments were performed in duplicates.
Recombinant immunotoxin binding specificity analysis by flow cytometry
6D8-PE38 RIT was labeled with an Alexa Fluor 488 microscale protein labeling kit, according to the manufacturer’s instructions (Thermo Fisher Scientific). Vero E6 cells were transiently transfected with pCAGGS-EBOV-GP1,2 or control pCAGGS-MARV-GP1,2. 48 h p.t., cells were dissociated with enzyme-free cell-dissociation buffer. Transfected cells were washed with phosphate-buffered saline (Thermo Fisher Scientific) and then incubated with phosphate-buffered saline containing 3% bovine serum albumin (Millipore Sigma) at 4°C for 30 min to block non-specific binding. The cells were then incubated with different concentrations of labeled 6D8-PE38 RIT at 4°C for 1 h. The binding of immunotoxin on the cell surface was analyzed by flow cytometry. All experiments were performed in duplicates.
Immunofluorescence assay
Huh-7 cells (4 x 104 cells/well, 96-well plate format) or MDMs (1 x 105 cells/well, 96-well plate format) were inoculated with EBOV at an MOI of 0.3 or 3. After 1 h of incubation at 37°C, viral inoculums were removed, and the cells were supplemented with DMEM containing 2% FBS. At various times post-inoculation, cell plates were fixed with 10% neutral buffered formalin (NBF, Thermo Fisher Scientific) for 24 h and then transferred from the BSL-4 to a BSL-2 laboratory. Without the permeabilization step, the plates were stained with mouse anti-EBOV GP1,2 mAb 6D8, followed by secondary Alexa Fluor 488-conjugated goat anti-mouse IgG antibody (Thermo Fisher Scientific). Cell nuclei were stained with Hoechst 33342 dye (Thermo Fisher Scientific). Fluorescent signal images were acquired with the Operetta high-content imaging system (PerkinElmer, Waltham, MA, USA). All experiments were performed in duplicates.
Inhibition of infectious virus production assay
Huh-7 cells (4 x 104 cells/well, 96-well plate format) or MDMs (1 x 105 cells/well, 96-well plate format) were inoculated with EBOV at an MOI of 0.3 or 3. After 1 h of incubation at 37°C, viral inoculums were removed and the cells were treated with increasing concentrations of 6D8-PE38 RIT, control RIT YC15-PE38 [19], mAb 6D8 or control mouse IgG. At 48 h post-exposure (p.e.), tissue culture supernatants were collected. Virus titers in tissue culture supernatants were determined by plaque assay, as described previously [18]. All experiments were performed in duplicates.
Data analysis
All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Statistically significant differences in viral titer were determined by unpaired Student t-test (*, P < 0.05, significant; **, P < 0.01, very significant; ***, P < 0.001, highly significant).
Results
Design and production of EBOV GP1,2-targeted recombinant immunotoxin 6D8-PE38
To test the potential and feasibility of the immunotoxin as a therapeutic concept, we chose mAb 6D8 to design an immunotoxin targeting EBOV GP1,2. Developed by the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), mAb 6D8 is a non-neutralizing mAb that recognizes a linear epitope in the mucin-like domain of GP1, 2’s GP1 subunit (amino acid residues 389–405: HNTPVYKLDISEATQVE; absent in GP1s of other ebolaviruses and filoviruses) [23]. mAb 6D8 specifically bound to the cells transfected with an EBOV GP1,2-expressing plasmid but not to cells transfected with a MARV GP1,2-expressing plasmid or empty vector control plasmid (Fig 2).

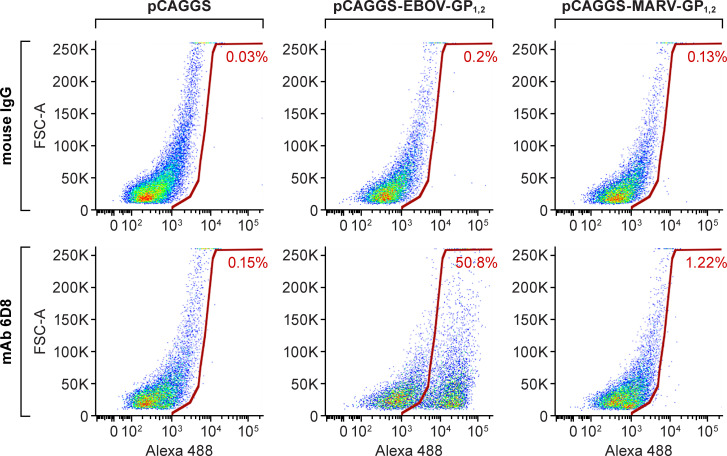
Recognition of EBOV GP1,2 expressed on the cell surface by mAb 6D8.
Vero E6 cells were transfected with plasmids pCAGGS-EBOV-GP1, 2, pCAGGS-MARV-GP1,2, or pCAGGS. After 48 h, cells were dissociated with enzyme-free cell-dissociation buffer and stained with mAb 6D8 or control mouse immunoglobulin G (IgG), followed by Alexa Fluor 488-conjugated goat anti-mouse IgG antibody. FSC-A: forward scatter area.
An scFv of mAb 6D8, which contains the variable regions of the heavy and the light chains of mAb 6D8, connected by a 15-amino-acid linker (Gly4Ser)3, was linked to PE38, which contains only the translocation and effector domains of PE. We expressed this 6D8-PE38 RIT in Escherichia coli and purified it from the inclusion body fraction through standard protocols of solubilization, denaturation, refolding, ion exchange, and size-exclusion chromatography [24]. We detected the expression of the 6D8-PE38 RIT in bacterial culture upon IPTG induction and in the inclusion body fraction (Fig 3). The size and purity of the 6D8-PE38 RIT (MW = 66 kDa) was demonstrated by SDS-PAGE and Coomassie staining (Fig 3).

Expression and purification of 6D8-PE38 recombinant immunotoxin targeting the EBOV GP1,2.
SDS-PAGE analysis of steps in purification of the 6D8-PE38 RIT. Lane 1: Molecular weight marker; Lane 2: Uninduced bacterial cell lysate; Lane 3: isopropyl-β-D-thiogalactopyranoside (IPTG)-induced bacterial cell lysate; Lane 4: Inclusion body preparation from induced cells; Lane 5: Purified 6D8-PE38 RIT.
Specific binding of 6D8-PE38 recombinant immunotoxin to cell surface-EBOV-GP
The specific binding of the 6D8-PE38 RIT to EBOV GP1,2, expressed on the cell surface, was evaluated by flow cytometry. As shown in Fig 4, 6D8-PE38 RIT bound to EBOV GP1,2, expressed on transfected Vero E6 cells in a dose-dependent manner. Moreover, the RIT did not bind to the control cells, demonstrating the binding specificity of 6D8-PE38 RIT.

6D8-PE38 recombinant immunotoxin specially binds to EBOV GP1,2-expressing cells.
6D8-PE38 RIT was labeled with Alexa Fluor 488. Vero E6 cells were transfected with pCAGGS-EBOV-GP1,2 or control pCAGGS-MARV-GP1,2 plasmids. After 48 h, transfected cells were incubated with the indicated concentrations of labeled 6D8-PE38 RIT. Cell-bound RIT was detected by flow cytometry. FSC-H: forward scatter height.
Inhibition of infectious EBOV production by 6D8-PE38 recombinant immunotoxin
To test the potential of the 6D8-PE38 RIT as a possible EBOV antiviral, we examined its effect on infectious virus production from cells infected with a wild-type EBOV obtained during the 2013–2016 EVD epidemic in Western Africa, caused by EBOV variant Makona [25, 26]. Since mAb 6D8 was generated with GP1,2 from the 1976 EBOV Yambuku variant as an immunogen [23], we first evaluated binding of mAb 6D8 to cells infected with EBOV variant Makona. Huh-7 cells or MDMs were exposed to live virus at an MOI of 0.3 or 3; mock-exposed cells served as negative controls. The expression of GP1,2 at 8, 24, and 48 h p.e. was assessed by staining with mAb 6D8 (Fig 5). GP1,2 expression was observed in both virus-exposed cell types in an MOI-dependent and time-dependent fashion. At 8 h p.e., GP1,2 expression was not detected in either cell type at either MOI; at 24 h p.e., GP1,2 expression was evident in both cell types, with higher levels at MOI 3; and at 48 h p.e., GP1,2 expression was robust in all cases. As expected, GP1,2 expression could not be detected in mock-infected cells.

Surface staining with mAb 6D8 of EBOV-infected Huh-7 cells and MDMs.
(A) Huh-7 cells or (B) MDMs were exposed to EBOV at an MOI of 0.3 or 3. At indicated times post-exposure, cells were stained with mAb 6D8. Fluorescence images were collected by high-content imaging.
With the confirmation of efficient mAb 6D8 binding to cells infected with EBOV, we next tested whether the 6D8-PE38 RIT was active against the infected cells. We used suppression of EBOV release as a readout of infected cell killing, as previously used in human immunodeficiency virus 1 (HIV-1) infection studies [27]. Huh-7 cells or MDMs were infected with EBOV at an MOI of 0.3 or 3. At 1 h p.e., the infected cells were washed to remove unbound virions and then treated with increasing concentrations of 6D8-PE38 RIT or control YC15-PE38 RIT. The amount of produced infectious virions was quantitated by plaque assay. We observed a dose-dependent reduction of infectious EBOV production from both cell types by 6D8-PE38 RIT, but not by the unrelated control YC15-PE38 RIT, in both Huh-7 cells (Fig 6A) and MDMs (Fig 6B). The observed potencies are comparable to reported data for other PE-based RITs with in vivo efficacy [28]. These effects cannot be explained by simple neutralization by the antibody component of the recombinant immunotoxin, since no reduction was observed with mAb 6D8 (Fig 6C), consistent with a previous report demonstrating the non-neutralizing effects of this antibody [23].

Effect of 6D8-PE38 recombinant immunotoxin on infectious EBOV production from infected cells.
(A) Huh-7 cells and (B) MDMs were exposed to EBOV at the indicated MOIs. After 1 h, viral inoculums were removed, and the cells were treated with increasing concentrations of 6D8-PE38 RIT or control YC15-PE38 RIT. At 48 h post-exposure, tissue culture supernatants were collected, and virus titers were measured by plaque assay. Data represent the means ± the standard deviations of results from triplicate wells. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 (Student t-test). (C) Huh-7 cells were exposed to EBOV at the indicated MOIs. After 1 h, viral inocula were removed, and the cells were treated with increasing concentrations of mAb 6D8 or control mouse IgG. At 48 h post-exposure, tissue culture supernatants were collected, and virus titers were measured by plaque assay. Data represent the means ± the standard deviations of results from triplicate wells.
These data demonstrate the efficacy and specificity of the 6D8-PE38 RIT against EBOV infection.
Discussion
The 2013–2016 EVD epidemic in Western Africa and recent outbreaks in the Democratic Republic of the Congo have highlighted the urgent need for effective antiviral therapeutics to meet future outbreaks. A number of investigational agents with promising activities in vitro and in animal models have been administered in the field and advanced to clinical studies [7, 29, 30]. Particularly promising are specific mAbs directed against EBOV GP1,2 [31, 32], used singly (e.g., mAb114) or in combination (e.g., REGN-EB3 mAb cocktail); the therapeutic potential of these antibodies has been the focus of several clinical trials [7, 33, 34].
This study is a first step toward an alternative EVD treatment strategy, using anti-EBOV antibodies for direct killing of virus target cells. Antibody-based target cell killing has been advanced in cancer treatment and is moving to the forefront of therapeutic approaches against diverse viral pathogens, including HIV-1. Specific efforts include adoptive transfer of T cells, e.g., natural virus-specific T cells expanded ex vivo [35] or genetically modified T cells expressing cloned T-cell receptors or chimeric antigen receptors [36, 37]; another T-cell-mediated technology involves bispecific T-cell-engaging antibodies [38]. Distinct from these technically complex cell-mediated strategies, RIT technology typically involves intravenous infusion of a purified protein for direct killing of infected cells [39, 40]; RITs targeting HIV-1 and other specific viral pathogens have been described [41–43]. We propose that immunotoxins, such as the 6D8-PE38 RIT reported herein, have potential therapeutic utility against EBOV. In this context, reported features of the original mAb 6D8 are noteworthy. This antibody strongly protected EBOV-exposed laboratory animals despite the absence of direct neutralizing activity; however, neutralization in the presence of complement was observed, suggesting that the in vivo protection reflected targeted killing of infected cells by either complement-mediated lysis or ADCC [23]. Indeed, Fc-mediated activities have been observed for many EBOV-protective antibodies [12, 44], including components of leading therapeutic cocktails, such as REGN-EB3 [45]. RIT technology, which is based on replacing the Fc domain of an antibody with a cytotoxic protein moiety, offers a direct approach for targeted killing of EBOV-infected cells, and our results indicate the efficacy and specificity of such an RIT against EBOV infection. It should be noted that the observed suppressive activity of the 6d8-PE38 RIT cannot be explained by neutralization, since mAb 6D8 is a non-neutralization antibody [23] and because the 6D8-PE38 RIT was added 1 h p.e., after removal of excess virus. A significant advantage of the 6D8-PE38 RIT, compared to the original mAb 6D8, is that the required dosage to achieve efficacy likely will be orders of magnitude lower. The in vivo efficacy range for a PE-based RIT is typically 0.05 mg/kg or less per dose [46], as contrasted with the 5–50 mg/kg range for mAb 6D8 [23] as well as other individual mAbs in efficacious anti-EBOV cocktails [7, 33, 34, 45, 47]. The mechanism of action of the 6D8-PE38 RIT tested here, which our data strongly suggest being preferential killing of EBOV-infected, will have to be evaluated further. Since EBOV, or over-expressed EBOV GP1,2, causes severe cytopathic effects in infected/transfected cells [48–50], we could not separate these effects from cytopathic effects induced by the RIT. It is thus formally possible that the RIT exerted its EBOV-inhibitory effect by other or additional means, for instance via steric hindrance caused by the cytotoxic (here, PE38) moiety. Such alternative or additional actions may also contribute to off-target effects of the RIT on various cell types that will have to be defined.
Cell-culture studies have revealed synergistic activities between particular combinations of EBOV-inhibiting drugs [18]. In considering the RIT, relevance was based on previous cell-culture studies with HIV-1 that demonstrated robust synergy between one agent that inhibits virus replication and another that directly kills infected cells. For instance, combination treatment with an HIV-1 reverse transcriptase inhibitor (azidothymidine [AZT] or didanosine [DDI]) plus an RIT (CD4-PE40) completely eliminated HIV-1 from cultures, a result not approached with either agent alone [51]. Similar cooperative activities were observed in a humanized mouse model; combination treatment with HIV-1 replication blockers (reverse transcriptase and protease inhibitors) plus RIT CD4-PE40 nearly completely prevented virus rebound after cessation of treatment, in marked contrast with the limited effect of either class of agents alone [51].
We hypothesize that RITs, similar to the proof-of-principle 6D8-PE38 RIT described here, will robustly complement EBOV-replication inhibitors. For example, an RIT might significantly enhance the therapeutic efficacy of a small-molecule RNA synthesis inhibitor, such as remdesivir, beyond what was observed clinically with remdesivir alone [7]. The molecular weights of mAbs and RITs are too large to pass through selectively permeable endothelial cell borders (e.g., blood-brain or blood-testis barriers). Hence, mAbs and RITs could only be used therapeutically to address acute EVD. However, in combination with EBOV-specific small-molecule antivirals that cross such barriers, acute and persistent EBOV infection in immune-privileged sites of EVD survivors [52] could be addressed simultaneously and synergistically [53]. In addition, recent studies suggest that RITs could be modified to enhance entry in privileged sites [54]. RITs could also be combined with anti-EBOV mAbs that act primarily by direct neutralization; these replication-blocking activities might be significantly complemented by the direct targeting cell-killing activity of the RIT. To achieve such a cooperative effect, mAb selection must avoid binding competition between the two antibody-based agents. mAb 6D8 binds to a linear epitope in the mucin-like domain of EBOV GP1,2 [23, 55], which is physically separate from the GP1,2 core, base, and glycan cap that harbors the binding sites for the potent neutralizing mAbs of current therapeutic interest [12, 45, 55–57]. In recent years, numerous EBOV-specific mAbs have been described with a broad variety of properties [12] that could be taken into account for the development of EBOV-specific RITs with improved affinity and/or more preferable GP1,2-binding sites. In addition, mAbs could be chosen that bind the GP1,2s of multiple ebolaviruses (e.g., those of Bundibugyo virus [BDBV], Sudan virus [SUDV], and/or Taï Forest virus [TAFV]), or even bind the GP1,2s of non-ebolavirus filoviruses, thereby resulting in broad-spectrum RITs.
Finally, we must consider general clinical concerns that have arisen during the extensive studies with anti-cancer RITs [39, 40]. The toxin moieties of clinically advanced RITs are generally derived from bacterial toxins. The immunogenicity of these nonhuman components has been a critical obstacle, prompting extensive de-immunization efforts through genetic modification [58]. This concern is minimized for EVD by the acute nature of the disease and the resulting short treatment window. Another obstacle for RIT therapy is the potential for dose-limiting adverse effects, including on-target toxicity against normal cells expressing the same target molecule and off-target systemic toxicity, particularly vascular leak syndrome associated with toxin-induced damage to endothelial cells. On-target toxicity would likely be minimal for the 6D8-PE38 RIT, since surface expression of the target antigen is limited to EBOV-infected cells. The extent of off-target toxicity varies amongst different RITs, and can be reduced by specific genetic modifications of the toxin domain as has been reported for several PE-based RITs [59–61]. We hypothesize that none of these obstacles is insurmountable and that proof-of-principle in vivo experiments ought to be performed to further evaluate the therapeutic potential of RITs against EVD.
Acknowledgements
We thank Hideki Ebihara (Laboratory of Virology, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH], Hamilton, Montana, United States of America [USA]) for providing human hepatocarcinoma Huh-7 cells. We thank John M. Dye (United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Maryland [MD], USA) for providing the heavy chain and light chain sequences of mAb 6D8. We also thank Anya Crane and Jiro Wada (Integrated Research Facility at Fort Detrick (IRF-Frederick), NIAID, NIH, Fort Detrick, Frederick, MD, USA) for editing the manuscript and assisting with figure preparation, respectively.
The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the U.S. Department of Health and Human Services, the US Army, or of the institutions and companies affiliated with the authors.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
 An immunotoxin targeting Ebola virus glycoprotein inhibits Ebola virus production from infected cells
An immunotoxin targeting Ebola virus glycoprotein inhibits Ebola virus production from infected cells
