
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Introduction
Campylobacter spp. are zoonotic bacteria that cause gastroenteritis in humans worldwide, whose main symptom is diarrhea. In certain cases, extra intestinal manifestations may occur, such as Guillain Barré syndrome. The bacteria cause severe diarrhea mostly in children and in immunocompromised individuals. This review aims to address the prevalence of Campylobacter spp. in humans in sub-Saharan Africa. It also aims to understand the impact of HIV in the prevalence, as well as to report data on antibiotic resistance and propose research priorities.
Methods
We followed PRISMA guidelines to find studies on the occurrence of Campylobacter spp. in humans in all countries from sub-Saharan Africa. Studies published between 2000 and 2020 were searched in PubMed, Cochrane Library, CINAHL, African Index Medicus, African Journals Online, Google Scholar and Science Direct. We have conducted a random-effect meta-analysis and calculated the proportion of resistant isolates to different antibiotics.
Results and discussion
We found 77 studies that described such occurrence in humans in 20 out of 53 sub-Saharan African countries. Campylobacter jejuni was the most prevalent species. Pooled prevalence was 9.9% (CI: 8.4%–11.6%). No major variations within the different sub-regions were found. Most studies reported Campylobacter spp. as the cause of diarrhea, mainly in children. Some studies reported the bacteria as a possible etiologic agent of acute flaccid paralysis and urinary tract infection. Campylobacter spp. presented a higher pooled prevalence in HIV infected patients, although not statistically significant. High proportions of resistant strains were reported for many antibiotics, including erythromycin and tetracycline.
Conclusion
Campylobacter spp. occur in sub-Saharan Africa, although information is scarce or inexistent for many countries. Research priorities should include investigation of the understudied species; extra intestinal manifestations; the impact of HIV infection and associated risk factors. Control strategies should be reinforced to contain the spread of this pathogen and drug resistance.
Introduction
Campylobacter spp. are a group of zoonotic gram-negative bacteria and the leading cause of human bacterial gastroenteritis [1]. In humans, they account for 5%-14% of all diarrheal disease in the world [2]. Cases of Campylobacter spp. infection have increased in North America, Europe, and Australia. Some studies indicate that Campylobacter spp. infections are also endemic in Africa, Asia, and the Middle East [2–5].
In the United States of America, the incidence of Campylobacter spp. infection in 2015 was 12.97 per 100,000 population [6]. In Europe, over 1.8 million Campylobacter spp. cases were reported from 2008 to 2016 [7]. The most frequently notified foodborne infection in Australia is from Campylobacter spp. with 16,968 notifications in 2010 (112.3 cases per 100,000) [8].
According to a systematic review and meta-analysis published in 2011 [9], Campylobacter spp. were identified as some of the most common bacterial gastrointestinal (GI) pathogens in sub-Saharan Africa, with an average of 8.3% in diarrheic and non-diarrheic patients seen in hospitals, primary health care centers or recruited in community cohorts. Other common GI pathogens identified in sub-Saharan Africa were: diarrheagenic E. coli species, enterotoxigenic E. coli, Shigella spp. and Salmonella spp. (30%, 15.4%, 10.5%, 8.4%, respectively) [9].
There are few surveillance systems in place for prevalence data on Campylobacter spp. in Africa. However, several reports are published. These bacteria are actually the most commonly reported zoonosis in developed and developing countries [5].
Poor hygiene, lack of adequate sanitation, direct contact between people and contaminated animals, drinking contaminated food and water are some sources of attribution of Campylobacter spp. infection in low-income countries [10], most of which are part of sub-Saharan Africa.
C. jejuni and less frequently, C. coli cause watery or bloody diarrhea, fever, abdominal cramps and vomiting. Although enteritis caused by these bacteria is sporadic and usually self-limiting, complications such as bacteremia, hepatitis, pancreatitis, lung infections, brain abscesses, meningitis and reactive arthritis can occur [3, 4]. Furthermore, the bacteria have been associated with inflammatory bowel disease and some types of cancer on the intestinal tract [5]. Other Campylobacter species have been isolated from humans with disease, such as: C. concisus, C. fetus, C. hyointestinalis, C. insulaenigrae, C. lari, C. sputorum, C. upsaliensis, C. curvus, C. gracilis, C. hominis, C. rectus, C. showae and C. ureolyticus [3, 11]. Infection by Campylobacter spp. is normally limited to children in developing countries [2, 3, 12]. Antibiotics can be considered for treatment in severe cases and in immunocompromised patients [2, 4].
Campylobacter spp. are commonly isolated from stools, and less commonly from blood. The optimal conditions for thermophilic Campylobacter species growth in culture medium, such as C. jejuni, C. coli, C. upsaliensis and C. lari, include a microaerophilic environment (5 to 10% of oxygen) and temperature between 40°C and 42°C. Non-thermophilic species (including C. fetus, C. concisus, C. curvus, C. hyointestinalis and other species) often grow better at 37°C [13, 14]. Another diagnostic test to detect the bacteria is Polymerase Chain Reaction (PCR) [5].
Although much is known about Campylobacter spp. in the developed world [15], there are few summarized data about epidemiology, clinical and diagnostic aspects as well as on antibiotic resistance of Campylobacter spp. in sub-Saharan Africa [16, 17]. In addition, due to the high prevalence of HIV infection in the region, the impact of this infection in the prevalence of campylobacteriosis is not clearly understood in sub-Saharan Africa.
The available review reports were either circumscribed to one country [18] or restricted to a single Campylobacter species [19, 20]. Further, to date, only one meta-analysis on the prevalence of Campylobacter spp. in sub-Saharan Africa has been conducted in 2011 [9]. However, updated data are needed. Thus, the aim of this review is to summarize data available regarding human Campylobacter spp. prevalence and drug resistance in sub-Saharan Africa from 2000 to 2020, as well as to recommend future research priorities.
Methods
Search strategy
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21] to summarize available data on human campylobacteriosis from each sub-Saharan African country. The United Nations macro-geographical definition of Africa was used to define the geographical boundaries of this review (https://unstats.un.org/unsd/methodology/m49/), as follows: a) Eastern Africa: British Indian Ocean Territory, Burundi, Comoros, Djibouti, Eritrea, Ethiopia, French Southern Territories, Kenya, Madagascar, Malawi, Mauritius, Mayotte, Mozambique, Réunion, Rwanda, Seychelles, Somalia, South Sudan, Uganda, United Republic of Tanzania, Zambia and Zimbabwe; b) Middle Africa: Angola, Cameroon, Central African Republic, Chad, Congo, Democratic Republic of the Congo, Equatorial Guinea, Gabon and Sao Tome and Principe; c) Southern Africa: Botswana, Eswatini (former Swaziland), Lesotho, Namibia and South Africa; d) Western Africa: Benin, Burkina Faso, Cabo Verde, Côte d’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Saint Helena, Senegal, Sierra Leone and Togo.
A complete study protocol is available in S1 File. PubMed, Cochrane Library, CINAHL, African Index Medicus, African Journals Online, Google Scholar and Science Direct were searched for studies published up to 13 March 2019 without language restrictions. An update of the database search was done on 25 March 2020 using the same set of search terms (detailed search strategy in S2 File). The PRISMA checklist is available in S1 Table.
Selection criteria of studies included:
The study population consisted of any group of people of all age groups in sub-Saharan Africa who had been tested for Campylobacter spp.;
Descriptive, cross-sectional studies, prospective, or retrospective studies and case reports and series in which the prevalence of Campylobacter spp. in any country in sub-Saharan Africa was reported;
Conference abstracts and
Only studies published since 2000.
The main exclusion criteria of studies included:
Review articles;
Countries not belonging to sub-Saharan Africa;
Experimental data.
The primary outcome in the review was the prevalence of Campylobacter spp. in countries from sub-Saharan Africa. The main secondary outcomes were the antibiotic resistance proportions.
Risk of bias (quality) assessment
Risk of bias was assessed separately for each eligible study, using an assessment tool with slight modifications [22]. Details of this assessment can be found in S2 Table. No study was excluded based on its quality.
Data extraction (selection and coding)
Titles and abstracts were screened for location, study population and general correlation with the research objectives. Full versions of potentially relevant articles were obtained to assess eligibility. Two researchers (DFH and SMSA) screened independently studies and determined study eligibility. Cross-references of the full text retrieved articles were also searched. Data were collected independently from each publication and captured using a standardized Word document form. Data were extracted from text, tables and figures.
Data analysis
In reports where the numerator and denominator of the study sample were available, prevalence data were calculated, if not already provided. When not presented in the manuscript, the 95% exact confidence intervals (CI) were calculated, using the “binom.test” function (“stats” package) in R 3.5.1.
A meta-analysis was performed using MetaXL version 5.3 (https://www.epigear.com/index_files/metaxl.html) (EpiGear International Pty Ltd, Australia). The software was used to produce the pooled estimates, forest plots, as well as to calculate the Cochran’s Q and p values. Study heterogeneity (Cochran’s Q) was evaluated by I2 (level of inconsistency) [23]. The I2 values above 75% were considered as a high degree of heterogeneity [24].
The pooled prevalence estimates of Campylobacter spp. infection and 95% CIs were calculated assuming a random-effect model. Subgroup analysis were conducted to sub-Saharan African regions; study subjects (patients with and without diarrhea; HIV infected and uninfected patients); years of publication and age. A separate pooled prevalence analysis was made to each Campylobacter species.
Regarding antibiotic resistance, the proportions of resistant Campylobacter spp. isolates were calculated dividing the number of resistant isolates by the number of tested isolates.
Results
Search results
Results from the search are summarized in Fig 1. Most records were published in English, except for one that was published in French. From the 77 total records [25–101], 70 were research articles, 1 was a PhD thesis, 3 were MSc dissertations and 3 were conference abstracts.

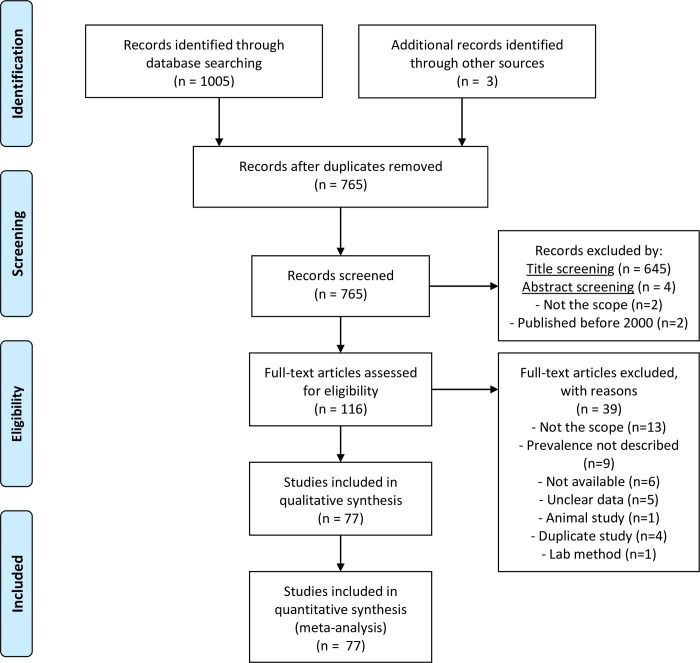
PRISMA flow diagram of study selection.
The studies were originated from 20 out of 53 sub-Saharan African countries, namely: Angola, Botswana, Burkina Faso, Côte d’Ivoire, Ethiopia, Ghana, Guinea Bissau, Kenya, Madagascar, Malawi, Mozambique, Niger, Nigeria, Rwanda, Senegal, South Africa, Tanzania, Uganda, Zambia and Zimbabwe. Overall quality assessment scores for risk of bias in studies included in the review ranged from three to ten. Of the total 77 studies assessed, one study had a score of 3, fifty were scored >7, of which twenty-five were scored >8. Twenty-six studies had a score between 4 and 7.
The majority of studies were based in Kenya (n = 13), Nigeria (n = 12), Tanzania (n = 9), South Africa (n = 8) and Ethiopia (n = 7). The number of samples per study ranged from 40 to 5635. Of the total 77 studies, 33 were recorded in the past 10 years (since 2010) and 6 studies did not report about data collection time frame. Prevalence ranged from 0% to 68%. Nine different Campylobacter spp. were identified, namely: C. jejuni, C. coli, C. lari, C. hyointestinalis, C. fetus, C. curvus, C. sputorum, C. concisus and C. upsaliensis. Nevertheless, Campylobacter species were not identified to species level in thirty studies. Table 1 provides an overview of the included studies.

| N (%) | |
|---|---|
| sub-Saharan Africa region | |
| Eastern Africa | 43 (55.8%) |
| Middle Africa | 1 (1.3%) |
| Southern Africa | 11 (14.3%) |
| Western Africa | 22 (28.6%) |
| Type of samples | |
| Feces | 75 (97.4%) |
| Urine and feces | 1 (1.3%) |
| Blood and feces | 1 (1.3%) |
| Setting | |
| Hospitals | 66 (85.7%) |
| Communities, farms or peri urban areas | 6 (7.8%) |
| Othersa | 4 (5.2%) |
| Only primary schools | 1 (1.3%) |
| Type of study | |
| Cross-sectional | 49 (63.6%) |
| Case-control | 20 (26.0%) |
| Cohort | 7 (9.1%) |
| Prospective | 1 (1.3%) |
| Human subjects age | |
| Children below 5 years | 39 (50.6%) |
| Children aged 10–15 years | 9 (11.7%) |
| Above 18 years | 2 (2.6%) |
| Random ages | 27 (35.1%) |
| Diagnostic methods | |
| Routine culture only | 12 (15.6%) |
| Routine culture and other methods | 47 (61.0%) |
| PCR only | 16 (20.8%) |
| Not reported/ not clear | 2 (2.6%) |
| Type of subjects | |
| With diarrhea | 34 (44.1%) |
| With or without diarrhea | 26 (33.8%) |
| Asymptomatic | 2 (2.6%) |
| Othersb | 15 (19.5%) |
N- Number of human studies.
a One study was based on laboratory records, one other was unclear and two studies were from both schools and hospitals;
b These included: malnourished patients; volunteers; patients with and without enteric complaints; patients with acute flaccid paralysis; patients with urinary tract infection and HIV infected patients.
S3 Table presents detailed information about Campylobacter spp. prevalence in studies found in sub-Saharan Africa. There were no outbreak reports.
Pooling estimates of Campylobacter spp. in humans
The pooled prevalence estimates of Campylobacter spp. in humans with individual studies are shown in a forest plot (Fig 2). We have excluded data on the control group from Nhampossa et al. 2015 [46] study because they were not clear. Overall, studies from sub-Saharan Africa revealed a pooled prevalence of 9.9% (95% CI: 8.4%–11.6%). There was a substantial heterogeneity among studies (I2 = 98%; p = 0.00).

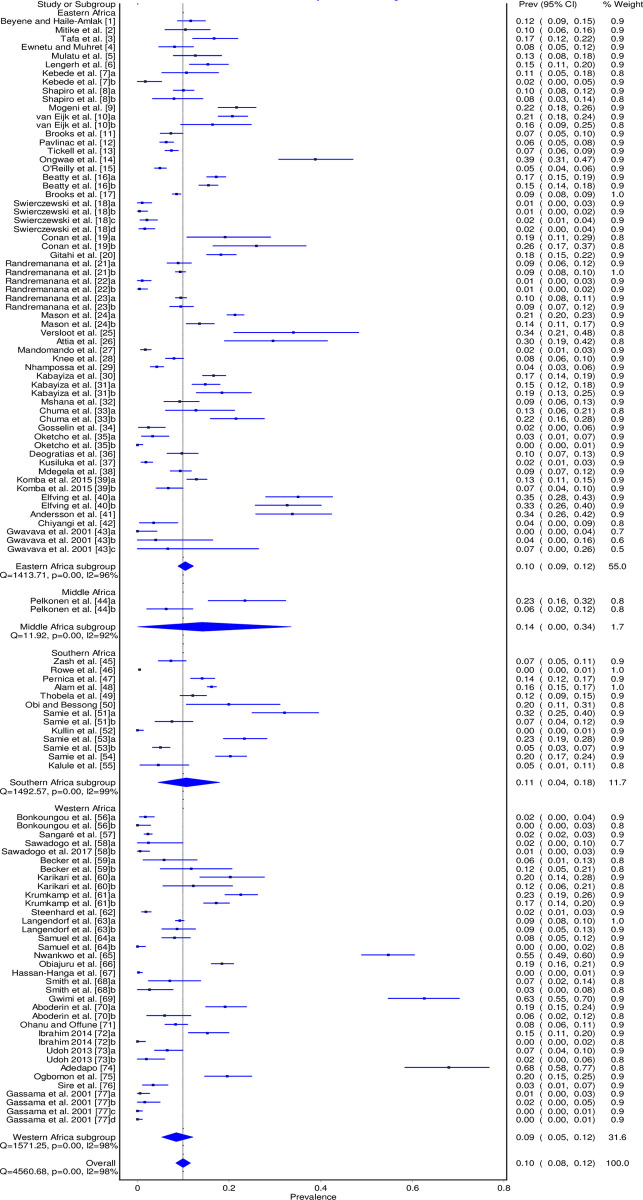
Forest plot of Campylobacter spp. pooled prevalence in humans in sub-Saharan Africa between 2000 and 2020.
The summary of the subgroup analysis is found in Table 2. The prevalence among the sub-regions varied between 8.5% (95% CI: 5.3% - 12.1%) and 14.1% (95% CI: 0.0% - 33.6%).

| Sample size | No. positive | No. of studies | Pooled Prev. | 95% CI | Cochran’s Q | Heterogeneity I2 (%) | p value | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 59,249 | 6,519 | 77 | 9.9 | 8.4–11.6 | 4,560.7 | 98 | 0.00 | 100 |
| Region | |||||||||
| Eastern Africa | 32,256 | 3,614 | 43 | 10.5 | 8.8–12.2 | 1,413.7 | 96 | 0.00 | 55.0 |
| Middle Africa | 194 | 29 | 1 | 14.1 | 0.0–33.6 | 11.9 | 92 | 0.00 | 1.7 |
| Southern Africa | 13,578 | 1,409 | 11 | 10.7 | 4.5–17.9 | 1,492.6 | 99 | 0.00 | 11.7 |
| Western Africa | 13,221 | 1,467 | 22 | 8.5 | 5.3–12.1 | 1,571.3 | 98 | 0.00 | 31.6 |
| Patients with or without diarrheaa | |||||||||
| With diarrhea | 32,965 | 3,749 | 60 | 10.2 | 8.6–11.9 | 1,473.3 | 96 | 0.00 | 68.9 |
| Without diarrhea | 9,143 | 939 | 28 | 6.5 | 4.1–9.2 | 635,1 | 95 | 0.00 | 31.1 |
| HIV positive or negative | |||||||||
| HIV positive | 1,387 | 190 | 13 | 10.8 | 3.9–19.2 | 331.6 | 95 | 0.00 | 56.2 |
| HIV negative | 5,273 | 413 | 9 | 8.4 | 4.6–13.0 | 277.8 | 96 | 0.00 | 43.8 |
| HIV positive patients: With or without diarrhea | |||||||||
| HIV with diarrhea | 916 | 115 | 11 | 10.7 | 5.1–17.6 | 85.5 | 88 | 0.00 | 71.7 |
| HIV without diarrhea | 364 | 4 | 5 | 1.4 | 0.1–3.2 | 5.6 | 29 | 0.23 | 28.3 |
| Years | |||||||||
| 2000–2005 | 3,644 | 278 | 10 | 5.5 | 3.0–8.5 | 206.3 | 92 | 0.00 | 15.0 |
| 2006–2010 | 22,169 | 2,260 | 16 | 9.2 | 5.5–13.4 | 1,986.6 | 99 | 0.00 | 19.3 |
| 2011–2015 | 27,026 | 3,049 | 29 | 9.5 | 7.5–11.7 | 1,432.5 | 97 | 0.00 | 40.8 |
| 2016–2020 | 6,410 | 932 | 22 | 14.7 | 9.9–19.9 | 866.8 | 97 | 0.00 | 24.9 |
| Age | |||||||||
| Below 15 years | 39,256 | 4,768 | 48 | 10.5 | 8.9–12.2 | 1,768.7 | 96 | 0.00 | 61.0 |
| Above 18 years | 295 | 15 | 2 | 4.6 | 0.6–9.8 | 12.1 | 67 | 0.02 | 3.6 |
| Random agesb | 19,698 | 1,736 | 27 | 9.5 | 6.2–13.1 | 2,405.1 | 98 | 0.00 | 35.4 |
| Study setting | |||||||||
| Communities | 7,884 | 759 | 6 | 11.7 | 7.3–16.5 | 304.7 | 97 | 0.00 | 8.3 |
| Hospitals | 48,968 | 5,422 | 66 | 9.5 | 7.8–11.4 | 4,089.7 | 98 | 0.00 | 84.6 |
| Primary schools | 580 | 106 | 1 | 18.3 | 15.2–21.5 | - | - | - | 0.9 |
| Others | 1,817 | 232 | 4 | 11.8 | 5.4–19.1 | 115.6 | 95 | 0.00 | 6.2 |
| Campylobacter spp. | |||||||||
| C. jejuni | 38,775 | 3,136 | 46 | 7.6 | 6.2–9.0 | 1,463.3 | 96 | 0.00 | 100 |
| C. coli | 28,343 | 632 | 32 | 3.4 | 2.4–4.4 | 714.2 | 95 | 0.00 | 100 |
| C. lari | 8,401 | 115 | 10 | 3.0 | 0.7–6.8 | 358.7 | 97 | 0.00 | 100 |
| C. hyointestinalis | 6,015 | 21 | 3 | 1.3 | 0.0–3.6 | 16.4 | 88 | 0.00 | 100 |
| C. fetus | 6,123 | 24 | 4 | 2.8 | 0.0–8.9 | 83.9 | 96 | 0.00 | 100 |
| C. curvus | 5,635 | 1 | 1 | 0.02 | 0.0–0.1 | - | - | - | 100 |
| C. sputorum | 5,635 | 1 | 1 | 0.02 | 0.0–0.1 | - | - | - | 100 |
| C. upsaliensis | 7,131 | 266 | 4 | 5.6 | 0.6–14.2 | 189.9 | 98 | 0.00 | 100 |
| C. concisus | 5,957 | 244 | 2 | 4.1 | 3.6–4.6 | 0.9 | 0 | 0.64 | 100 |
a Number of studies is greater than 77 because 26 studies included both groups with and without diarrhea.
b Random ages refers to studies that included patients from a wide range of ages and to 3 studies where age-related data were not reported.
No statistically significant differences were found in Campylobacter spp. prevalence in patients with and without diarrhea, as well as between HIV infected patients and HIV uninfected ones. When comparing HIV-infected patients, Campylobacter spp. prevalence was significantly higher in patients with diarrhea when compared to non-diarrheic HIV patients (10.7% (95% CI: 5.1% - 17.6%) and 1.4% (95% CI: 0.1% - 3.2%)), respectively. There was an increase in Campylobacter spp. pooled prevalence over time and a higher prevalence was found in children under 15 years, although not statistically significant as well.
C. jejuni was the most tested species and with the highest pooled prevalence (7.6%). Table 3 presents the pooled prevalence of Campylobacter species with subgroup analysis by the presence of diarrhea and by sub-Saharan African region. The table does not include C. curvus and C. sputorum because these species presented the lowest prevalence (0.02% for both) and were tested in the same study from South Africa [67].

| C. jejuni | W | C. coli | W | C. lari | W | C. hyointestinalis | W | C. fetus | W | C. upsaliensis | W | C. concisus | W | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with or without diarrhea | ||||||||||||||
| With | 8.3 (6.3–10.4) | 76.4 | 2.5 (1.7–3.6) | 72.4 | 0.9 (0.5–1.5) | 71.9 | 1.3 (0.2–3.3) | 100 | 1.7 (0.1–4.6) | 100 | - | - | 3.8 (1.2–7.3) | 50.3 |
| Without | 4.9 (1.4–9.3) | 23.6 | 2.6 (1.1–4.3) | 27.6 | 2.0 (0.9–3.2) | 28.1 | - | - | - | - | - | - | 2.6 (0.5–5.6) | 49.7 |
| sub-Saharan African region | ||||||||||||||
| EA | 7.5 (5.6–9.6) | 47.8 | 2.2 (1.5–3.0) | 45.6 | 1.3 (0.8–1.9) | 37.3 | - | - | - | - | - | - | - | |
| MA | - | - | - | - | - | - | - | - | - | - | - | - | ||
| SA | 9.9 (6.0–14.2) | 11.9 | 3.5 (1.1–6.5) | 15.2 | 0.0 (0.0–0.1) | 9.7 | 0.2 (0.1–0.4) | 38.7 | 0.0 (0.0–0.1) | 26.4 | 3.2 (3.5–4.5) | 26.3 | 4.1 (3.6–4.6) | 100 |
| WA | 6.9 (4.3–9.8) | 40.3 | 4.7 (1.6–8.5) | 39.2 | 6.5 (0.2–16.0) | 53.0 | 2.0 (0.5–4.6) | 61.3 | 3.9 (0.0–12.9) | 73.6 | 7.1 (0.0–29.7) | 73.7 | - | - |
EA–Eastern Africa; MA–Middle Africa; SA–Southern Africa; WA: Western Africa; W—Weight
Resistance of Campylobacter spp. isolates to antibiotics
From the 77 studies included in this systematic review, a total of 31 reported antibiotic resistance [25, 30, 32, 33, 36, 37, 44, 48, 50, 57, 59, 61, 64, 65, 69, 70, 73–75, 77, 80, 81, 84, 85, 87, 89, 91, 94–96, 99]. Data were from Eastern, Southern and Western Africa. S4 Table provides detailed information for antibiotic resistance within each study.
Antibiotic resistance was screened for C. jejuni (916 isolates), C. coli (154), C. lari (44), C. upsaliensis (33), C. fetus (16) and other Campylobacter spp. not identified to species level (534). Isolates were tested to a total of 48 different antibiotics. Table 4 presents the summary of Campylobacter spp. resistance to the 5 most tested antibiotics.

| Region | Proportion % (number of resistant isolates) | ||||
|---|---|---|---|---|---|
| Erythromycin | Tetracycline | Ciprofloxacin | Nalidixic acid | Gentamicin | |
| Eastern Africa | 54% (335/625) | 44% (267/605) | 21% (122/575) | 36% (199/555) | 39% (119/304) |
| Southern Africa | 70% (157/225) | 30% (68/225) | 12% (30/254) | 52% (116/225) | 19% (42/225) |
| Western Africa | 13% (65/512) | 49% (172/353) | 11% (34/304) | 24% (66/279) | 24% (194/490) |
| sub-Saharan Africa | 41% (557/1,362) | 43% (507/1,183) | 16% (186/1,133) | 36% (381/1,059) | 35% (355/1,019) |
The highest proportion of resistant isolates among the 10 most tested antibiotics (S4 Table), was for ampicillin (63%), trimethoprim sulfamethoxazole (49%), tetracycline (43%) and erythromycin (41%). However, many other antibiotics presented a high proportion of resistant isolates as presented in S4 Table.
Multidrug resistance, referred as resistance to more than two drugs, was reported in a total of seven studies [36, 37, 57, 59, 70, 80, 84].
Discussion
To our best knowledge, this is the most recent systematic review and meta-analysis that summarized and analyzed data about the prevalence, distribution and antibiotic resistance of Campylobacter species in sub-Saharan Africa.
We found a pooled prevalence of Campylobacter spp. in humans of 9.9%, with a greater representation of patients with diarrhea. This prevalence is close to an average prevalence of 8.3% previously reported in the region [9].
Most of the reports have focused on diarrhea-related comorbidities, although the differences in the prevalence of Campylobacter spp. in patients with and without diarrhea were not statistically significant.
It is understandable that most of the studies were based in Eastern Africa (55.8%) that has the majority of countries of sub-Saharan African region (22 countries, 41.5%). These data are consistent with the sample size because Eastern Africa presented the largest sample size and weight (n = 32,256, weight = 55.0%). Middle Africa, with the lowest weight in the meta-analysis (1.7%) is the second region with the less countries in sub-Saharan Africa (9/53 countries). Thus, there was a low representativeness of this region in this review.
In Eastern Africa, Kenya and Tanzania had most studies (13 and 9, respectively). Accordingly, Kenya had the highest percentage of samples analyzed in the sub-region, followed by Madagascar (42%, and 20%, respectively). However, Tanzania had on average fewer samples analyzed per publication than Madagascar, but the later had a greater weight in the meta-analysis.
The presence of Campylobacter spp. in asymptomatic individuals [34–36, 38–41, 49, 51, 53, 58, 63, 71, 73, 76, 78, 79, 81, 82, 85, 89, 91, 92, 94, 95, 99–101] is noteworthy, both due to the considerable number of reports, as well as because it appears to occur frequently in sub-Saharan Africa. It may result from continued exposure probably at low doses [40]. This exposure may be due to poor hygienic-sanitary conditions [36, 38, 41, 58], including contact with reservoir animals such as chickens [36], and has probably resulted in developed immunity against the pathogen [38, 40, 41]. In certain cases, the asymptomatic carriage could be consequence of a resolved infection in which the bacteria continues to be eliminated in the feces [41, 79].
Campylobacter spp. are known to be excreted for up to 12 weeks and even to 40 weeks after infection [102]. Considering the common sanitary conditions in sub-Saharan Africa, it is more likely that the asymptomatic carriage is a result of developed protective immunity [4], meanwhile, when the individual is exposed to high doses of the bacteria, a symptomatic infection may occur [40]. Although there is an asymptomatic population, Campylobacter spp. have been proven to cause diarrhea in some of the studies included in this review [25, 41, 53, 63, 71, 73, 81, 85, 91, 94].
The findings of this study demonstrated no statistically significant differences in the prevalence of Campylobacter spp. in both HIV positive and negative patients. However, when analyzing particularly HIV infected patients, the ones with diarrhea presented a higher prevalence of Campylobacter spp. when compared to HIV patients without diarrhea. In view of this, Campylobacter spp. may be predominantly symptomatic in HIV-positive patients; and this can be supported by previous reports that showed the association of HIV with the prevalence and/or severity of campylobacteriosis [77, 101, 103–105]. This needs to be clarified, especially for the sub-Saharan African context, as it bears 66% of new HIV infections worldwide [106]. Thus, in order to understand the impact of HIV in campylobacteriosis, future studies should include HIV serological status data, including CD4 cell count and/or viral load and if possible the intake or not of highly active antiretroviral treatment (HAART).
The increasing prevalence of Campylobacter spp. over time in sub-Saharan Africa is in concordance with the global data [3, 4]. These findings can be due to the expanded use of molecular techniques for Campylobacter spp. detection or because more susceptible patients were studied. Regardless of these assumptions, this increased prevalence should raise the concern of health authorities in regard to these bacteria.
The pooled prevalence among the sub-regions suggests that the pathogen has a nearly uniform epidemiology in the sub-Saharan region, which can be explained by the similar hygienic-sanitary conditions. Fact remains that Middle Africa revealed the highest pooled prevalence (14.1%) most likely because only one study contributed with a few amount of data. The lowest prevalence found in Western Africa, although not quite different from other regions, may be the result of some control measures in place aiming at hygiene promotion and improving sanitation in rural and urban areas, with a main contribution from Senegal and Burkina Faso [107, 108], as well as from Guinea-Bissau, Côte d’Ivoire, and Niger [109].
The high pooled prevalence of Campylobacter spp. in children, particularly those under 15 years is also in agreement with the findings worldwide. It is known that children, mainly the ones under 5 years are the most affected by diarrhea caused by Campylobacter spp. [2, 3]. In the meta-analysis this group presented a greatest weight (61.0%), but it was difficult to compare to other age categories due to the uneven way of reporting data.
Despite the fact that the majority of studies tested Campylobacter spp. occurrence in stool as cause of diarrhea, one study tested that type of sample with the purpose of knowing whether these bacteria were the cause of acute flaccid paralysis [68]. Analysis of samples other than stool are important, although they were few in the region, particularly for urine [80] and blood [62]. Despite these few data, it was noticed that the bacteria were present in patients diagnosed with Urinary Tract Infection (UTI) [80]. Further research is needed to identify the presence of Campylobacter spp. in diseases or symptoms other than gastroenteritis, including bacteremia and UTI. These studies would contribute to identify the tissues or organs to which the bacteria often spreads, along with the clinical presentation.
The substantial heterogeneity among studies (I2 = 98%) can be explained by the different types of studies, settings (rural, urban, hospitals), inclusion criteria, sample size, detection methods, data collection time-period and identified species.
It was to be expected that the majority of studies were hospital-based (85.7%), considering that these are less costly, easier and faster to perform. However, studies at community level are important to comprehensively understand Campylobacter spp. association with different factors related to environment, hygiene practices, presence of animals, and to analyze in more detail the presence of the pathogen in symptomatic and asymptomatic groups.
The fact that a higher prevalence was detected in schools and communities can be possibly explained by the poor hygiene conditions in those settings, which contribute to human contamination. Nevertheless, these studies presented a small amount of data when compared to studies carried out in hospitals and the difference was not statistically significant.
Notwithstanding the fact that cross-sectional and case-control studies provide useful epidemiological information, cohort studies provide the strongest scientific evidence, but they were less frequent in sub-Saharan African region (9.1%) when compared to cross-sectional (63.6%) and case-control (26.0%). Accordingly, cohort and case-control tested fewer samples when compared to cross-sectional studies (6,200, 13,313 and 39,336) respectively.
The identification of Campylobacter spp. through culture was the most common (15.6% and 9,489 samples, in addition to culture coupled with other methods (61.0% and 40,436 samples)), when compared with other PCR alone (20.8% and 8,570 samples). Culture method has limitations because Campylobacter spp. can become unviable during transport and processing. They can also survive as viable but non culturable forms that will not grow on selective media [110]. These facts can lead to false-negative results. Besides that, certain culture methods cannot isolate a wide variety of species. For example, Campylobacter ureolyticus cannot be detected on Preston agar under microaerobic conditions at 42°C [111]. Selective culture media containing antibiotics, the common method for culturing Campylobacter spp. from feces can be unable to detect some species including C. hyointestinalis, C. fetus and C. upsaliensis, which are sensitive to antibiotics used in these media [112]. Probably for these reasons there was a misrepresentation of Campylobacter species other than C. jejuni and C. coli.
Culture is a more time-consuming method and with fewer sensitivity [113], compared to modern molecular methods such as PCR. However, PCR results must also be analyzed with caution, as the method can detect fragments of DNA that may not correspond to a current infection [63].
C. jejuni was the most searched and reported species. It presented the highest prevalence mainly in patients with diarrhea when comparing with other Campylobacter spp. Nonetheless, C. coli also exhibited a notable prevalence (3.4%) that was approximate from the other species such as C. upsaliensis, C. concisus, and C. lari (5.6%, 4.1% and 3.0%, respectively). These data must be interpreted cautiously in view of the fact that these three species were tested in a fewer amount of samples. Moreover, C. curvus and C. sputorum were less commonly searched and reported [67].
The contribution of C. coli, C. lari and C. concisus for diarrhea in humans in sub-Saharan Africa does not appear to be prominent as the differences in prevalence in case and control groups was not statistically significant. The role of other Campylobacter species on the onset of diarrhea is not clear, since it was not possible to compare diarrheic and non-diarrheic groups, although they were often isolated in patients with gastrointestinal complaints.
Although 33 out of 53 sub-Saharan African countries had no data, it is very likely that Campylobacter species occur there, given the fact that these bacteria are found in the nearby countries in the four sub-regions.
In general, some studies demonstrated that Campylobacter spp. prevalence can be worsened due to other comorbidities such as malnutrition [42, 43, 98], HIV/AIDS [77, 89], malaria [54], giardiasis [82], and, rotavirus and norovirus infection in children [41]. In some studies, Campylobacter spp. prevalence was clearly associated with a younger age [33, 38, 40, 56, 57].
Risk factors that may have contributed to the bacteria prevalence were identified in some studies, and included: living in densely populated localities [51]; consumption of chicken meat [57, 94], pre-prepared salad [57], raw food [81]; canned milk [94]; drinking contaminated water [70, 94, 114]; inadequate hygienic-sanitary conditions [27, 35, 45, 51, 90, 95]; malnourishment [70]; contact with persons with diarrhea in the household [26]; and contact with animals in households [37, 77, 95] such as cats [25, 26, 70], dogs [25, 70], chickens [70, 94], goats [35], sheep [94], cattle [55], pigeons [70] and pigs [90].
The antibiotic resistance is a matter of concern due to the greater risk for treatment failure [80]. The antibiotic resistance of Campylobacter spp. isolated from animals in sub-Saharan Africa has already been reported [115]. This is of great concern due to frequent contact with animals that can transfer resistance genes to human Campylobacter spp. isolates and worsen the situation [116]. Furthermore, humans can acquire pathogens with resistance directly from animals and animal products [117, 118].
This review found resistance to antibiotics used for treatment of campylobacteriosis, including erythromycin (41%) which is considered the drug of choice for clinical treatment of campylobacteriosis [119], as well as to tetracycline and gentamicin (43% and 35%) which are used in treatment of systemic infections [119]. Ciprofloxacin resistance proportion (16%), although considerable and reported globally [120], was not as prominent as of other antibiotics. Ciprofloxacin is also among the clinical used drugs for treatment of campylobacteriosis [119]. Among the most tested antibiotics, ampicillin revealed to have a higher resistance proportion (63%) (S4 Table). The resistant scenario is thought to be the result of indiscriminate use both by humans and in animal husbandry [57, 89].
In general, Campylobacter spp. may not have been so extensively studied in all countries from sub-Saharan Africa because they are not part of routine lab investigations for etiology of diarrhea in the countries such as Burkina Faso [76]. Some studies screened for other enteric pathogens such as Escherichia coli and Salmonella spp. in addition to Campylobacter spp. In some of these studies Campylobacter spp. where the most frequent bacterial pathogens [25, 26, 32, 40, 59, 64, 69, 73, 81, 87, 101], while not in other studies [30, 31, 61, 62, 65, 76, 88, 100].
Taking into consideration the findings of this systematic review and meta-analysis, we outline below research priorities for future Campylobacter spp. studies in sub-Saharan Africa. First we propose conducting of clinical, epidemiological and molecular studies, reflecting different seasonal variations and risk factors for infection. Studies should include antibiotic resistance data and search for antibiotics alternatives, as well as for molecules that can be candidates for new drugs [121]. We suggest the use of molecular techniques in replacement or in combination with culture, in order to provide the real burden of Campylobacter spp. infection and to characterize the genetic variants that occur in the region since these variations affect antibiotic resistance and pathogenicity [122]. Point of care diagnosis should be developed as well. Research questions should be broadened to find out the role of Campylobacter spp. in diseases other-than diarrhea, as well as to identify Campylobacter spp. other than C. jejuni and C. coli. Special attention should be given to conduct HIV-related research, specifically to analyze whether there is a relationship with the severity of disease caused by Campylobacter spp. and the degree of immunosuppression or viral load. It would also be important to know if HIV patients are at more risk for a bloodstream infection than HIV uninfected patients; and whether HAART therapy has a protective effect in campylobacteriosis.
Although we have systematized data concerning the occurrence of Campylobacter spp. and their resistance to antibiotics, this study presents the following limitations: a comparison of gender was not performed due to insufficient data in many studies; it was not possible to compare the prevalence in both urban and rural areas for the same reason; the age subgroup comparison was not sufficiently comprehensive due to heterogeneous and unreported data; antibiotics resistance data may have not covered all published studies in the region because the selected studies had to include prevalence data (that was the primary outcome of this review); and the last point is that it was not possible to compare resistance patterns by the different species due to the inconsistent data reported within each study.
Conclusions
Campylobacter spp. occur in humans in sub-Saharan Africa and presented a pooled prevalence of 9.9% (8.4%– 11.6%). No major prevalence variations were found within the sub-regions. These bacteria prevail mainly as an important causative agent of gastrointestinal disorders, particularly diarrhea. In addition, extra-intestinal infections such as acute flaccid paralysis and urinary tract infection occur, although data are not widely comprehensive.
Of the total nine isolated species, the one with broadest epidemiological understanding is C jejuni. Species like C. curvus, C. sputorum and C. concisus are understudied, thus deserve attention in further research, as they may be important in the etiology of diarrhea. HIV infection may contribute to the onset of campylobacteriosis in patients, since patients with HIV are often symptomatic when infected with Campylobacter spp.
Resistance to antibiotics is a matter of concern in the region, which may be due to the indiscriminate use both in the treatment of diarrhea and in animal husbandry. Consequently cross-contamination occurs, which increases the resistance proportion.
As research priorities, we propose the implementation of surveillance systems for common Campylobacter spp. in order to quantify the disease burden; obtaining data from the countries where they were not available from, and to study the less common species. The clinical presentation, including the prevalence of extra intestinal complications and risk factors for campylobacteriosis should also be addressed in future studies. Considering the high prevalence of HIV in the continent, epidemiological studies of Campylobacter spp. should compare the prevalence and severity of disease in both HIV positive and negative patients, as well as the influence of the viral load, CD4 cell count and HAART therapy. Finally we recommend the search for molecules that can be candidates for new antibiotics and to characterize the different Campylobacter spp. genotypes existent in the sub-Saharan African region.
Acknowledgements
We thank Professor Virgílio do Rosário, retired Professor from Instituto de Higiene e Medicina Tropical, Universidade Nova the Lisboa for helping to draft and edit the manuscript. Furthermore, we are thankful to Professor Kim Barrett, from the University of California San Diego, USA, for continuous mentorship.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
 A systematic review and meta-analysis reveal that Campylobacter spp. and antibiotic resistance are widespread in humans in sub-Saharan Africa
A systematic review and meta-analysis reveal that Campylobacter spp. and antibiotic resistance are widespread in humans in sub-Saharan Africa
