
These authors contributed equally to this work.
- Altmetric
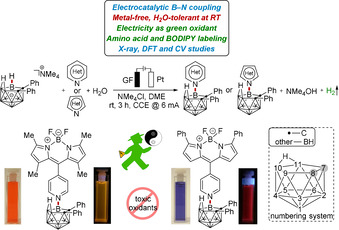
Electrocatalyzed oxidative B−H nitrogenations of nido‐carborane (nido‐7,8‐C2B9H12 −) with N‐heterocycles have been established, enabling the preparation of various N‐substituted nido‐carboranes without chemical oxidants or metal catalyst under ambient conditions. The electrolysis manifold occurred with high levels of efficiency as well as chemo‐ and position‐ selectivity, employing sustainable electricity as the sole oxidant. The strategy set the stage for a user‐friendly access to novel amino acid and fluorogenic boron‐dipyrrin (BODIPY)‐labeled nido‐carborane hybrids.
Metal‐free electrocatalyzed B−H nitrogenation is used to synthesize various N‐heterocyclic nido‐carboranes.
Introduction
Carboranes—polyhedral boron‐carbon molecular clusters—possess unique properties, such as the icosahedron geometry, enriched boron content and delocalized three‐dimensional aromaticity. [1] These features render carboranes valuable building blocks for applications to supramolecular design or nanomaterials, [2] optoelectronics, [3] boron neutron capture therapy (BNCT) agents [4] and organometallic coordination chemistry. [5] As a consequence, a variety of transition metal‐catalyzed regioselective B−H functionalization has emerged as a useful tool for the derivatization of closo‐carboranes. [6] Despite considerable progress, B−H functionalization of nido‐carborane (7,8‐C2B9H12 −) remain in its infancy. [7] One major challenge is represented by the negatively charged cluster, which prevents the B−H bond from undergoing nucleophilic substitutions. In recent years, nitrogen‐containing carboranes have received increasing attention, since they bear major potential in drug discovery [8] as well as catalysis, [9] with key contributions by the groups of Spokoyny, [10] Xie, [11] Teixidor [12] and Yan, [13] among others. [14] Inspite of indisputable advances, oxidative cage B−H functionalizations largely require transition metal catalysts [15] and stoichiometric amounts of chemical oxidants, such as toxic and/or expensive copper(II) or silver(I) salts, [16] which compromises the sustainable nature and generality of this approach.
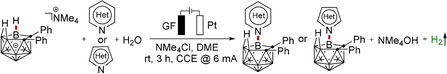
In recent years, the use of electricity as a redox agent to facilitate chemical reactions has been recognized as an increasingly viable, environmentally‐friendly strategy. [17] While significant recent impetus was gained by the merger of electrocatalysis with organometallic C−H activation, [18] electrochemical regioselective cage B−H functionalization continues to be scarce, with one electrochemical thiocyanation of nido‐carboranes and one very recently reported example of copper‐catalyzed electrochemical B−H oxygenation of o‐carborane. [19] Within our program on sustainable electrochemical bond activation, [20] we have now devised a strategy for unprecedented electrochemical regioselective cage B−H nitrogenation of nido‐carborane in a dehydrogenative manner, assembling a variety of N‐heterocycle‐, amino acid‐ and BODIPY‐labeled nido‐carboranes (Figure 1). Notable features of our findings include 1) electrochemical B−N coupling, 2) effective B−H nitrogenation devoid of chemical oxidants, 3) amino acid‐ and BODIPY‐labeled nido‐carborane, 4) H2O as the reactant for hydrogen evolution reaction (HER) and 5) selective B−H nitrogenations with electricity as the sole oxidant.

Electrooxidative cage B−H nitrogenation of nido‐carborane.
Results and Discussion
We initiated our studies by probing various reaction conditions for the envisioned electrochemical‐catalyzed B−N coupling of nido‐carborane 1 a with pyridine 2 a at room temperature in an operationally simple undivided cell setup equipped with a GF (Graphite Felt) anode and a Pt‐plate cathode (Table 1 and Table S1). After considerable preliminary experimentation, we were delighted to observe that the desired B‐pyridine nido‐carborane product 3 aa was obtained in 60 % yield in DME/H2O as the reaction medium (entries 1–4). Further electrolyte optimization indicated that NMe4Cl was best (entries 5–7). Increasing the amount of H2O to 1 mL led to a significant decrease in the yield (entries 8–9), while reducing it to 0.2 mL increased the efficiency to 83 % isolated yield of product 3 aa (entry 10). Control experiments confirmed the essential role of the H2O, the electricity, the NMe4Cl additive and the GF anode (entries 11–16).

|
Entry |
Electrolyte |
Solvent |
Yield [%][b] |
|---|---|---|---|
|
1 |
– |
MeOH/H2O |
8 %[c] |
|
2 |
– |
THF/H2O |
36 %[c] |
|
3 |
– |
CH3CN/H2O |
40 %[c] |
|
4 |
– |
DME/H2O |
60 %[c] |
|
5 |
nBuNPF6 |
DME/H2O |
20 %[c] |
|
6 |
nBuNBF4 |
DME/H2O |
63 %[c] |
|
7 |
NMe4Cl |
DME/H2O |
65 %[c] |
|
8 |
NMe4Cl |
DME/H2O |
32 %[d] |
|
9 |
NMe4Cl |
DME/H2O |
50 %[e] |
|
10 |
NMe4Cl |
DME/H2O |
87 % (83 %)[f] |
|
11 |
NMe4Cl |
DME |
10 % |
|
12 |
NMe4Cl |
DME/H2O |
–[g] |
|
13 |
– |
DME/H2O |
67 % |
|
14 |
KCl |
DME/H2O |
75 % |
|
15 |
NaCl |
DME/H2O |
70 % |
|
16 |
NMe4Cl |
DME/H2O |
73 %[h] |
[a] Reaction conditions: 1 a (0.10 mmol), 2 a (0.30 mmol), electrolyte (2 equiv.), DME (4.0 mL), H2O (0.2 mL), 25 °C, 3 h. [b] Yield was determined by 1H NMR with CH2Br2 as the standard. [c] H2O (0.5 mL). [d] H2O (1.0 mL). [e] DME (5.0 mL), H2O (1.0 mL). [f] Isolated yields in parenthesis. [g] No electricity. [h] Pt‐plate as anode. DME=1,2‐Dimethoxyethane, THF=Tetrahydrofuran.
With the optimized reaction conditions in hand, we probed its versatility for the B−N coupling of nido‐carboranes 1 with different N‐heterocyclic substrates 2 (Scheme 1). Electron‐rich as well as electron‐deficient groups on the pyridine 2 were amenable to the electrocatalyzed B−H oxidation coupling, providing the corresponding products in good to excellent yields (3 aa–3 am). Thereby, a variety of synthetically useful functional groups, such as ester (3 ah), amide (3 ai), chloro (3 aj) and bromo (3 ak), were fully tolerated, which could prove instrumental for further late‐stage manipulations. In addition, various isoquinolines (2 n–2 q) and even the carbazole‐substituted pyridine (2 r), afforded the corresponding electro‐oxidative B−N coupling product in good to excellent yields (3 an–3 ar). Notably, the free NH2‐amino group (2 d, 2 l) was also tolerated under the mild electro‐oxidative conditions, athough the amine‐substituted and Boc‐protected amino isoquinoline products (3 ap–3 aq) were isolated in somewhat lower yields. Interestingly, the Steglich catalyst 4‐dimethylaminopyridine (DMAP) was also a competent pyridine derivative in the electrochemical reaction, providing the DMAP decorated nido‐carborane product (3 am) with good efficacy.

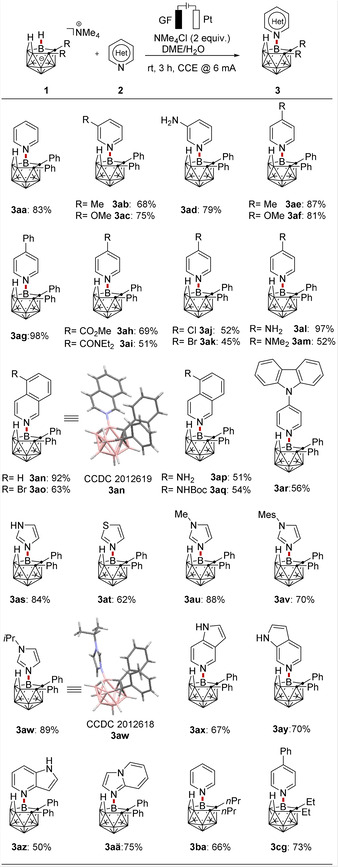
Electrooxidative B−H nitrogenation of nido‐carborane 1 with N‐heterocycles 2.
The robustness of the electrocatalyzed B−N bond formation at room temperature was next evaluated by other N‐heterocyclic substrates, such as imidazole with N=11.47 in MeCN according to Mayr's scale, [21] N‐substituted derivatives of imidazoles, thiazole and azaindoles, giving the corresponding B−N products in good to excellent yields (3 as–3 aä). In addition, dialkyl substituted nido‐carboranes also efficiently underwent the electrochemical transformation to provide the corresponding B−N coupling products 3 ba–3 cg. Thus, the oxidant‐ and catalyst‐free electrochemical oxidative B−N coupling provides a new route to a convenient and versatile synthesis of N‐heterocycle‐substituted nido‐carboranes. The connectivity of the products (3 an and 3 aw) was unambiguously verified by X‐ray single crystal diffraction analysis. [23]
Encouraged by the unique efficiency of the electrocatalyzed metal‐free B−N oxidative coupling with various N‐heterocyclic substrates, we became intrigued to explore the late‐stage amino acid and BODIPY diversification of structurally complex nido‐carboranes (Scheme 2). Both amino acid‐ and BODIPY‐labeled pyridine proved to be suitable substrates (5 aa–5 ad).

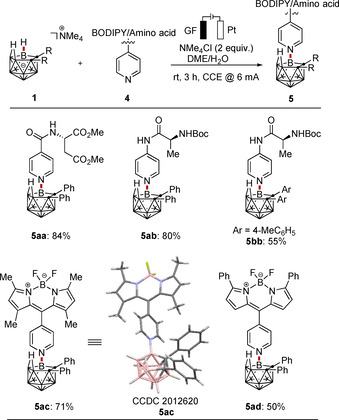
Electrooxidative B−N nitrogenation with amino acids and BODIPY pyridines.
The strategy was not restricted to intermolecular transformations. Indeed, the intramolecular B−N couplings of nido‐carborane 6 was likewise accomplished (Scheme 3), and either aryl or alkyl substituents at the cage‐carbon site afforded comparable results (7 a–7 b). Moreover, the molecular structure of the products (5 ac and 7 b) was again unambiguously verified by single‐crystal X‐ray diffraction. [23]

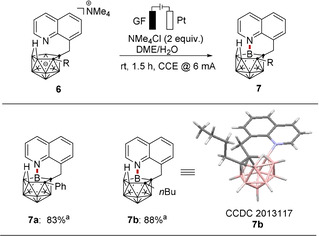
Electrooxidative intramolecular B−N annulation of 6.
The high efficacy of the electrocatalyzed B−H activation for the synthesis of N‐heterocyclic nido‐carboranes motivated us to delineate its mode of action. To this end, an intermolecular competition experiment between pyridine and imidazole revealed a slight preference for pyridine, likely due to the higher nucleophilicity of pyridine when compared to imidazole (pyridine: N=11.05 in H2O, imidazole: N=9.63 in H2O) [21] (Scheme 4). Furthermore, we probed the electrochemical B−H activation by means of cyclovoltammetric analysis of the nido‐carborane (Figure 2). Thus, we observed an irreversible oxidation of the nido‐carborane at E p/2=0.56 V vs. Ag/Ag+ at ambient temperature, which is indicative of a direct oxidation of the nido‐carborane under electrochemical condition. Furthermore, the calculated half‐wave oxidation potential of 1 a using DFT computation at the B97D3/def2‐QZVP+CPCM(DME)//B97D3/def2‐TZVP level of theory is in good agreement with the one obtained by our CV studies (exp: =0.87 V vs. SCE, calc: =0.86 V vs. SCE). [22] Subsequently, we analyzed the thermal and chemical stability of product 3 aa. Thus, we found that compound 3 aa (0.4 mL [D6]DMSO) was rather stable, when being heated to 120 °C for 10 h with only minor decomposition. Likewise, a solution of product 3 aa featured excellent stability in aqueous media as judged by 11B NMR spectroscopy, while showing reduced stability in strongly acidic or alkaline environments (SI Figure S1–S5).

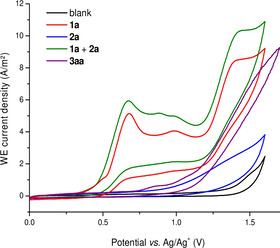
Cyclic voltammograms at 100 mV s−1, nBu4NPF6 (0.1 M in DME), concentration of substrates 1 mM.

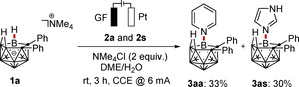
Competition experiments.
The optical properties of the thus‐obtained novel BODIPY‐labeled nido‐carborane 5 ac and 5 ad were studied in detail by UV/Vis absorption and fluorescence spectroscopy in various solvents (Table 2). The unprecedented BODIPY‐labeled nido‐carboranes exhibited very intense absorption in the UV and visible region, with an absorption maxima between 507–582 nm and high Stokes shift, resulting in an intense red to purple color. This could be rationalized by a possible donor‐acceptor‐donor structure of the compounds 5 ac and 5 ad, with the nido‐carborane core being a considerable electron acceptor. These spectroscopic data indicated the unique potential applications of the BODIPY‐labeled nido‐carborane compounds in pharmaceuticals, luminescent materials and bioimaging.

|
Compd |
Solvent |
Maxλabs [nm] |
Maxλem [nm] |
Stokes shift [cm−1] |
ϵmax [M−1 cm−1] |
|---|---|---|---|---|---|
|
5 ac |
DCM |
512 |
569 |
1956 |
68 812 |
|
CHCl3 |
513 |
567 |
1856 |
69 790 | |
|
Actone |
507 |
559 |
1834 |
73 746 | |
|
DMF |
509 |
561 |
1821 |
69 557 | |
|
THF |
509 |
562 |
1852 |
72 549 | |
|
5 ad |
DCM |
577 |
634 |
1558 |
63 459 |
|
CHCl3 |
582 |
640 |
1557 |
60 882 | |
|
Actone |
570 |
623 |
1492 |
65 681 | |
|
DMF |
574 |
602 |
810 |
58 557 | |
|
THF |
575 |
630 |
1518 |
67 718 |
Based on DFT, CV studies and literature reports, [13] a plausible reaction mechanism is proposed in Scheme 5, which commences with an anodic single electron‐transfer (SET) process from nido‐carborane ainon to form intermediate I, followed by the oxidation to generate intermediate II. Subsequently, deprotonation of the bridge proton results in the formation of cage‐open carborane intermediate III. Finally, the pyridine undergoes nucleophilic attack on the electron deficient B(9/11)‐H site of the intermediate III with consecutive transfer of H to the B(10) and B(11) forming a new bridge proton. In addition, molecular H2 is generated as the by‐product through cathodic proton reduction, which was confirmed by head‐space GC analysis. [22]

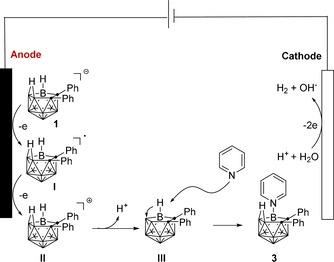
Proposed reaction mechanism.
Conclusion
In summary, reagent and catalyst‐free electrocatalyzed direct B−N oxidative couplings of nido‐carborane with N‐heterocyclic compound have been achieved at room temperature with molecular hydrogen as the sole by‐product. This approach features mild reaction conditions and high tolerance of functional groups leading to various amino acid‐ and BODIPY‐labeled nido‐carboranes, thereby offering a new platform for the design and synthesis of N‐substituted nido‐carborane by environmentally‐benign electricity. A plausible mechanism was established by cyclic voltammetry studies and computation. The thus‐obtained BODIPY‐labeled nido‐carborane displayed improved spectroscopic features.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Generous support by the DFG (Gottfried‐Wilhelm‐Leibniz prize to L. A.), the CSC (fellowship to L. Y.), and the DAAD (fellowship to B. B. J.) is gratefully acknowledged. We also thank Dr. Christopher Golz (University Göttingen) for support with the x‐ray diffraction analysis. Open access funding enabled and organized by Projekt DEAL.
References
1
1a
1b
1b
1c
2
2a
2c
2c
2d
2e
2e
2f
2g
2h
2i
3
3a
3b
4
4a
5
5a
5b
5b
5c
5c
5e
5e
5f
5g
6
6a
6d
6f
7
7a
7b
7c
7d
7f
7g
7h
8
8a
8b
9
9b
9c
10
12
13
13
14
14a
14a
14b
14c
14d
15
15a
15c
15c
15d
15f
15g
15h
15i
15k
15k
15l
15n
15o
16
16a
16d
16e
16e
17
17e
17g
18
18b
18c
18d
19
19b
20
20b
21
22
23
 Electrochemical B−H Nitrogenation: Access to Amino Acid and BODIPY‐Labeled nido‐Carboranes
Electrochemical B−H Nitrogenation: Access to Amino Acid and BODIPY‐Labeled nido‐Carboranes
