![The Reduced Nitridogermanates(III) Ca<sub>6</sub>[Ge<sub>2</sub>N<sub>6</sub>] and Sr<sub>6</sub>[Ge<sub>2</sub>N<sub>6</sub>] with Ge−Ge Bonds](https://storage.googleapis.com/nova-demo-unsecured-files/unsecured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/cover.png)
- Altmetric
The first nitridogermanates(III) Ca6[Ge2N6] and Sr6[Ge2N6] were synthesized from sodium flux and structurally characterized by powder and single crystal X‐ray diffraction, respectively. They crystallize isostructurally to each other and homeotypic to Ca6[Cr2N6]H in space group R 3‾ . They feature unprecedented, mutually isolated, ethane‐like [GeIII 2N6]12− anions in a staggered conformation. The compounds are semiconductors according to resistivity measurements and electronic structure calculations, yielding band gaps of 1.1 eV for Ca6[Ge2N6] and 0.2 eV for Sr6[Ge2N6].
The nitridogermanates(III), Ca6[Ge2N6] and Sr6[Ge2N6], contain the hitherto unknown molecular anion [Ge2N6]12− featuring a Ge−Ge‐bond and thus represent the first members of a novel class of compounds virtually unknown in the nitride chemistry of tetrel elements.
Introduction
The binary nitrides of the heavier tetrels silicon, germanium, tin and lead show a clear trend with respect to the stability of the tetrel‐nitrogen bond. Silicon nitride Si3N4 is a well‐known, highly inert compound that undergoes significant thermal decomposition only at temperatures above 1500 °C. [1] It has a large number of industrial and specialty applications due to its high hardness and thermal stability. Germanium nitride Ge3N4 is still a rather stable nitride, however, it decomposes around 600 °C in vacuum. [2] The crystal structures of the low‐pressure modifications of both silicon and germanium nitride consist of three‐dimensional networks of corner‐sharing SiN4 or GeN4 tetrahedra. There also exists a high‐pressure spinel modification of both,[ 3 , 4 ] in which two thirds of the tetrel atoms are coordinated octahedrally by nitrogen. Tin nitride Sn3N4 was so far exclusively observed in this modification. It is obtainable at atmospheric pressure, but starts to decompose around 300 °C. [5] Lead nitride, to the best of our knowledge, has not been shown to exist as of yet.
Due to the intermediate stability of the germanium‐nitrogen bond, Ge is unique among the group 14 elements when it comes to ternary nitrides. As discussed above, the heavier elements tin and lead are very reluctant to form bonds with nitrogen at all, with NaSnIIN currently being the only known ternary nitridostannate. [6] Silicon on the other hand is so strongly attracted to nitrogen that [SiIVN4]8− tetrahedra and their condensates are usually the only structural units found in nitridosilicates. [7] Exceptions from this rule include the (oxido)nitridosilicate Ce16(Si15O6N32) containing SiN6 octahedra and particularly SrSi6N8 and BaSi6N8, which feature SiIII−SiIII bonds next to dominating vertex‐sharing SiIVN4 tetrahedra.[ 8 , 9 , 10 ] Nitridogermanates exhibit a much richer structural chemistry than known for nitridosilicates or ‐stannates. Building blocks can include bent [GeIIN2]4− anions,[ 11 , 12 , 13 ] trigonal planar [GeIVN3]5− units, [14] as well as [GeIVN4]8− tetrahedra.[ 11 , 15 ] As with the silicates, these latter tetrahedra can condense via corner‐sharing to yield extended units or via edge‐sharing, resulting in [Ge2 IVN6]10− units. [16] Instances of nitridogermanate units coexisting with both isolated nitride ions and germanide Zintl ions have also been shown to exist.[ 13 , 17 , 18 ]
Here we present a hitherto unknown structural unit, the [GeIII 2N6]12− anion, featuring two germanium(III) atoms connected by a direct covalent bond and three nitride ligands each. In the title compounds, the [GeIII 2N6]12− anion is arranged in a staggered conformation, consistent with point group , within the crystal structure arrangement it retains full m (D 3d) symmetry, even though the crystal structure as a whole does not. This anion is notably distinct from the [GeIV 2N6]10− unit formed by two edge‐sharing tetrahedra found in Sr5[Ge2N6]. [16] Similar anionic units were revealed, for example, in a class of alkali metal chalcogenidotetrelates with the general formula A 6[Tt 2 Ch 6] and A=Na, K, Cs; Tt=Si, Ge, Sn; Ch=S, Se, Te.[ 19 , 20 , 21 , 22 , 23 ] Each of those compounds features ethane‐analogue [Tt 2 Ch 6]6− ions. Related compounds in the nitridometalate chemistry of transition metals include the homeotypic hydride Ca6[Cr2N6]H, [24] as well as Sr2Li6[Mn2N6] and Ca2Li6[Mn2N6],[ 25 , 26 ] which contain [MnIV 2N6]10− anions in a similar rhombohedral packing motif.
Results and Discussion
Dark grey single phase microcrystalline Ca6[Ge2N6] was obtained from calcium nitride Ca3N2, elemental Ge and sodium azide in a molar ratio n(Ca):n(Ge):n(N) of 3:1:3.7 in sealed tantalum ampoules at 800 °C. Sr6[Ge2N6] was synthesized by reacting strontium nitride Sr2N, germanium powder and sodium azide in a molar ratio n(Sr):n(Ge):n(N) of 3:1:3.5 in sealed niobium ampoules at 720 °C, using molten sodium flux. In each of three batches using this method, a small quantity of black Sr6[Ge2N6] single crystals was found, alongside mostly black, rod‐shaped Sr2[GeN2] crystals, yellow platelets of Sr5[Ge2N6] and red Sr7[GeN6] crystals.[ 12 , 15 , 16 ] In a later attempt to synthesize Sr6[Ge2N6], elemental germanium was replaced in the synthesis by germanium nitride Ge3N4 while keeping the overall nitrogen amount identical by reducing the sodium azide quantity. All other products resulted in similar proportions as before, but no Sr6[Ge2N6] was found in this batch. This suggests that Sr6[Ge2N6] might form as an intermediate during intercalation of strontium and concomitant nitridation of elemental germanium, retaining an isolated Ge−Ge bond. More detailed information on synthesis and crystal structure determinations can be found in the supporting information.
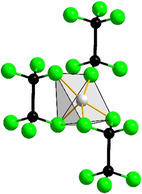
The title compounds crystallize homeotypic to Ca6[Cr2N6]H, [24] which features ethane‐like [Cr2N6]11− anions as well as hydride anions. The hydride ions are coordinated octahedrally by six calcium atoms. However, the site at (0, 0, 0), where hydride is found in Ca6[Cr2N6]H, is unoccupied in the title compounds. The distance from the vacant site to each neighboring cation amounts to 2.96 Å for Sr6[Ge2N6] and 2.85 Å for Ca6[Ge2N6], which would leave ample space for an anion such as N3− or H−. However, no significant residual electron density was found in this position, which is consistent with the charge balance of the compounds leaving no room for additional charged constituents. As depicted in Figure 1, this arrangement leads to a distorted square pyramidal coordination for the alkaline earth metal ions, where a distorted octahedral coordination complemented by H− was reported for Ca6[Cr2N6]H.

![Two projections of the extended unit cell of the title compounds (left, center) and coordination of the alkaline earth metal ions (right).
[33]](/dataresources/secured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/assets/ANIE-60-7691-g004.jpg)
Two projections of the extended unit cell of the title compounds (left, center) and coordination of the alkaline earth metal ions (right). [33]
Table 1 lists the most characteristic bond distances and angles in the title compounds. Perhaps unexpectedly, the Ge−Ge bond distances are significantly longer in Sr6[Ge2N6] than in Ca6[Ge2N6]. Though the difference may seem rather large, both values are within the range of Ge−Ge single bond distances reported for similar germanium(III) compounds, for example, the alkali metal chalcogenidogermanates with Ge−Ge bond distances as short as 2.43 Å in Na6[Ge2Se6] or as long as 2.58 Å in K6[Ge2S6].[ 22 , 23 ] Even longer distances are found in SrGe, a Zintl phase featuring infinite (Ge2−) chains with a bond length of 2.63 Å. [27] Lastly, the distance between two germanium atoms bridged by two nitrogen atoms in Sr5[GeIV 2N6] is reported at 2.61 Å, [16] which makes it only slightly longer than the Ge‐Ge distance in Sr6[Ge2N6].

|
|
Ca6[Ge2N6] |
Sr6[Ge2N6] |
|---|---|---|
|
d avg(A−N) [Å] |
2.515 |
2.666 |
|
d min(A−N) [Å] |
2.409(12) |
2.594(3) |
|
d max(A−N) [Å] |
2.728(13) |
2.803(3) |
|
d(Ge−Ge) [Å] |
2.419(5) |
2.595(1) |
|
d(Ge−N) [Å] |
1.891(18) |
1.908(1) |
|
α(Ge‐Ge‐N) [°] |
108.7(4) |
107.1(1) |
|
α(N‐Ge‐N) [°] |
110.3(7) |
111.7(1) |
The Ge−N bond distances found for the title compounds are similar to those of nitridogermanates(IV) with a tetrahedral coordination of germanium, such as Sr7[GeN4]N2 with d(Ge‐N)=1.92 Å, [15] Sr5[Ge2N6] with 1.84 Å< d(Ge‐N) <1.99 Å, [16] and Ca4[GeN4] with 1.89 Å< d(Ge‐N) <1.95 Å. [11] Nitridogermanates(II) generally contain slightly shorter Ge‐N distances, typically ranging between 1.84 Å and 1.89 Å,[ 13 , 17 , 18 ] with Sr3Ge[GeN2] being the only outlier with a reported Ge‐N distance of 1.94 Å. [12]
Figure 2 depicts IR and Raman spectra of Ca6[Ge2N6] and Sr6[Ge2N6]. In accordance to point group D 3d of the ethane‐like anion, three clearly distinguishable regions are visible: the ν(GeGe) mode (A1g), two Raman active modes for δ(GeN3) (A1g, Eg), and four ν(Ge‐N) modes in IR (A2u, Eu) and Raman (A1g, Eg) spectra. [28] The Ge‐Ge stretching mode is close to 300 cm−1 which was reported for elemental germanium, [29] despite the different bond lengths. Deformation modes δ(GeN3) agree well with those of various Ge3N4 modifications. [30] In contrast, stretching modes ν(GeN) are shifted to lower frequencies in comparison to Ge3N4. This corresponds well to the longer Ge−N bonds of terminal N atoms in [Ge2N6]12− and shorter bonds for the Ge‐N‐Ge bridging unit in Ge3N4. [31] A similar situation is observed for other isolated molecular anions such as [GeN2]4− and [GeN4]8− whose ν(GeN) modes accord well with the title compounds (Figure S2, Table S5).

![IR and Raman spectra of microcrystalline powders of Ca6[Ge2N6] (top), and a crystal of Sr6[Ge2N6] (bottom).](/dataresources/secured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/assets/ANIE-60-7691-g001.jpg)
IR and Raman spectra of microcrystalline powders of Ca6[Ge2N6] (top), and a crystal of Sr6[Ge2N6] (bottom).
Measurements of the temperature dependence of the magnetization were performed via SQUID magnetometry for magnetic fields up to 7 T and temperatures between 1.8 and 350 K. They indicate that Ca6[Ge2N6] is diamagnetic—see the supplementary material for details—which is consistent with what can be expected from the constituent elements and bonding. Electrical resistivity measurements were performed with a sapphire pressure cell from about 150 K to room temperature. Measurements of the resistivity of a Ca6[Ge2N6] sample exhibit activated semiconducting‐like behavior consistent with the sample being diamagnetic.
The electronic structure calculations show that both Ca6[Ge2N6] and Sr6[Ge2N6] are semiconductors with band gaps of 1.1 and 0.2 eV, respectively (see Figure 3). The densities of states (DOS) of both compounds are very similar throughout the whole valence band region. There are two very narrow bands at around −7.5 and −6.0 eV, respectively. The largest contributions to the former are from germanium and nitrogen s states, while N 2p is the largest contributor to the latter ahead of the s states. The higher energy regions are structured into three segments with the following approximate boundaries: [−4.4, −3.1], [−2.9, −1.0] and [−1.0, 0.0] eV (top of the valence band is set to 0 eV). The largest contributions come from the N 2p states, with Ca or Sr contributions (mainly from the 3d or 4d states, respectively) becoming significant in the last two segments. Most of the Ge 4p contributions are in the segment ranging from −4.4 to −3.1 eV. In both phases, the alkaline earth metal elements dominate the conduction bands.

![Total and partial electronic density of states computed for Ca6[Ge2N6] and Sr6[Ge2N6]. The band gaps are 1.1 eV and 0.2 eV, respectively.](/dataresources/secured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/assets/ANIE-60-7691-g003.jpg)
Total and partial electronic density of states computed for Ca6[Ge2N6] and Sr6[Ge2N6]. The band gaps are 1.1 eV and 0.2 eV, respectively.
The similarities between the two compounds extend to their effective charges and chemical bonding situation, as well. The effective charges computed based on the QTAIM approach yield positive charges amounting to 1.37 for calcium, 1.36 for strontium and 1.14 for germanium, in both cases. Hence, nitrogen atoms hold an effective charge of −1.75 in Ca6[Ge2N6] and −1.74 in Sr6[Ge2N6]. These high effective charge values imply significant ionic interaction contribution to the cohesion in the title compounds. The topological analysis of the ELI reveals the chemical bonding situation in the hexagonal unit cell as depicted in Figure 4. The organization of the two types of bonds in the [Ge2N6]12− ion is shown in Figure 5. The two‐center Ge−Ge bond has an electron population of n b Ge‐Ge=2.72 and 2.70 in Ca6[Ge2N6] and Sr6[Ge2N6], respectively. The volume of the Ge−Ge bond basin, V b Ge‐Ge, in Sr6[Ge2N6] (13.67 Å3) is larger than that in Ca6[Ge2N6] (11.91 Å3), however when expressed as a fraction of the unit cell volume, these values become comparable, 1.73 % versus 1.75 %, respectively. Additionally, if we define density of bonding electrons as ρ b Ge‐Ge=n b Ge‐Ge/ V b Ge‐Ge, then we find that ρ b Ge‐Ge in Ca6[Ge2N6] (0.228 e− Å−3) is higher than that in Sr6[Ge2N6] (0.198 e− Å−3) reflecting, essentially, the effect of difference in unit cell volumes rather than difference in Ge−Ge bond distances. The other bond is a highly polar Ge−N bond at which each of the five alkaline earth metal atoms coordinating to the nitrogen atom participates at a level less than 1 %. There are six such bonds per formula unit (alternatively one per N atom). The electron population of the bond basin is 7.50 in Ca6[Ge2N6] and 7.48 in Sr6[Ge2N6] with the largest contributions coming from the N atoms, at 6.62 and 6.59 electrons, respectively. Ge contributions are 0.57 and 0.58 electrons, while individual alkaline earth metal atoms provide only about 0.06 electrons so that total Ca or Sr contribution amounts to 0.30 and 0.31, respectively.

![The ELI distribution in the hexagonal unit cell of Sr6[Ge2N6] with the isosurface value of 1.38. The two‐dimensional plot of ELI in the (a,c) plane highlights the structuring of the penultimate shell (n=4) of the Sr atoms.](/dataresources/secured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/assets/ANIE-60-7691-g006.jpg)
The ELI distribution in the hexagonal unit cell of Sr6[Ge2N6] with the isosurface value of 1.38. The two‐dimensional plot of ELI in the (a,c) plane highlights the structuring of the penultimate shell (n=4) of the Sr atoms.

![The ELI distribution with isosurface value of 1.385 around the [Ge2N6]12− ion displays how the Ge−Ge and N−Ge bonds are organized spatially. The N atoms are located inside the isosurface regions representing the N−Ge bonds.](/dataresources/secured/content-1766030327506-9142851b-6f16-4768-8166-ddafde939c4b/assets/ANIE-60-7691-g002.jpg)
The ELI distribution with isosurface value of 1.385 around the [Ge2N6]12− ion displays how the Ge−Ge and N−Ge bonds are organized spatially. The N atoms are located inside the isosurface regions representing the N−Ge bonds.
ELI valence region attractors and the associated basins involve the genuine valence electrons of the atoms. The electrons occupying the Ca 3d or Sr 4d states belong to the penultimate shell (principal quantum number equal to 3 and 4, respectively). The participation of these electrons in the chemical bonding can be concluded if the distribution of the ELI in the penultimate shell deviates from spherical symmetry, a situation referred to as structuring. [32] Based on the electronic DOS (Figure 3), the occupancies of the Ca 3d and Sr 4d states are computed as 0.54 and 0.65 electrons, respectively. The implied participation of these electrons in chemical bonding is identified in Figure 4 for the exemplary case of Sr6[Ge2N6].
Conclusion
Ca6[Ge2N6] and Sr6[Ge2N6] are two new compounds containing the hitherto unknown molecular anion [Ge2N6]12− and particularly the first nitridogermanates(III), a novel class of compounds virtually unknown in nitride chemistry of the tetrels. Both compounds crystallize homeotypic to Ca6[Cr2N6]H, although the hydride site is left unoccupied. Like the majority of nitridogermanates reported so far, the anions in Ca6[Ge2N6] and Sr6[Ge2N6] are isolated from each other and do not condense to form chains, layers or three‐dimensional networks, as would be very typical for the related nitridosilicates.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank Steffen Hückmann for collecting the powder X‐ray diffraction data, Dr. Falk Lissner for collecting the single crystal X‐ray diffraction data. A.O. thanks Ulrike Nitzsche from IFW Dresden, Germany, for technical help in electronic structure calculations. Open access funding enabled and organized by Projekt DEAL.
References
1
2
3
4
5
6
7
7
8
8
9
9
10
13
14
16
17
18
19
20
21
21
22
23
24
25
25
26
27
28
29
30
31
32
33
 The Reduced Nitridogermanates(III) Ca6[Ge2N6] and Sr6[Ge2N6] with Ge−Ge Bonds
The Reduced Nitridogermanates(III) Ca6[Ge2N6] and Sr6[Ge2N6] with Ge−Ge Bonds
