
These authors contributed equally to this work.
- Altmetric
Recent work has shown that chemical release during the fundamental cellular process of exocytosis in model cell lines is not all‐or‐none. We tested this theory for vesicular release from single pancreatic beta cells. The vesicles in these cells release insulin, but also serotonin, which is detectible with amperometric methods. Traditionally, it is assumed that exocytosis in beta cells is all‐or‐none. Here, we use a multidisciplinary approach involving nanoscale amperometric chemical methods to explore the chemical nature of insulin exocytosis. We amperometrically quantified the number of serotonin molecules stored inside of individual nanoscale vesicles (39 317±1611) in the cell cytoplasm before exocytosis and the number of serotonin molecules released from single cells (13 310±1127) for each stimulated exocytosis event. Thus, beta cells release only one‐third of their granule content, clearly supporting partial release in this system. We discuss these observations in the context of type‐2 diabetes.
Exocytosis from beta cells monitored by amperometry is compared to the vesicle content assessed from intracellular vesicle impact cytometry to determine if partial release is dominant. Serotonin was used as an electroactive proxy for insulin as it is co‐released. The fraction of release from insulin‐containing granules in beta cells was found to be at most 34 %, providing a new perspective on insulin release for diabetes research.
Insulin is a metabolic hormone produced and released by the pancreatic islets. Each islet contains several different endocrine cell types with different responsibilities in the regulation of systemic metabolism. Among these are the beta cells which secrete insulin that lowers blood glucose levels. [1]
Insulin is stored as a hexamer complex with Zn ions (INS6Zn2) inside secretory nano granules or vesicles (50–300 nm diameter). [2] Generally, when glucose levels increase, beta cells start to secrete insulin by the process of exocytosis to decrease glucose levels. During rapid exocytosis (<50 ms), the vesicles dock to and fuse with the plasma membrane. This involves the formation of a fusion pore through which some or all of their cargo is released into the extracellular space. In the extracellular space, the insulin complex dissociates into insulin monomers, which acts on the target organs to promote glucose storage. Inadequate exocytotic release of insulin leads to type‐2 diabetes. [3] Hence, a detailed understanding of exocytosis in beta cells may help to establish the underlying molecular and cellular causes of the disease.
Until recently, exocytosis in general was thought to be principally an all‐or‐none event. More recently kiss‐ and‐ run and partial release modes have also been defined. [4] Partial release has been shown to be the predominant mode of release in PC12 and adrenal chromaffin cells[ 4b , 4c , 5 ] as well as in the neurons of Drosophila larvae. [5e] Subsequent work has shown that the exocytotic process can be regulated at the level of a single release event by varying the fraction released.[ 5a , 5b , 6 ] The partial release concept has been supported with TIRF and super‐resolution STED microscopy at adrenal chromaffin cells. [7]
In this paper, we use electrochemical methods to investigate whether exocytosis in beta cells is all or partial, a critical issue in determining the amount of hormone released. Many techniques have been used to study exocytosis at beta cells including immunosorbent assays, radioimmunoassays, fluorescence microscopy, chemiluminescence, and electron microscopy. [8] These methods have low temporal resolution, and they do not address the question whether the release process is all‐or‐none or partial. To fill this gap, amperometry with micro/nanoelectrodes is currently the only method with both high temporal resolution (≤ms) and the quantitative ability to count target molecules in single vesicles or during exocytotic release. [9] Amperometry at PC12 cells has recently been used to examine a repeated stimulation paradigm to observe plastic changes in the fraction released during exocytosis. [6f]
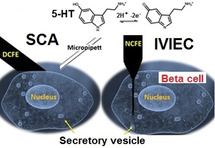
For intracellular analysis, we have used amperometry at a nanotip electrode to carry out intracellular vesicle impact electrochemical cytometry (IVIEC) to detect vesicle content in situ. [5] The IVIEC and SCA approaches are shown in Figure 1. In both cases, we chose to measure serotonin (5‐hydroxytryptamine(5‐HT)), [10] as it was difficult to electroanalyze insulin and 5‐HT is loaded in beta cell vesicles. 5‐HT is easily oxidized and detected at CFEs and as it is co‐released with insulin is an excellent surrogate of insulin during beta cells exocytosis. [11]

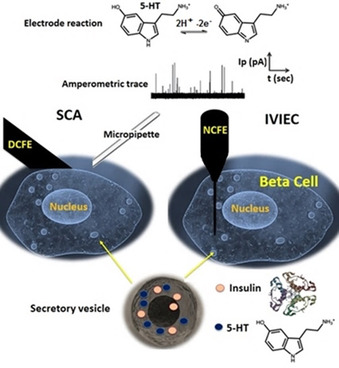
Schematic illustration of amperometry measurements of exocytosis by disk carbon fibre electrode (DCFE) and vesicle contents by nano‐tip carbon fibre electrode (NCFE) from 5‐HT loaded beta cells.
The vesicle cargo of beta cells generally includes the insulin hexamer complex, free Zn2+ ions, C‐peptide, ATP, 5‐HT and a small amount of gamma‐aminobutyric acid (GABA). [1a] The risk of any interference in amperometric measurement is therefore negligible. Generally, trace amounts of 5‐HT are stored by pancreatic beta cells, but levels are low and basal levels cannot be detected by amperometry. [11d] To solve this, beta cells were preloaded by 5‐HT via incubation and selective uptake into secretory vesicles has been demonstrated.[ 11d , 12 ]
Serotonin that is secreted during exocytosis or in single vesicles can be quantified by oxidation (+700 mV vs. Ag/AgCl) at the surface of a disk carbon fibre electrode (CFE) (Figure 1). Single‐cell amperometry (SCA) is used to quantify exocytosis. Here we quantitatively measure all the released catecholamine for vesicles under the electrode and this has been verified by comparing electrodes of different sizes. [6f] Additionally, we only use events with rapid rise times (25 % to 75 % <0.5 ms) to avoid measuring release from outside the electrode.
Recent work has shown that chemical release during the fundamental cellular process of exocytosis in model cell lines is not all or none. We tested this theory for vesicular release from single pancreatic beta cells. The vesicles in these cells release insulin, but also serotonin, which is detectible with amperometric methods. Traditionally, it is assumed that exocytosis in beta cells is all‐or‐none. Here we use a multidisciplinary approach involving nanoscale amperometric chemical methods to explore the chemical nature of insulin exocytosis. We amperometrically quantified the number of serotonin molecules stored inside of individual nanoscale vesicles (39 317±1611) in the cell cytoplasm before exocytosis and the number of serotonin molecules released from single cells (13 310±1127) for each stimulated exocytosis event. Thus, beta cells release only one‐third of their granule content, clearly supporting partial release in this system. We discuss these observations in the context of type‐2 diabetes.
To measure 5‐HT stored inside vesicles, a nanoscale tip CFE is inserted into the cell (Figure 1) is used where the vesicles adsorb on the electrode surface, rupture due to electroporation, and their contents are oxidized. This approach can be used to quantify the total electroactive content of each vesicle.[ 5a , 13 ]
For amperometric experiments, primary mouse pancreatic beta cells (cell isolation is described in detail in the supporting information (SI)) were incubated in tissue culture RPMI 1640 media containing 0.5 mM 5‐HT and 1 mM 5‐hydroxytryptophan (serotonin precursor) at 37° C, 5 % CO2 for 16–18 h. The 5‐hydroxytryptophan is enzymatically converted to 5‐HT after it enters the cytoplasm.[ 11d , 11e ] Just before amperometric measurements, the extracellular medium was changed to Krebs buffer solution (SI). Measurements were performed at 37 °C. Nano‐tip CFEs (see SI for fabrication method and sizes) were used to quantify the amount of stored 5‐HT in intracellular vesicles. [6] The nano‐tip electrode was placed on the top of a single unstimulated cell and pushed gently into it with a micromanipulator while applying a voltage to the electrode and recording current (Figure 1). In IVIEC, cytoplasmic vesicles adsorb on the electrode surface and open by electroporation.[ 5 , 6 ] Bursting vesicles expose their contents, allowing 5‐HT to be electrochemically detected and quantified (Figure 2 A,B).

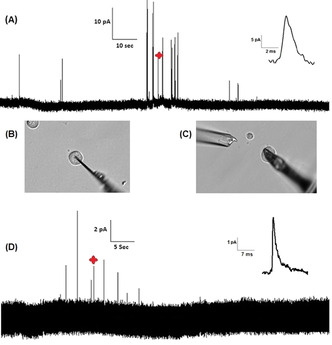
A) Representative amperometric trace for intracellular vesicle impact electrochemical cytometry from 5‐HT loaded mouse primary beta cell (inset: amplified amperometric current spike marked in the amperometric trace). Optical micrographs showing the experimental setups for B) IVIEC and C) SCA measurements. D) Representative amperometric trace for single cell amperometry from 5‐HT loaded mouse primary beta cell. Detection of exocytosis was carried out by applying 700 mV vs. Ag/AgCl. Cells were stimulated by application of 60 mM K+ stimulating solution. (inset: amplified amperometric current spike marked in the amperometric trace).
To quantify released 5‐HT from single cell during exocytosis, a disk CFE (fabrication and size described in SI) was positioned gently over the top of a single beta cell (Figure 1). Then, we used a nanopipette (Figure 2 C) to locally elevate the extracellular K+ concentration. As the beta cell membrane potential is principally determined by the K+ permeability this rapidly depolarizes the beta cell, thereby activating voltage‐gated Ca2+ channels, [3] initiating exocytosis events recorded as amperometric current spikes (Figure 2 C,D).
For both amperometric measurements, the number of 5‐HT molecules can be quantified with Faraday's law N=Q/n F, [6] where Q is the charge from the time integral amperometric spikes, N the number of molecules released from single vesicle, F is the Faraday constant, and n is the number of electrons exchanged in the oxidation reaction (n=2 for 5‐HT). We used SCA and IVIEC to compare the amount of 5‐HT released via exocytosis to the total vesicular 5‐HT content and the results are presented in a normalized frequency histogram (Figure 1S, Supporting Information). A large range of vesicles with different content ranging up to ≈400 000 5‐HT molecules was observed with IVIEC (Figure 1S‐A), while up to ≈120 000 molecules were released via exocytosis. Interestingly, the distributions observed for exocytotic release versus vesicular content suggest that the fraction of 5‐HT molecules released during exocytosis differs for individual vesicles.
The large range of distributions for 5‐HT molecules stored in vesicles might result from factors such as different preloading rates in individual vesicles, the vesicle size, and variable efficiencies between cells in the production of 5‐HT from the 5‐hydroxytryptophan precursor. IVIEC measurements (Figure 1S‐A), show a smaller number of events with higher 5‐HT content. Consequently, we examined the data by dividing it into three subgroups. We then used the mean of the medians (to minimize the influence of the cell‐to‐cell and vesicle‐to‐vesicle variation) of the amount of 5‐HT to compare and calculate the fraction released via exocytosis (Figure 1S).
The fraction released for 5‐HT in the different ranges was estimated by using the median of each range separately (Figure 1S). The results show that the fraction released ranges from 20 to 34 %, and fractional release from vesicles with higher molecular content is decreased. Although there is a wide range of vesicular content from IVIEC, most vesicles (approx. 60–70 %) contain less than 140 000 molecules. We therefore focused on the range in which the majority of events are located to decrease deviations and possible errors in the estimation of the fraction of 5‐HT molecules released.
A comparison of IVIEC data (choosing the reduced size range with data points from 0–140 000) to the SCA data is presented in Figure 3. The number of stored 5‐HT molecules was higher (39 317±1611 molecules, mean of medians ± SEM, 558 spikes from 51 cells) in comparison to released 5‐HT (13 310±1127 molecules, mean of medians ± SEM, 1442 spikes from 26 cells). These data show that even when omitting the larger content vesicles only 34 % of the 5‐HT content is released during beta cell exocytosis. Thus, it is justifiable to conclude that a large portion of full exocytosis events from pancreatic beta cells undergo a process whereby only a fraction of the vesicular cargo is released via the fusion pore. If anything, by excluding the vesicles with the highest molecular content, we report a lower estimate of the fractional release. These findings echo observations in chromaffin and PC12 cells, as well as exocytosis from nerve cells in Drosophila larvae.[ 4b , 4c , 5e , 6b , 6d , 6f ]

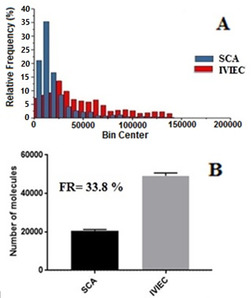
A) Histograms of released 5‐HT of exocytosis by SCA (blue, cells number=26) and vesicle content by IVIEC (red, cells number=51) from preloaded mouse primary beta cells. B) Mean of median of number of molecules detected by SCA and IVIEC in the range 0–140 000 (inset: fraction released (FR) for 5‐HT from beta cells).
In summary, we used two sensitive electrochemical methods, to obtain the fraction of 5‐HT (as a proxy for insulin) released during exocytosis at the single‐cell level with a goal of determining if release from beta cells is partial. The number of 5‐HT molecules released during exocytosis is ≈34 % of the stored content or lower. We conclude that partial release must be an important mechanism of exocytosis from beta cells. If this occurs for a small molecule like 5‐HT that will pass with relative ease through the partially open fusion pore, then it is likely that that the release of large molecules (such as the insulin hexamer) will be impeded more strongly. The same is true if the release is in part hindered by binding to a protein dense core in the vesicle as the larger insulin is expected to bind more tightly. This quantitative finding is highly relevant for the understanding of the causes of type‐2 diabetes, a metabolic disorder resulting from insufficient insulin release. [3] It is possible that beta cells physiologically regulate insulin release with exquisite precision by controlling release at the level of individual exocytosis events and that this regulation becomes impaired in type‐2 diabetes. These data therefore raise the tantalising possibility that pharmacological intervention can be used to increase the fraction released during beta cell exocytosis and lead to new therapies for type‐2 diabetes.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We acknowledge funding from the European Research Council (Advanced Grant), the Knut and Alice Wallenberg Foundation, the Swedish Research Council (VR). AH acknowledges funding from Sweden's Innovation Agency (Vinnova) and the Swedish Strategy Group for EU‐coordination. Also, AH thanks Alicia Lork for critical comments on the manuscript. NRG was supported by the European Foundation for the Study of Diabetes‐Rising Star Program and Novo Nordisk Foundation‐Young Investigator Program.
References
1
2
2a
3
3c
3d
4
4c
4c
4d
4e
5
5a
5a
5b
5b
5c
5d
5e
5e
5f
5f
6
6b
6b
6e
6e
7
7a
7b
8
8a
8b
8b
8c
9
9a
10
10a
10b
10d
11
11b
11d
11f
11g
12
 Nanoscale Amperometry Reveals that Only a Fraction of Vesicular Serotonin Content is Released During Exocytosis from Beta Cells
Nanoscale Amperometry Reveals that Only a Fraction of Vesicular Serotonin Content is Released During Exocytosis from Beta Cells
