
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Accurate and rapid diagnosis of Acanthamoeba keratitis (AK) is difficult. Although the diagnostic procedure for AK has improved, further development and effective diagnostic tool utilization for AK need to continue. Chorismate mutase is a key regulatory enzyme involved in the shikimate pathway, a metabolic pathway absent in mammals but central for amino acid biosynthesis in bacteria, fungi, algae, and plants. In this study, we describe the identification and production of a polyclonal peptide antibody targeting chorismate mutase secreted by A. castellanii, which could be used for AK diagnosis. Western blot was performed using the protein lysates and conditioned media of the human corneal epithelial (HCE) cells, non-pathogenic Acanthamoeba, pathogenic Acanthamoeba, clinical isolate of Acanthamoeba spp., and other causes of keratitis such as Fusarium solani, Pseudomonas aeruginosa, and Staphylococcus aureus. Polyclonal antibodies raised against A. castellanii chorismate mutase specifically interacted with lysates of Acanthamoeba origin and their culture media, while such interactions were not observed from other samples. Acanthamoeba-specificity of chorismate mutase was also confirmed using immunocytochemistry after co-culturing Acanthamoeba with HCE cells. Specific binding of the chorismate mutase antibody to Acanthamoeba was observed, which were absent in the case of HCE cells. These results indicate that the chorismate mutase antibody of Acanthamoeba may serve as a method for rapid and differential Acanthamoeba identification.
Introduction
Acanthamoeba keratitis (AK) is a painful and sight-threatening infection caused by several free-living amoebae belonging to the genus Acanthamoeba [1]. Over the past few decades, the incidence rates of AK have been increasing and several risk factors associated with AK have been delineated, which includes contact lens usage and corneal trauma [2]. Although early detection and diagnosis of AK could lead to successful treatment, accurately diagnosing AK remains difficult and this has frequently resulted in ocular Acanthamoeba infection being misdiagnosed as an infection of viral, bacteria, or fungal origin [1–3]. Inaccurate or delayed diagnosis can lead to AK treatment failure. Current diagnostic methods for AK primarily rely on microbiological culture and microscopic identification, while histochemical staining and PCR-based diagnosis are also available [4–7]. Nevertheless, these methods require corneal scrapings that inflict immense pain to the patients during the sample acquisition process and as such, a non-invasive diagnostic method encompassing a high degree of sensitivity and Acanthamoeba specificity is desired.
The potential of highly specific antibody-based Acanthamoeba detection methods has previously been reported [8, 9]. Several studies involving specific antibodies against Acanthamoeba spp. for AK diagnosis have reported interesting results. Acanthamoeba-specific lacrimal IgA and serum IgG were detected from both AK patients and healthy subjects, with lesser quantities of lacrimal IgA being reported from the former of the two [10]. Four antibody clones that specifically bind to Acanthamoeba spp. were isolated from a bacteriophage display library. Flow cytometry and immunofluorescence data revealed that the clone HPPG6 demonstrated specific binding to A. palestinensis [8]. Monoclonal antibodies targeting A. castellanii have been produced and their interactions with Acanthamoeba trophozoite or cyst were confirmed by flow cytometric analysis [9]. A monoclonal antibody raised against a mannose-binding protein of A. culbertsoni exhibited cross-reactivity with other Acanthamoeba spp. [11]. Although these earlier findings highlighted the importance of antibodies for AK diagnosis, most of the aforementioned studies did not investigate the interspecies interaction that may occur between Acanthamoeba-specific antibodies and other causative agents of keratitis. Recently, an immunochromatography-based assay kit using fluorescent silica nanoparticles was developed for rapid AK diagnosis [12]. Ease of use and rapid Acanthamoeba detection are the major advantages of this method, but the kit suffers from low sensitivity for cyst detection. Furthermore, the Acanthamoeba-specificity of this detection method requires elucidation. To this extent, developing an AK diagnostic method that encompasses the favorable features of these earlier works whilst demonstrating Acanthamoeba-specificity would be promising.
In this study, we sought to develop a rapid and simple diagnostic method for AK using Acanthamoeba-specific antibodies. We isolated the chorismate mutase protein from the secretory proteins of pathogenic A. castellanii. Chorismate mutase is an enzyme that catalyzes the chemical reaction of chorismate to prephenate in the shikimate pathway, which are subsequently used for producing the amino acids phenylalanine and tyrosine [13, 14]. This pathway, originally discovered in plants, was later reported to be present in various other organisms such as the bacterium Mycobacterium tuberculosis, fungi, and parasites [14–18]. Since the chorismate mutase is absent in humans and other mammals, this enzyme could serve as a potential target for antimicrobial agent development [19, 20]. The present study aimed to discover a chorismate mutase-specific antibody of A. castellanii origin, which can be used for Acanthamoeba identification. The results of this study were supported by western blot analysis and immunocytochemistry.
Materials and methods
Cell culture
Acanthamoeba, human corneal epithelial (HCE) cells, Fusarium solani, Pseudomonas aeruginosa, and Staphylococcus aureus were prepared as previously described [21]. Briefly, both of the non-pathogenic (ATCC 30011; yeast culture isolate) and pathogenic (ATCC 30868; human corneal isolate) strains of Acanthamoeba castellanii Castellani were purchased from the American Type Culture Collection (ATCC). A keratitis sample isolated from an anonymized patient at the Department of Ophthalmology, Kyung Hee University Hospital (Seoul, Republic of Korea) was sent to our lab to confirm whether the keratitis sample was of Acanthamoebic origin. The clinical isolate shared 98% sequence homology with Acanthamoeba spp. based on the complete 18s rDNA sequence. All three Acanthamoeba strains were axenically cultured in PYG medium at 25°C and after 5 days, the culture media was collected. HCE cells were incubated at 37°C with 5% CO2 in endothelial cell growth media (KGM BulletKit; Lonza, Portsmouth, NH, USA). P. aeruginosa and S. aureus were cultured in Brain Heart Infusion media at 37°C, while F. solani was cultured in Sabouraud Dextrose (SD) media at 25°C. The culture media of F. solani, P. aeruginosa, and S. aureus were harvested after cultivating for 1 day.
Cloning and peptide antibody production of chorismate mutase
Gene sequence of the A. castellanii Neff chorismate mutase (GenBank accession no: XM_004352878) was used to design the primers for chorismate mutase, which were subsequently used to amplify the coding sequence of A. castellanii Castellanii chorismate mutase by polymerase chain reaction. The primer information is as follows: 5’-ATGCGCTTCCTGCTCGCCTT-3’ (forward), and 5’-TCAATCGGCGTGCTGGACGG-3’ (reverse). The peptide sequence for the immunogen is as follows: NH2-C-RLQVETLNSEFNAGLPVPVQHAD-COOH. Synthesized peptide and the antibody raised against the peptide were purchased from AbFRONTIER (AbFRONTIER, Seoul, Korea). Briefly, two NZW rabbits were injected intradermally with 1.0 mg of peptide-KLH conjugates in complete Freund’s adjuvant on days 0, and 0.5 mg of peptide in incomplete Freund’s adjuvant on days 28, 42, and 56. The animals were bled on days 35 and 49. After the 3rd immunization, antisera titer was assessed using an indirect ELISA with the peptide-BSA conjugates as coating antigens until the titer plateaued. After the last immunization, blood samples were acquired by cardiac puncture from each rabbit.
Acanthamoeba specificity of the chorismate mutase antibody by western blot
Acanthamoeba-specificity of the chorismate mutase was confirmed using western blot as previously described [21]. Protein samples from the whole cell lysates and the culture media of Acanthamoeba spp., HCE cells, F. solani, P. aeruginosa, and S. aureus were prepared using a lysis buffer. After determining the concentration of each protein sample, 20 μg of cell lysates and 5 μg of conditioned media were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking the membrane with 5% skim milk in TBST for 1 h at RT, membrane was incubated overnight at 4°C with the chorismate mutase polyclonal antibody (1:1000). Membrane was washed with TBST, then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1:5000) (Sigma-Aldrich, St. Louis, MO, USA) at RT for 1 h. Bands were developed using enhanced chemiluminescence (ECL; Thermo Fisher, MA, USA) on an x-ray film in the darkroom.
Immunocytochemistry
HCE cells were seeded at a density of 3 × 105 cells/well on a six-well plate and incubated in KGM BulletKit media at 37°C for 24 h. After incubation, A. castellanii (ATCC 30868) trophozoites were added to HCE cells at a density of 5 × 105 cells/well, and incubated at 37°C for 4 h. After incubation, HCE cells and A. castellanii were fixed with 100% methanol for 5 min at RT and permeabilized using 0.2% Triton X-100 in phosphate-buffered saline (PBST) for 10 min at RT. After washing with PBS, cells were blocked using 1% bovine serum albumin in PBS for 30 min at RT. The cells were incubated overnight at 4°C with chorismate mutase antibody (1:200), washed with PBST, and subsequently incubated with mouse-anti-rabbit IgG-CFL-488 antibody (1:400) (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at RT. Cells were washed with 1X PBS and stained with VECTASHIELD® Mounting Medium with DAPI (Abcam, Burlingame, CA, USA). Images were acquired using fluorescent microscopy (Leica DMi8, Wetzlar, Germany).
Enzyme-linked immunosorbent assay (ELISA)
IgG antibody levels specific to A. castellanii were determined by ELISA. A checkerboard titration of each antigen and serum were designed for the optimal antigen concentration (100 to 0.0001 μg/μl) and serum dilutions (1:5 to 1:5000). Cell lysates and the conditioned media of Acanthamoeba were used as coating antigens. Briefly, in 96-well microplates, each well was coated with the antigen dissolved in carbonate coating buffer (0.1 M sodium carbonate, pH 9.5) at 4°C overnight. Plates were washed three times with PBS containing 0.05% Tween 20 (PBST) and blocked with 0.2% gelatin for 1 h at 37°C. All antibodies were diluted in PBS. Diluted samples were incubated at 37°C for 1 h and plates were washed. HRP-conjugated goat anti-rabbit IgG (Cusabio Co. Ltd, Wuhan, China) was added at 1:1000 dilution and incubated for 1 h at 37°C. O-phenylenediamine (OPD; Zymed, San Francisco, CA) was dissolved in citrate-phosphate buffer (pH 5.0) with 0.03% H2O2 and subsequently used as substrate. The optical density values at 450 nm were read using an EZ Read 400 microplate reader (Biochrom Ltd., Cambridge, UK). Sera of naïve rabbits were used as negative control.
Results
Identification of chorismate mutase in A. castellanii
Secretory proteins isolated from both non-pathogenic (Fig 1A) and pathogenic (Fig 1B) A. castellanii were resolved by SDS-PAGE and compared for analysis. As illustrated in Fig 1, several protein bands that were specifically present only in the pathogenic Acanthamoeba strain were observed. One such protein, as indicated by the arrow in Fig 1B, was selected for identification. Mass spectrometry and NCBI database search results revealed that the selected protein showed the highest similarity with the chorismate mutase of A. castellanii Neff. In this study, the full-length open reading frame of chorismate mutase from pathogenic A. castellanii Castellani was identified by polymerase chain reaction (GenBank accession number MN630520). The gene consists of 585 bp and encodes 194 amino acids with a calculated mass 21.34 kDa. Protein homology search results revealed that this protein showed 96.9% similarity with chorismate mutase 1 (putative) of A. castellanii Neff and 71.6% similarity with chorismate mutase 2 (subfamily protein) of A. castellanii Neff (Fig 2 and Table 1). Peptide analysis was performed using the SignalP 4.1 server database, which predicted the presence of the signal peptide at amino acid positions 1 to 19.

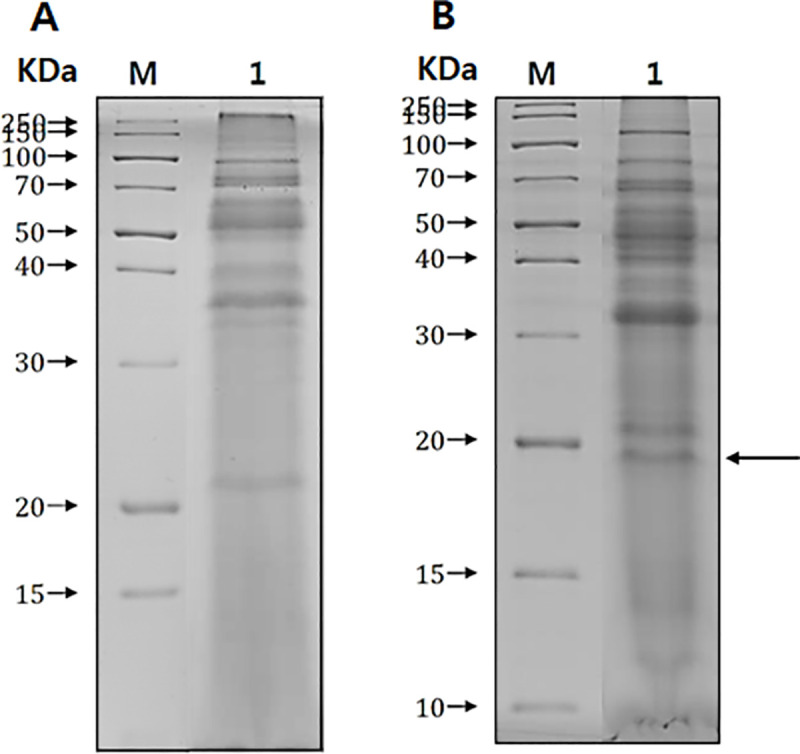
Secretory proteins of A. castellanii.
Secretory proteins between non-pathogenic Acanthamoeba (ATCC 30011) (A) and pathogenic Acanthamoeba (ATCC 30868) (B) were compared by SDS-PAGE. M; protein size marker, lane 1; conditioned media of Acanthamoeba. An arrow indicates a specific protein band of pathogenic Acanthamoeba origin.

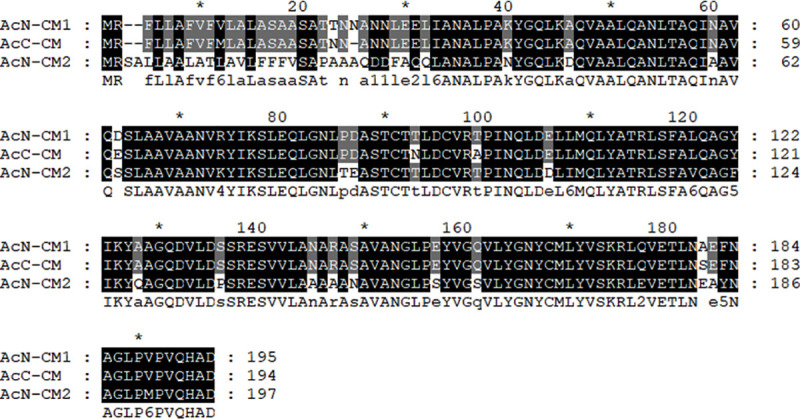
Alignment of the chorismate mutase amino acid sequences of Acanthamoeba spp..
Aligned amino acid sequences of A. castellanii Neff chorismate mutase 1 (ELR23397), A. castellanii Neff chorismate mutase 2 (ELR15898), and A. castellanii Castellani chorismate mutase (MN630520) were compared. ClustalX multiple sequence alignment was used to produce the alignment, and the degree of conservation is represented by different shading. AcN; Acanthamoeba castellanii Neff, AcC; Acanthamoeba castellanii Castellani, CM; chorismate mutase.

| Name | Accession No. | Length (aa) | Identities |
|---|---|---|---|
| AcC-CM | MN630520 | 194 | 194 (100%) |
| AcN-CM1 | ELR23397 | 195 | 189 (96.9%) |
| AcN-CM2 | ELR15898 | 197 | 144 (71.6%) |
| Fo-CM | EWY95915 | 223 | 34 (13.7%) |
| Fo-CM2 | EWY95914 | 266 | 37 (13.3%) |
| Pa-CM | OHQ59631 | 185 | 36 (16.0%) |
| Pa-CM2 | KJJ16793 | 365 | 37 (9.3%) |
| Sa-CM | OHS92057 | 363 | 30 (6.7%) |
AcC; Acanthamoeba castellanii Castellani, CM; chorismate mutase, AcN; Acanthamoeba castellanii Neff, Fo; Fusarium oxysporum, Pa; Pseudomonas aeruginosa, Sa; Staphylococcus aureus.
Production of chorismate mutase antibody
To assess the applicability of the chorismate mutase protein for AK diagnosis, we produced a polyclonal peptide antibody against the chorismate mutase of A. castellanii. Based on the hydrophobicity and antigenicity analysis profiles, two highly immunogenic epitopes were identified at amino acid position 127–134 and 172–194. Amino acid sequences of chorismate mutase from Acanthamoeba was compared with that of other causes of keratitis such as Fusarium spp., Pseudomonas aeruginosa, and Staphylococcus aureus. Protein homology search results revealed that chorismate mutase of Acanthamoeba showed 13.7%, 13.3%, 16.0%, 9.3%, and 6.7% similarity with chorismate mutase 1 or 2 of F. oxysporum, P. aeruginosa, and S. aureus, each respectively (Fig 3 and Table 1). Based on the amino acid homology search, amino acids at positions 172–194 were selected for peptide antibody production (boxed area in Fig 3).

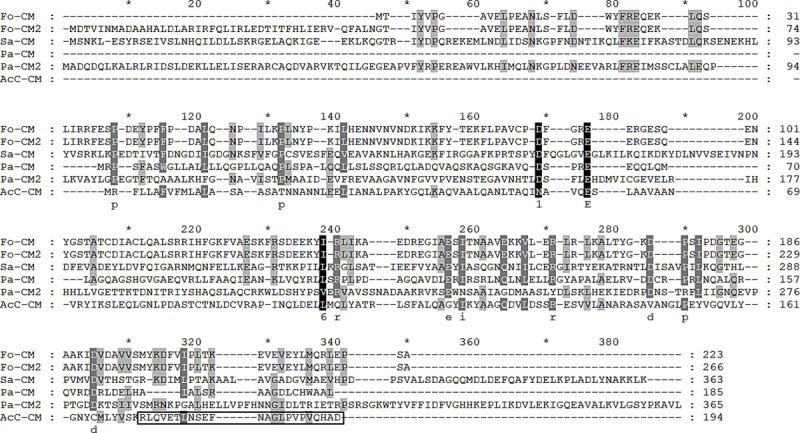
Alignment of the chorismate mutase amino acid sequences from different organisms.
Aligned amino acid sequences of F. oxysprum chorismate mutase (EWY95915), F. oxysprum chorismate mutase 2 (EWY95914), S. aureus chorismate mutase (OHS92057), P. aeruginosa chorismate mutase (OHQ59631), P. aeruginosa chorismate mutase 2 (KJJ16793), and A. castellanii Castellani chorismate mutase (MN630520) were compared. The boxed sequence corresponds to the antigen peptides used to raise the anti-chorismate mutase polyclonal antibody. Fo; Fusarium oxysporum, Sa; Staphylococcus aureus, Pa; Pseudomonas aeruginosa.
Confirming the specificity of a chorismate mutase peptide antibody
The specificity of the chorismate mutase antibody was demonstrated by western blot analysis using cell lysates and conditioned media of HCE cells, non-pathogenic Acanthamoeba, pathogenic Acanthamoeba, Acanthamoeba spp. isolated from a clinical sample, F. solani, P. aeruginosa, and S. aureus (Fig 4). Western blot results revealed that the chorismate mutase antibody did not react with HCE cell lysate and conditioned media (lane 1 of Fig 4A and 4B) but reacted with Acanthamoeba spp. cell lysate and conditioned media (lanes 2 to 4 of Fig 4A and 4B). The estimated sizes of the chorismate mutase protein and the secretory chorismate mutase in conditioned media were 21.34 kDa and 19.34 kDa, respectively. While both of these were detected from the conditioned media of Acanthamoeba spp., their presence was not confirmed in HCE cells, F. solani, P. aeruginosa, and S. aureus lysates (Fig 4B). Several non-specific bands were detected in the P. aeruginosa cell lysates, S. aureus cell lysate and its conditioned media (lane 7 of Fig 4A and 4B).

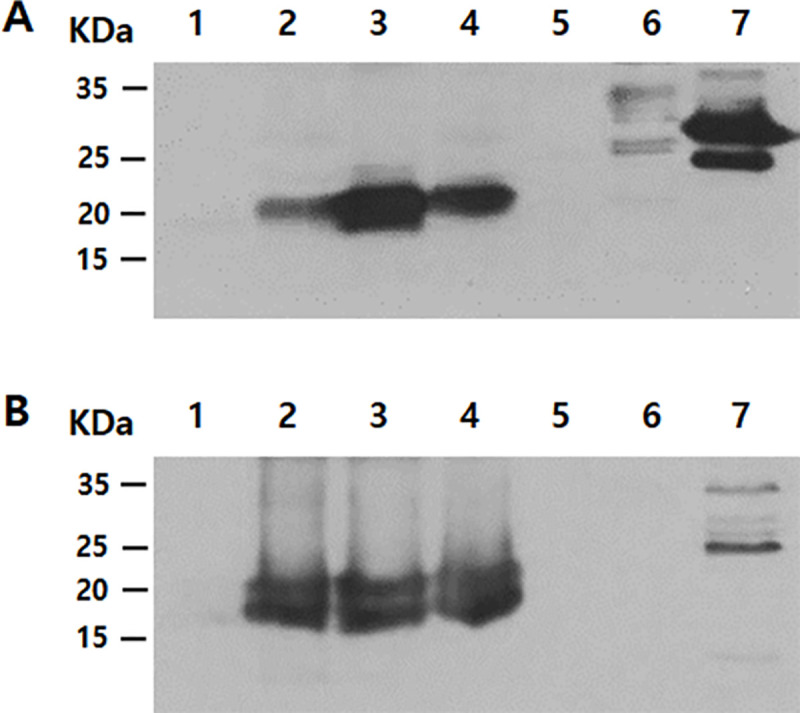
Specific antibody response of chorismate mutase.
The differential diagnosis of Acanthamoeba keratitis was confirmed by western blot analysis of cell lysates (A) and conditioned media (B) using the polyclonal peptide antibody raised against chorismate mutase of A. castellanii. Lane 1: HCE cells, lane 2: non-pathogenic Acanthamoeba, lane 3: pathogenic Acanthamoeba, lane 4: clinical isolate of Acanthamoeba, lane 5: F. solani, lane 6: P. aeruginosa, and lane 7: S. aureus.
To further validate the chorismate mutase antibody specificity, immunocytochemistry was performed using HCE cells were cultured with A. castelannii (Fig 5). As expected, DAPI staining revealed the cellular nucleic acid contents of HCE cells (Fig 5B), whereas HCE cells remained unstained when incubated with chorismate mutase antibody (Fig 5C). On the contrary, strong interaction between the chorismate mutase antibody and Acanthamoebae trophozoites was observed (Fig 5C). Faint DAPI staining was observed from Acanthamoeba, which could be attributed to its smaller size in comparison to HCE cells. A. castellanii cysts were also detected with chorismate mutase antibody (Fig 6A), however, F. solani, P. aeruginosa, and S. aureus were not detected with that antibody (Fig 6B–6D). Overall, the chorismate mutase antibody demonstrated its potential for selective and differential detection of Acanthamoeba via immunocytochemistry.

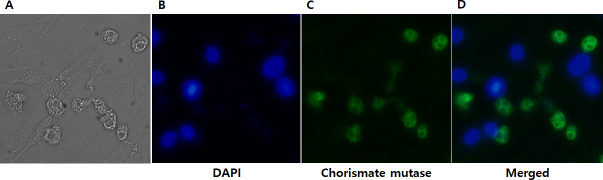
Immunocytochemistry using the A. castellanii chorismate mutase antibody.
HCE cells cultured with A. castellanii trophozoites for 4 h were visualized under a fluorescent microscope. Bright-field (A), DAPI-stained (B), indirect immunocytochemical labeling (C), and merged images (D) were acquired at 400x magnification.

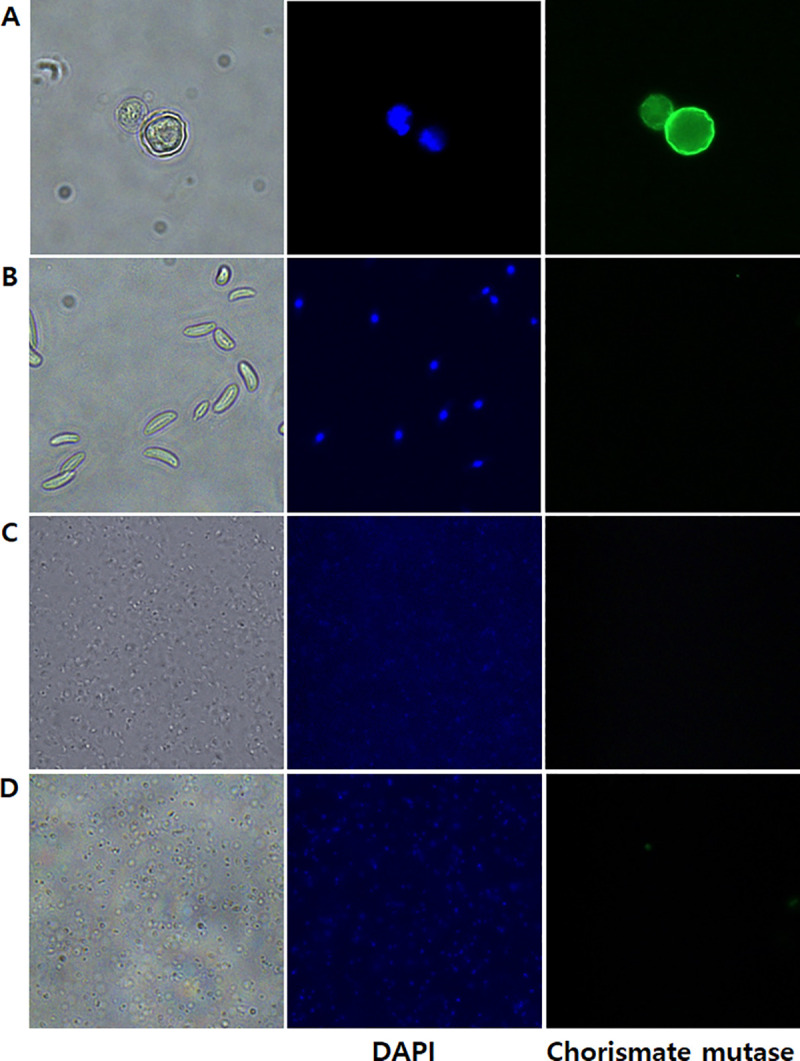
Confirming the species-specificity of the A. castellanii chorismate mutase antibody using immunocytochemistry.
A. castellanii cysts (A), F. solani (B), P. aeruginosa (C), and S. aureus (D) were visualized under a fluorescence microscope. Bright-field, DAPI-stained, and indirect immunocytochemical labeling images were acquired at 400x magnification.
ELISA titer of anti-chorismate mutase antibody
As seen in Fig 7, serological analysis of immune sera indicated that a rabbit injected with A. castellanii chorismate mutase possessed Acanthamoeba-specific IgG. High IgG antibody titers were observed from both A. castellanii cell lysates (1/5000) and its conditioned media (1/5000) (Fig 7A and 7B). In addition, Acanthamoeba-specific IgG was demonstrated to be highly sensitive, with a detection limit as low as 0.1 ng for both Acanthamoeba cell lysate and the conditioned media (Fig 7C and 7D).

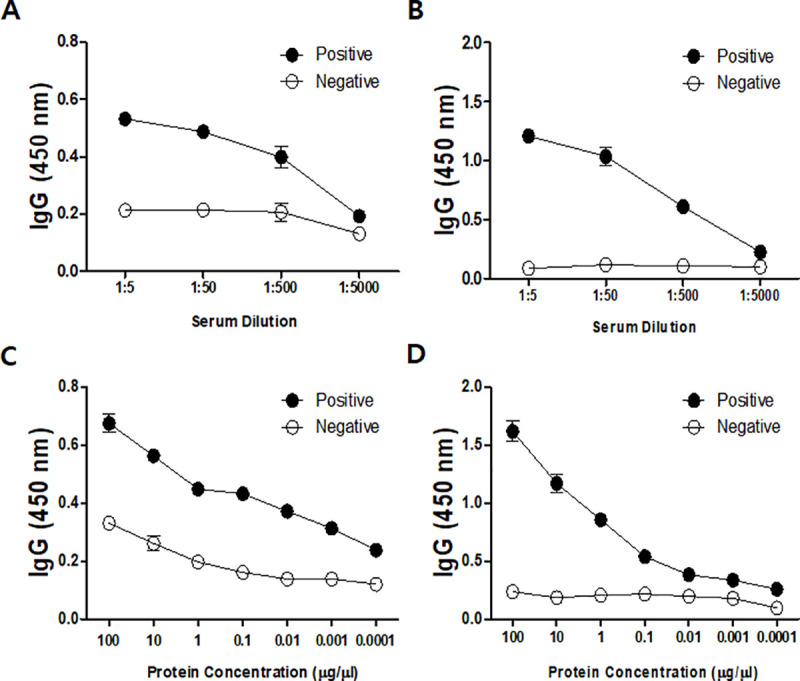
A. castellanii chorismate mutase-specific IgG response.
The chorismate mutase polyclonal antibody was titrated using A. castellanii cell lysates (A) and conditioned media (B). Sensitivity of the antibody was also determined using cell lysates (C) and conditioned media (D). positive; immunized serum, negative; non-immuned serum.
Discussion
Specific detection and early diagnosis of Acanthamoeba infection are critical for an effective AK treatment. In recent years, culturing of live Acanthamoeba and amplifying its DNA by PCR have become the principal procedure for detecting Acanthamoeba and diagnosis of AK [12]. Nevertheless, pain infliction during the corneal sample acquisition process can be discomforting to the patients. Identifying a reliable method enabling rapid and accurate detection of Acanthamoeba has become an utmost priority, as delayed or inaccurate diagnosis consequentially leads to successful treatment failure with ensuing visual impairment.
In this study, we produced a chorismate mutase peptide antibody against A. castellanii, and confirmed the specific detection of Acanthamoeba among HCE cells and other causes of keratitis (Figs 4 and 5). This antibody could be suitable for detecting the presence of Acanthamoeba in human ocular infections. Chorismate mutase, though absent in mammals, is linked to the shikimate pathway, an essential process for the biosynthesis of aromatic compounds in bacteria, fungi, algae, and plants [22]. This enzyme is present in other microbial pathogens that contribute to AK such as Fusarium spp., Pseudomonas spp., and Staphyloccus spp. Amino acid sequence homology results have revealed that compared to the chorismate mutase of A. castelanii, the sequence homologies observed from Fusarium, Pseudomonas, and Staphylococcus were extremely low (Fig 3 and Table 1).
Previously, it was reported that Acanthamoeba must be present as trophozoites for binding to HCE cells since cysts are non-infective and do not facilitate cell binding [23–25]. Based on this rationale, immunocytochemistry analysis was conducted using only A. castellanii trophozoites and HCE cells (Fig 5). However, we confirmed that chorismate mutase antibody bound to A. castellanii cysts (Fig 6). Although our results demonstrated Acanthamoeba-specificity of the chorismate mutase antibody, confirming our results using other Acanthamoeba spp. that cause AK, and various clinical samples isolated from AK patients would further validate our findings.
Most of the time, corneal scrapes or corneal biopsy specimens are used to culture or identify Acanthamoeba for AK diagnosis [26]. In the future, corneal scrape sample acquisition for AK diagnosis could be replaced with other non-invasive methods such as corneal cell secretions or tears. A. castellanii has been found to secrete a large amount of chorismate mutase proteins (Figs 1 and 4B), which signifies the potential application of chorismate mutase antibody reported in this study for AK diagnosis. Two distinct bands were observed in the Acanthamoeba conditioned media. One possible explanation for this phenomenon is due to the signal peptide cleavage of the secretory protein, a 19 amino acids long peptide with a molecular weight of approximately 2 kDa. However, highly diluted protein samples by tears can limit the detection capabilities of the chorismate mutase antibody, which can lead to false-negative results. A. castellanii chorismate mutase-specific IgG revealed that the antibody was highly sensitive and could identify A. castellanii even at low concentrations (Fig 7C). For this study, we prepared 900 μg of protein cell lysate from 8 × 105 trophozoites, implying that 0.1 ng of protein was prepared from a single trophozoite, and the lowest detection level of the antibody could be estimated as one trophozoite. Additional studies investigating the concentration of chorismate mutase in the patients’ tears and the minimum detectable concentration of the protein are needed before applying findings of the present study for AK diagnosis.
In conclusion, we produced an antibody against the secretory protein chorismate mutase of A. castellanii for the rapid and accurate identification of Acanthamoeba. Our in vitro studies suggest that this antibody is highly sensitive, indicating its potential application for differential AK diagnosis.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
 Chorismate mutase peptide antibody enables specific detection of Acanthamoeba
Chorismate mutase peptide antibody enables specific detection of Acanthamoeba
