
A.E.M.B. and H.M. contributed equally.
A.v.S. currently works at Haaglanden Medical Center, the Hague.
Members of the ACES Study Group and their institutional affiliations are listed in Table S6.
- Altmetric
Objective
Diagnosing autoimmune encephalitis (AIE) is difficult in patients with less fulminant diseases such as epilepsy. However, recognition is important, as patients require immunotherapy. This study aims to identify antibodies in patients with focal epilepsy of unknown etiology, and to create a score to preselect patients requiring testing.
Methods
In this prospective, multicenter cohort study, adults with focal epilepsy of unknown etiology, without recognized AIE, were included, between December 2014 and December 2017, and followed for 1 year. Serum, and if available cerebrospinal fluid, were analyzed using different laboratory techniques. The ACES score was created using factors favoring an autoimmune etiology of seizures (AES), as determined by multivariate logistic regression. The model was externally validated and evaluated using the Concordance (C) statistic.
Results
We included 582 patients, with median epilepsy duration of 8 years (interquartile range = 2–18). Twenty patients (3.4%) had AES, of whom 3 had anti–leucine‐rich glioma inactivated 1, 3 had anti–contactin‐associated protein‐like 2, 1 had anti–N‐methyl‐D‐aspartate receptor, and 13 had anti–glutamic acid decarboxylase 65 (enzyme‐linked immunosorbent assay concentrations >10,000IU/ml). Risk factors for AES were temporal magnetic resonance imaging hyperintensities (odds ratio [OR] = 255.3, 95% confidence interval [CI] = 19.6–3332.2, p < 0.0001), autoimmune diseases (OR = 13.31, 95% CI = 3.1–56.6, p = 0.0005), behavioral changes (OR 12.3, 95% CI = 3.2–49.9, p = 0.0003), autonomic symptoms (OR = 13.3, 95% CI = 3.1–56.6, p = 0.0005), cognitive symptoms (OR = 30.6, 95% CI = 2.4–382.7, p = 0.009), and speech problems (OR = 9.6, 95% CI = 2.0–46.7, p = 0.005). The internally validated C statistic was 0.95, and 0.92 in the validation cohort (n = 128). Assigning each factor 1 point, an antibodies contributing to focal epilepsy signs and symptoms (ACES) score ≥ 2 had a sensitivity of 100% to detect AES, and a specificity of 84.9%.
Interpretation
Specific signs point toward AES in focal epilepsy of unknown etiology. The ACES score (cutoff ≥ 2) is useful to select patients requiring antibody testing. ANN NEUROL 2021;89:698–710
Autoimmune encephalitis (AIE) associated with neuronal antibodies is a severe but treatable neurological disease. Seizures occur frequently in patients with AIE (50–95%), often in combination with other symptoms, such as cognitive symptoms, behavioral changes, and autonomic dysfunction. 1 , 2 , 3 , 4 Seizures are often resistant to antiseizure medication (ASM), whereas the response to immunotherapy is good. 5 , 6 Most patients have fulminant encephalitis with prominent seizures.
Neuronal antibodies have also been reported in patients with epilepsy (14–31%). 7 , 8 , 9 Results from these studies have ensured that patients with less rapidly progressive encephalitis are being recognized as well. Nevertheless, in most of these studies, patients had short epilepsy duration, and most of them had signs and symptoms of encephalitis. Interestingly, some of the mentioned studies report patients with epilepsy without fulminant encephalitis or even any sign of encephalitis. To complicate interpretation, some of these studies describe a variety of antibodies, some pathogenic, but others with questionable clinical relevance. 10 , 11
An important category comprises neuronal antibody–positive epilepsy patients without other encephalitis signs, because underdiagnosis is likely. It is essential to recognize these patients early and to perform antibody testing in preselected patients. At the same time, testing needs to be rigorous, confirming results using different tests, to avoid false positives or clinically irrelevant results. Similarly, it is important to limit the number of patients who require testing, for the sake of specificity and cost‐effectiveness.
The aim of our prospective, multicenter study was to identify neuronal antibodies in a comprehensive cohort of patients with focal epilepsy of unknown etiology, and without, or with unrecognized, signs of encephalitis. We have developed a clinical score, based on the prospectively collected data of patients with focal epilepsy of unknown etiology, that can be used to guide autoimmune etiology of seizures (AES) screening. This antibodies contributing to focal epilepsy signs and symptoms (ACES) score has subsequently been validated in a second, external cohort.
Patients and Methods
Study Design, Participants, and Definitions
In this prospective, multicenter, observational cohort study, adults with focal epilepsy of unknown etiology were included by epileptologists between December 2014 and December 2017. Patients included in this study had been referred to (tertiary) epilepsy centers by neurologists who had no particular suspicion of AIE. Patients were included in the Netherlands, from tertiary epilepsy centers and from dedicated epilepsy centers in academic hospitals, and one general hospital with an epilepsy referral center. We requested all epileptologists (1) to include patients with epilepsy with or without additional symptoms, but without suspicion of encephalitis; and (2) to exclude patients strongly suspected of having AIE. Patients with epilepsy with known infectious, genetic, or metabolic etiologies were excluded. Patients with a structural lesion on brain magnetic resonance imaging (MRI) at inclusion were excluded, whereas patients with mesial temporal sclerosis, or with T2/fluid‐attenuated inversion recovery (FLAIR) hyperintensities mainly in the mesial temporal lobe, both unilateral and bilateral, were not, as these MRI findings might be associated with AIE. 12
International League against Epilepsy guidelines were used to define focal seizures, an unknown etiology of epilepsy, and drug‐resistant epilepsy. 13 The study was registered at ClinicalTrials.gov (NCT02802475).
Patients were classified as (1) definite AES if a known neuronal antibody was detected in serum and/or cerebrospinal fluid (CSF), and if results were confirmed with a different laboratory test; (2) probable AES if a known neuronal antibody was detected in serum and/or CSF, but if we were unable to confirm the finding, or when the diagnostic criteria for seronegative AIE were met 14 ; (3) possible AES, if serum or CSF showed similar staining patterns on immunohistochemistry (IHC), but without known antibody; and (4) non‐AES.
Procedures
The including epileptologist or the coordinating investigator (M.A.A.M.d.B.) completed a case record form at enrollment, containing information about patient characteristics, epilepsy characteristics, presence of autoimmune‐associated clinical signs or symptoms (disorders of memory, sleep, behavior, speech, movement, or the autonomic system), previous and current medication (including ASM), current level of functioning (measured with modified Rankin Scale [mRS] 15 ), and results from prior ancillary testing (including MRI, electroencephalogram [EEG], and if available CSF analysis). Blood was drawn, and if CSF analysis was performed, this was collected as well.
Patients were prospectively followed for 1 year (last follow‐up in December 2018). At 1, 4, 8, and 12 months after inclusion, the treating physician or coordinating investigator collected data about seizure type, monthly seizure frequency, types of ASM used, and mRS. Final diagnosis was obtained at last follow‐up.
Laboratory Methods
All samples were screened for immunoreactivity with IHC, as previously described. 16 Subsequently, all samples with a positive or questionable IHC result were tested more extensively. A combination of laboratory techniques was used, depending on the staining pattern and the clinical phenotype. Commercial cell‐based assay (CBA; Euroimmun, Lübeck, Germany) was used to detect anti–N‐methyl‐D‐aspartate receptor (anti‐NMDAR), anti–α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor (anti‐AMPAR), anti–γ‐aminobutyric acid B receptor (anti‐GABABR), anti–leucine‐rich glioma‐inactivated 1 (anti‐LGI1), anti–contactin‐associated protein‐ like 2 (anti‐CASPR2), and anti–glutamic acid decarboxylase 65 (anti‐GAD65). Anti‐GAD65 was tested with enzyme‐linked immunosorbent assay (ELISA; Medizym anti‐GAD 96, Medipan, Berlin, Germany) as well, to quantify antibody concentration. An in‐house CBA with live cells was used to detect anti‐GABAAR, anti‐GABABR, anti‐AMPAR, and anti–dipeptidyl‐peptidase–like protein 6. Radioimmunoassay (RIA; VGKC Antibody Assay RIA, DLD Diagnostika, Hamburg, Germany) was used to detect voltage‐gated potassium channel complex antibodies (anti‐VGKC), and immunoblotting was used to detect onconeural antibodies (anti‐Hu/Yo/Ri/Tr/amphyphysin/CV2/Ma1/Ma2; Euroimmun). We considered serum anti‐GAD65 relevant if the ELISA concentration was >10,000IU/ml. Only samples tested positive by ELISA and with a compatible positive staining on IHC were considered positive. 17 In addition, all serum samples were screened for the presence of anti–glycine receptor (anti‐GlyR) with an in‐house CBA with live cells, as these antibodies cannot be visualized with IHC. 18 , 19 All positive results were confirmed in separate experiments.
Samples scored questionable or positive on IHC, but without a known antibody, were tested more extensively using immunocytochemistry with live hippocampal cell cultures. 16
A subgroup of patients considered for epilepsy surgery were side by side tested by CBA, ELISA, and RIA for antibodies, as a part of a standardized protocol. In addition, patients with an ACES score of 2 or more were tested by commercial CBA and ELISA post hoc.
Statistical Analysis
Dutch Cohort
Patients were divided into 3 groups, based on antibody test results: (1) antibodies targeting extracellular neuronal antigens, (2) high‐concentration anti‐GAD65, and (3) non‐AES. Comparisons between the 3 groups (extracellular‐AES, GAD‐AES, and non‐AES) were performed with the Fisher–Freeman–Halton test, the Pearson Chi‐square test, or the Kruskal–Wallis test, when appropriate. To correct for multiple testing, we only considered p values < 0.002 to be relevant (Bonferroni). Exploratory, post hoc in‐between analysis using the Fisher exact test and Mann–Whitney U test was used to visualize group differences.
The patients with probable or possible AES were not included in the analysis, and are only described on an exploratory basis, as their etiology could not be confirmed with certainty. We repeated analyses assigning these patients to the non‐AES group to assess the effects of exclusion.
A multivariate logistic regression model was used to identify independent risk factors pointing toward AES (comparing both extracellular‐AES and GAD‐AES to non‐AES). Antibody status was used as dependent variable. Missing values (0.8% of the total number of values) were imputed. Multiple imputation was used to create 5 sample sets, with antibody status included as covariate. We excluded the factor “family history of autoimmune diseases,” as we identified overt recall bias (lack of reliable information in many patients without AES). The univariate regression and multivariate regression models were constructed using the imputed data. Variables with a p value < 0.007 in univariate analysis (corrected for multiple testing) were included in the multivariate logistic regression model. To derive the ACES score, variables were eliminated using a backward likelihood ratio method with cutoff p value of 0.05. The selected risk factors were used on the original and imputed data to check the accuracy. Each risk factor was assigned 1 point to create the ACES score (range = 0–6 points). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the nonimputed dataset. Percentages were visualized with 95% confidence interval (CI) assuming a Poisson distribution.
The systematic difference in direction between the Antibody Prevalence in Epilepsy and Encephalopathy (APE), APE2 and ACES scores were compared using McNemar paired test. Internal validation with bootstrapping was used to estimate the degree of optimism in the ACES score. In addition, Firth logistic regression was used. We performed a sensitivity analysis to assess the validity of our model allocating the excluded patients with probable or possible AES to the non‐AES group (to be conservative).
Czech Cohort
External validation was performed in a Czech cohort consisting of 128 temporal lobe epilepsy patients. 20 In the validation cohort, samples were tested by commercial CBAs for anti‐NMDAR, anti‐AMPAR, anti‐GABABR, anti‐CASPR2, and anti‐LGI1 (Euroimmun) and immunoblot (PNS 11 Line Assay, Ravo Diagnostika, Freiburg, Germany), confirmed by RIA for anti‐GAD65 (CentAK anti‐GAD65 M, Medipan GMBH, Berlin, Germany). All positive and questionable samples had been tested by IHC (F.L.). For uniformity, all questionable and positive samples were additionally tested by CBA, ELISA, and IHC in Rotterdam (M.J.T.). All 6 risk factors used in the ACES score had been collected prospectively in the Czech cohort, and were retrieved from the database to calculate the ACES score.
The model performance in this validation cohort was described by discriminatory value, using the Concordance (C) statistic (area under the curve of the receiver operating curve) on the ACES model. Statistical analysis was performed using SPSS Statistics 21 (IBM, Armonk, NY), R statistical software version 3.6.2 (rms library), and Prism 8.0.1 (GraphPad Software, San Diego, CA) for Windows.
Ethics Committee Approval
The institutional review board of the Erasmus MC University Medical Center approved the study protocol (MEC‐2014‐463). Written informed consent was obtained from all patients.
Results
Patients: Dutch Cohort
Informed consent was obtained in 618 patients, of whom 36 (6%) were excluded (Fig 1). Of 582 included patients with focal epilepsy of unknown etiology, 48% were male. The median age at inclusion was 44 years (interquartile range [IQR] = 29–58, range = 18–89). Ten percent of all included patients had a history of autoimmune diseases. Median epilepsy duration was 8 years (IQR = 2–18, range = 0.1–75), whereas 66 patients (11%) had an epilepsy duration of <1 year. Patients were treated with a median of 1 ASM at inclusion (IQR = 1–2, range = 0–5).

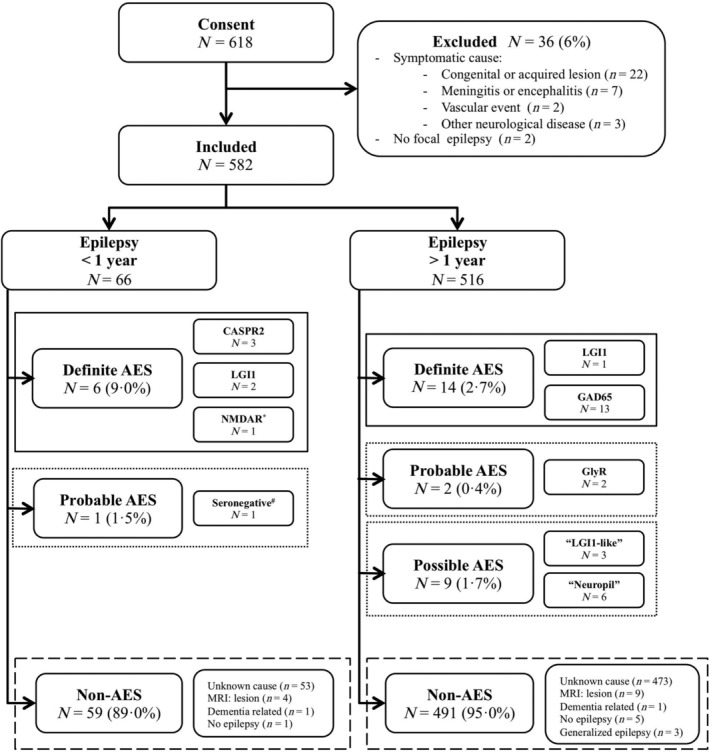
Flowchart with follow‐up diagnosis of all included patients. *This patient had focal onset epilepsy with sporadically occurring focal to bilateral tonic–clonic seizures, and had a recently discovered esophagus carcinoma. Eleven months after inclusion, his seizure frequency increased, and he developed psychotic symptoms. Cerebrospinal fluid was positive for anti–N‐methyl‐D‐aspartate receptor (NMDAR), confirmed with live hippocampal neurons. #According to the criteria. 14 AES = autoimmune etiology of seizures; CASPR2 = contactin‐associated protein‐like 2; GAD65 = glutamic acid decarboxylase 65; GlyR = glycine receptor; LGI1 = leucine‐rich glioma‐inactivated 1; MRI = magnetic resonance imaging.
MRI was normal in 532 patients (91%), whereas T2/FLAIR hyperintensities of the mesial temporal lobe were observed in 14 patients (2%), and 36 patients (6%) had mesial temporal sclerosis. The EEG showed epileptic discharges in 389 patients (67%). In 362 of these 389 patients, EEG reports were available. In 23% (n = 83), seizures had a multifocal onset, whereas in 77% (n = 279) there was a focal seizure onset, including temporal (39%, n = 108), frontotemporal (39%, n = 110), and extratemporal (20%, n = 57) localization.
Results of Laboratory Studies
Serum was collected from all patients, and CSF was collected from 46 patients (8%). In 20 of all 582 patients (3.4%), neuronal antibodies related to AES were detected (definite AES), including anti‐LGI1 (n = 3), anti‐CASPR2 (n = 3), anti‐NMDAR (n = 1), and high‐concentration anti‐GAD65 (n = 13). Of the 66 patients with epilepsy for less than 1 year at inclusion, 6 had antibodies (9%; see Fig 1).
Three patients of all 582 patients were scored as probable autoimmune. One patient, with a neuropil staining pattern on IHC, fulfilled the criteria of seronegative AIE, 14 whereas in serum of the other 2 patients anti‐GlyR were detected using CBA. Nine patients had a positive IHC, but no known antibody was identified, nor was immunocytochemistry positive in any of these samples; these patients were considered possible autoimmune. Serum and CSF of 3 of these 9 patients showed a similar “LGI1‐like” staining pattern on IHC. In the other 6 patients, IHC showed a diffuse neuropil staining pattern. No additional antibodies were detected in the patients with questionable IHC staining patterns and negative live neurons (n = 36). In addition, 186 IHC negative samples were tested by commercial antibody testing, partly in parallel, and often as part of screening for epilepsy surgery (n = 104). The other samples were tested because of high ACES scores (ACES ≥2, n = 82; post hoc testing). No antibodies were found in these samples.
Comparison between AES and Non‐AES
Comparison of patients with antibodies against extracellular antigens (LGI1, CASPR2, NMDAR; n = 7), patients with anti‐GAD65 (n = 13), and non‐AES patients (n = 550; Table S1), showed that patients with AES (antibodies against both extracellular antigens and GAD65) more frequently had drug‐resistant epilepsy (p = 0.002) and uni‐ or bilateral T2/FLAIR hyperintensities of the mesial temporal lobe (p < 0.0001), had higher mRS at inclusion (p < 0.0001), and more often had cognitive symptoms (p < 0.0001) and behavioral changes (p < 0.0001; Fig 2). Post hoc in‐between analysis showed that patients with antibodies against extracellular antigens were older at disease onset, were more frequently male, had shorter epilepsy durations, and more frequently had autonomic symptoms than patients with anti‐GAD65 or non‐AES patients. Patients with high‐concentration anti‐GAD65 were all female and more often had other autoimmune diseases, speech problems (word finding difficulties, non‐fluent speech), and muscle stiffness than patients with antibodies against extracellular antigens or non‐AES patients.

![Overview of the characteristics, signs, and symptoms that occurred more often in patients with neuronal antibodies. *p between 0.05 and 0.005, **p between 0.005 and 0.0001, ***p < 0.0001. The top p values correspond to the values visualized in Table S1. The lighter‐colored lines visualize significance of the post hoc in‐between analysis. (A) Factors that differed significantly between autoimmune etiology (AE) of seizures and non‐AE. (B) Values that differed between the extracellular antigen group and the non‐AE group. (C) Values that differed between the GAD65 and non‐AE groups. In order of appearance, the y‐axis shows: (1) years (age at onset), (2) cumulative percentage (all bar diagrams), (3) months and years (0–12, 1–80 respectively; epilepsy duration). In the bar diagrams, the x‐axis shows the raw values. CASPR2 = contactin‐associated protein‐like 2; GAD65 = glutamic acid decarboxylase 65; LGI1 = leucine‐rich glioma‐inactivated 1; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NMDAR = N‐methyl‐D‐aspartate receptor. [Color figure can be viewed at www.annalsofneurology.org]](/dataresources/secured/content-1766029909547-59b46bf8-d6bb-45e3-98b0-1f9876e10b8c/assets/ANA-89-698-g002.jpg)
Overview of the characteristics, signs, and symptoms that occurred more often in patients with neuronal antibodies. *p between 0.05 and 0.005, **p between 0.005 and 0.0001, ***p < 0.0001. The top p values correspond to the values visualized in Table S1. The lighter‐colored lines visualize significance of the post hoc in‐between analysis. (A) Factors that differed significantly between autoimmune etiology (AE) of seizures and non‐AE. (B) Values that differed between the extracellular antigen group and the non‐AE group. (C) Values that differed between the GAD65 and non‐AE groups. In order of appearance, the y‐axis shows: (1) years (age at onset), (2) cumulative percentage (all bar diagrams), (3) months and years (0–12, 1–80 respectively; epilepsy duration). In the bar diagrams, the x‐axis shows the raw values. CASPR2 = contactin‐associated protein‐like 2; GAD65 = glutamic acid decarboxylase 65; LGI1 = leucine‐rich glioma‐inactivated 1; MRI = magnetic resonance imaging; mRS = modified Rankin Scale; NMDAR = N‐methyl‐D‐aspartate receptor. [Color figure can be viewed at www.annalsofneurology.org]
ACES Score
Eight variables showed a statistically significant association with antibody status in univariate analysis: drug‐resistant epilepsy, autoimmune diseases, behavioral changes, cognitive symptoms, muscle stiffness, speech problems, autonomic symptoms, and MRI hyperintensities of the mesial temporal lobe (Table 1 and Fig 2). The multivariate model included the following factors: autoimmune diseases, behavioral changes, cognitive symptoms, speech problems, autonomic symptoms, and MRI hyperintensities of the mesial temporal lobe (see Table 1). The internally validated C statistic was 0.95. To correct for optimism, penalized modeling was performed showing similar odds ratios for all factors; all remained significant and independent risk factors (data not shown).

| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) a | p a | Adjusted Odds Ratio (95% CI) a | p a | |
| Drug‐resistant epilepsy | 7.1 (2.1–24.5) | 0.002 | — | — |
| Autoimmune diseases | 8.2 (3.2–20.7) | <0.0001 | 13.3 (3.1–56.6) | 0.0005 |
| Behavioral changes | 11.0 (4.1–29.3) | <0.0001 | 12.6 (3.2–49.9) | 0.0003 |
| Cognitive symptoms | 31.8 (4.4–229.9) | 0.001 | 30.6 (2.4–382.7) | 0.009 |
| Muscle stiffness | 7.3 (1.9–28.7) | 0.004 | — | — |
| Speech problems | 12.8 (4.1–40.0) | <0.0001 | 9.6 (2.0–46.7) | 0.005 |
| Autonomic symptoms | 22.1 (6.4–75.5) | <0.0001 | 23.3 (3.8–143.3) | 0.001 |
| Temporal MRI hyperintensities | 16.9 (4.6–62.1) | <0.0001 | 255.3 (19.6–3,332.2) | <0.0001 |
The table only shows the data of patients with autoimmune etiology of seizures (n = 20), and with no evidence for autoimmunity (n = 550).
a Numbers shown are from the cohort data after imputation.
CI = confidence interval; MRI = magnetic resonance imaging.
The external validation cohort consisted of 128 temporal lobe epilepsy patients, of whom 7 had neuronal antibodies (5.5%, compared to 3.4% in the Dutch cohort, p = 0.30). Data of the Dutch and Czech cohort are compared in Table S2, and results of the Czech antibody‐positive patients are shown in Table S3. The C statistic of our model for the external validation cohort was 0.92, showing that the (overall) discriminative performance is similar in the validation cohort.
The 6 independent risk factors were used to create the ACES score, each factor assigning 1 point. Evaluating different cutoff values for the ACES score (Fig 3) in the original cohort, 19.4% (95% CI = 11.4–29.6) of the patients with a score ≥ 2 had antibodies (PPV), whereas none of the patients with < 2 risk factors had antibodies, resulting in an NPV of 100% (95% CI = 81.4–100). Sensitivity of an ACES score of ≥ 2 was 100% (95% CI = 81.4–100), and specificity was 84.9% (95% CI = 67.9–100; Table 2). No patient with an ACES score ≥ 2, but without AES (n = 83), was identified by commercial tests, outside the planned screening algorithm (see Fig 3B). In addition, we reviewed data of 55 Dutch anti‐NMDAR, 43 anti‐LGI1, 19 anti‐GAD65, and 14 anti‐CASPR2 patients with seizures. All of them, except 1 patient with anti‐LGI1 (ACES score = 1), had an ACES score of 2 or more. Using the same cutoff for the ACES score, 37 patients in the Czech validation cohort had an ACES score ≥ 2 (29%). All patients with AES were identified (see Fig 3C). 20

![Autoimmune etiology of seizures (AES) patients as distributed by ACES score. The number of AES patients by ACES score (A) are provided for both the original, Dutch cohort (B) and the Czech validation cohort (C). Only the data of the patients with antibodies targeting extracellular antigens, anti–glutamic acid decarboxylase 65 (GAD65), and no evidence for autoimmunity are shown. The numbers in the bar diagrams correspond with the numbers of patients of each specific group. All patients with an ACES score of ≥2 without AES were tested by commercial cell‐based assay and enzyme‐linked immunosorbent assay post hoc, and all were negative. MRI = magnetic resonance imaging. [Color figure can be viewed at www.annalsofneurology.org]](/dataresources/secured/content-1766029909547-59b46bf8-d6bb-45e3-98b0-1f9876e10b8c/assets/ANA-89-698-g003.jpg)
Autoimmune etiology of seizures (AES) patients as distributed by ACES score. The number of AES patients by ACES score (A) are provided for both the original, Dutch cohort (B) and the Czech validation cohort (C). Only the data of the patients with antibodies targeting extracellular antigens, anti–glutamic acid decarboxylase 65 (GAD65), and no evidence for autoimmunity are shown. The numbers in the bar diagrams correspond with the numbers of patients of each specific group. All patients with an ACES score of ≥2 without AES were tested by commercial cell‐based assay and enzyme‐linked immunosorbent assay post hoc, and all were negative. MRI = magnetic resonance imaging. [Color figure can be viewed at www.annalsofneurology.org]

| Cutoff a | n (%) b | PPV (95% CI) | NPV (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| ≥1 point | 305 (53%) | 6.5 (2.8–13.1) | 100 (81.4–100) | 100 (81.4–100) | 48.2 (36.5–63.6) |
| ≥2 point | 103 (18%) | 19.4 (12.2–29.7) | 100 (81.4–100) | 100 (81.4–100) | 84.9 (67.9–100) |
| ≥3 point | 22 (4%) | 54.5 (41.4–70.5) | 98.5 (80.5–100) | 60 (45.8–77.2) | 98.2 (80.5–100) |
| ≥4 point | 1 (0.2%) | 100 (81.4–100) | 96.7 (78.7–100) | 5 (1.6–11.7) | 100 (81.4–100) |
a The six factors include: autoimmune diseases, behavioral changes, cognitive symptoms, speech problems, autonomic symptoms, temporal magnetic resonance imaging hyperintensities.
b N = 570.
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Results from the sensitivity analysis (allocating the 12 patients with probable or possible AES [3 and 9 patients, respectively] to the non‐AES group) showed comparable odds ratios (a sensitivity of 100% and specificity of 84.7%).
Comparing the ACES score (of both cohorts) to the prior published APE/APE2 score, 8 , 21 showed that the APE/APE2 score detected 8 of 10 patients with antibodies against extracellular proteins, and 7 of 17 patients with anti‐GAD65. All scores performed well in patients with a short history of epilepsy, but the ACES score scored better in patients with chronic epilepsy (p = 0.0015; Fig 4).

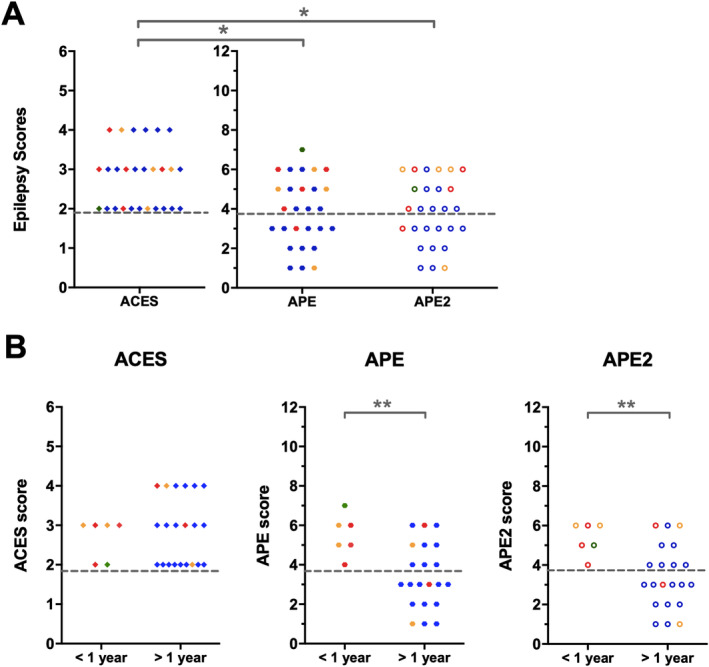
Comparison of the ACES score with the Antibody Prevalence in Epilepsy and Encephalopathy (APE) and APE2 score. (A) Visualization of the epilepsy scores per patient, combining the Dutch and Czech cohorts, shows that all patients are identified by the ACES score, whereas a considerable percentage is not identified by the APE or APE2 score. (B) Dissection by duration of epilepsy shows that the difference in performance is caused by the patients with epilepsy for >1 year. * p < 0.05, **p < 0.01. The colors refer to the antibodies identified: red, contactin‐associated protein‐like 2; orange, leucine‐rich glioma‐inactivated 1; green, N‐methyl‐D‐aspartate receptor; blue, glutamic acid decarboxylase 65.
Clinical Characteristics of the Dutch Cohort
Looking very carefully, additional subtle signs or symptoms of encephalitis/encephalopathy were present at inclusion in 19 of 20 patients with AES. All patients with anti‐LGI1 had subtle characteristic signs, including cognitive symptoms, insomnia, hyponatremia, and behavioral changes, and 2 of 3 patients had faciobrachial dystonic seizures (FBDS). 22 Two of 3 patients with anti‐CASPR2 had other core signs or symptoms, 23 albeit mild, including cognitive symptoms, peripheral nerve hyperexcitability, insomnia, autonomic symptoms, and cerebellar symptoms, and 1 patient developed neuropathic pain after inclusion. In both the anti‐LGI1 and anti‐CASPR2 patients, some signs were identified in retrospect (insomnia, FBDS, peripheral nerve hyperexcitability, cerebellar symptoms), whereas others were observed but remained unrecognized as AES‐related (because of their subtleness, and concomitant start of ASM). During follow‐up, 1 patient with focal epilepsy with sporadically occurring tonic–clonic seizures and recently diagnosed esophagus carcinoma developed hallucinations, refractory epilepsy, and psychosis. Anti‐NMDAR was detected in his CSF 11 months after inclusion, whereas his serum was negative at baseline, but also at 11 months after inclusion.
All 13 women with anti‐GAD65 had a serum concentration > 10,000 IU/ml (median serum concentration = 466,000 IU/ml, median CSF concentration = 4,600IU/ml). Five anti‐GAD65 patients had diabetes mellitus type 1 (38%). All patients had focal onset seizures, most frequently with sensory onset (déjà vu episodes; 9/13). Interestingly, in 4 patients, temporal seizures were clearly provoked by music. Oligoclonal bands unique to the CSF were tested in 8 patients, of whom 5 tested positive.
In the “probable autoimmune” group, 1 patient developed frequent seizures. Shortly afterward, he suffered from abdominal symptoms and weight loss, which turned out to be Crohn's disease. He then continued to deteriorate, with progressive cognitive symptoms. He was treated with steroids and adalimumab, leading to (slow) cognitive improvement and cessation of seizures. He met the “seronegative AIE” criteria. 14 The 2 patients with anti‐GlyR were a middle‐aged male with drug‐resistant epilepsy and a young woman with sporadic seizures without other signs.
In the “possible autoimmune” group, all 3 women with a similar “LGI1‐like” pattern on IHC had daily drug‐resistant focal onset seizures. In 2 of these 3 women, ASMs were withdrawn because of ineffectiveness. The 9 patients with neuropil staining on IHC had variable clinical phenotypes. All clinical characteristics of the patients with definite, probable, and possible AES are shown in Tables S4 and S5.
In 550 patients, there was no evidence for autoimmunity at last follow‐up. Of these 550 patients, 96% were diagnosed as focal epilepsy with unknown etiology, whereas in the other patients ancillary testing led to another diagnosis, including a symptomatic lesion on revised or new MRI (n = 13), generalized epilepsy (n = 3), dementia and seizures (n = 2), and no epilepsy (n = 2).
Immunotherapy Responses
All 6 patients with anti‐LGI1 and anti‐CASPR2 encephalitis were treated with immunotherapy (Fig 5A, B) and became seizure‐free (1 anti‐LGI1 encephalitis patient became seizure‐free at 13 months, just outside the study period), as did the anti‐NMDAR encephalitis patient. Ten of the 13 anti‐GAD65 patients were treated with immunotherapy; 5 of them had a remarkable decrease in their seizure frequency (see Fig 5C; 50–95%), deviating from their seizure frequency the year before, whereas in the other 5 patients no seizure frequency reduction was observed (see Fig 5D). Three patients refused immunotherapy (reasons: less severe disease [n = 2] or fear of side effects [n = 1]).

![Individual treatment responses of patients with anti–leucine‐rich glioma‐inactivated 1 (LGI1), anti–contactin‐associated protein‐like 2 (CASPR2), and anti–glutamic acid decarboxylase 65 (GAD65). The figures visualize the seizure frequency over time per antibody: (A) anti‐LGI1, (B) anti‐CASPR2, (C) anti‐GAD65 with treatment response, (D) anti‐GAD65 without treatment response (both black), or untreated (gray). The Roman numerals correspond to the numbers shown in Table S4. All patients were treated with antiseizure medications (ASM). The figure only shows ASM changes that led to a decrease in seizure frequency. AED = antiepileptic drug; IVIg = intravenous immunoglobulin; −T12 = 12 months before inclusion, T0 = inclusion date, T1 = 1 month after inclusion, T4 = 4 months after inclusion, T8 = 8 months after inclusion, T12 = 12 months after inclusion. [Color figure can be viewed at www.annalsofneurology.org]](/dataresources/secured/content-1766029909547-59b46bf8-d6bb-45e3-98b0-1f9876e10b8c/assets/ANA-89-698-g005.jpg)
Individual treatment responses of patients with anti–leucine‐rich glioma‐inactivated 1 (LGI1), anti–contactin‐associated protein‐like 2 (CASPR2), and anti–glutamic acid decarboxylase 65 (GAD65). The figures visualize the seizure frequency over time per antibody: (A) anti‐LGI1, (B) anti‐CASPR2, (C) anti‐GAD65 with treatment response, (D) anti‐GAD65 without treatment response (both black), or untreated (gray). The Roman numerals correspond to the numbers shown in Table S4. All patients were treated with antiseizure medications (ASM). The figure only shows ASM changes that led to a decrease in seizure frequency. AED = antiepileptic drug; IVIg = intravenous immunoglobulin; −T12 = 12 months before inclusion, T0 = inclusion date, T1 = 1 month after inclusion, T4 = 4 months after inclusion, T8 = 8 months after inclusion, T12 = 12 months after inclusion. [Color figure can be viewed at www.annalsofneurology.org]
Discussion
In this prospective, multicenter cohort study, we show that a small, but relevant, proportion of patients with focal epilepsy of presumed unknown etiology have neuronal antibodies. All patients with AES had unrecognized signs of encephalitis. Identifying these patients is crucial, because seizures respond better to immunotherapy than to ASM, which is reflected by seizure freedom in all patients with antibodies against extracellular proteins after immunotherapy. For recognition of AES patients, we have provided and validated a simple clinical score that helps physicians in selecting patients who should be screened for neuronal antibodies.
In the current study, neuronal antibodies were found in 3.4% of patients, whereas other studies describe higher numbers of >10%. 7 , 8 The most important issue explaining this discrepancy is the difference in patient selection. The patients included in our study were referred to epilepsy centers by neurologists who had no suspicion of AIE. In retrospect, most of the patients with AES had subtle signs or symptoms of AIE, but these signs were unrecognized as being related to AIE. For example, insomnia, muscle stiffness, or mild cognitive problems were often considered to be side effects of ASM. In addition, patients were only included in the AES group when substantial evidence for autoimmunity for the detected antibodies was available. In a smaller prospective cohort of patients with temporal lobe epilepsy, the same antibodies were identified as in the ACES study, 20 whereas others found a greater variability of antibodies. 7 , 8 Some of the described antibodies are pathogenic, like anti‐NMDAR, and anti‐GABABR, but occurred in highly selected patients with clear encephalitis. However, experiments to confirm antibody results were lacking in some studies, although it is an essential necessity when screening large cohorts, to avoid false positive results. Prior studies also describe antibodies with a debatable role in neuroinflammation, including low‐concentration anti‐GAD65, and double negative anti‐VGKC, nowadays considered clinically irrelevant by most. 10 , 11
The Czech study 20 was used to externally validate the results. This study was chosen because it was of a considerable size, investigated chronic epilepsy as well (median duration even longer), and did not included patients referred for overt encephalitis. In contrast to our study, all patients had temporal epilepsy, and they more often had mesiotemporal sclerosis. Otherwise, the epidemiological and clinical characteristics were largely similar. Reassuringly, the antibody frequency and type of antibodies (anti‐LGI1, anti‐CASPR2, anti‐GAD65) were within the same range.
We found anti‐LGI1, anti‐CASPR2, and anti‐GAD65. This is a comprehensible observation, because the clinical phenotypes of patients with these syndromes can be more prolonged and less aggressive, and diagnosis is more likely to be delayed or missed than in patients with clear encephalitis. 1 , 12 , 22 Although clinical phenotypes related to these antibodies can be less serious, immunotherapy is superior to ASM, 5 , 17 which makes diagnosis in an early phase important. Thirteen women had high anti‐GAD65 concentrations. Earlier performed laboratory studies were unable to reveal the pathogenicity of anti‐GAD65. 23 However, patients with high concentrations have overlapping and well‐defined neurological syndromes, and comparable seizure characteristics, not observed in low‐concentration patients. 17 Almost 75% of the high‐concentration anti‐GAD65 women had déjà vu episodes. In addition, one‐quarter had musicogenic epilepsy, meaning temporal seizures clearly triggered by specific music or songs, whereas the prevalence of musicogenic epilepsy in other non‐AES patients is very low. Musicogenic epilepsy has been described before in anti‐GAD65 encephalopathy. 24 Concerning immunotherapy responses, in contrast to the patients with anti‐LGI1 or anti‐CASPR2, no patient became seizure‐free. However, in one‐half of the treated patients, a long‐term epilepsy frequency reduction of up to 95% was observed, although these patients had been refractory to ASMs for a significant time. This makes regression to the mean or natural history as the explanation for the epilepsy frequency reduction highly unlikely. In support, seizure response was accompanied by a serological response.
The finding that only anti‐LGI1, anti‐CASPR2, and anti‐GAD65 occurred suggests that screening for these antibodies only is generally sufficient in patients with focal epilepsy of unknown etiology without clear encephalitis. If anti‐LGI1 is detected in serum and the phenotype fits, diagnosis can be made, and treatment can be started. 12 If serum is positive for anti‐CASPR2, CSF should be tested as well. 22 If the anti‐GAD65 ELISA concentration is >10,000IU/mL, this can be considered clinically relevant. 17 This is roughly comparable to an RIA titer of 20nmol/L 8 or 2,000U/mL. 24 In case of deterioration of symptoms or suggestive signs or symptoms, antibody testing should be expanded, taking into account the clinical phenotype.
We identified 6 independent risk factors pointing toward AES. The assessed risk factors were predefined before the study started. The ACES score was created assigning all factors 1 point instead of creating a value‐weighted score. The easier the score, the more likely it is to be used in clinical settings. In addition, as our score is meant to identify those who need antibody testing, our primary aim was to create a sensitive score. Although a weighted score would provide better model performance, it would not increase the sensitivity (already optimal if at least 2 factors of the ACES score were present). Lastly, some of the odds ratios showed large confidence intervals due to the low frequency in the control group of the original cohort. By not assigning a weighted value, we lower the risk of overfitting. Using a cutoff value of 2 for the ACES score, all antibody‐positive patients were identified, but antibody testing was unrevealing in 14%. It should therefore not be used to diagnose AES, but to guide selection of patients for antibody screening.
The factors included in the ACES score partially overlap with those used in the APE/APE2 score. 8 , 21 Nevertheless, there are important differences, which makes sense as, judging by the published papers of Dubey et al, the scores serve different populations. Our study included patients with focal epilepsy, all without overt encephalitis. In addition, our ACES score was validated in another chronic epilepsy cohort, and showed very good discrimination. In this study, we confirm that the APE/APE2 score is useful to detect patients in a (sub)acute setting with antibodies against extracellular neuronal antigens. However, almost 70% of chronic patients would have been missed using this score, underlining the importance of a score that is applicable to patients with variable disease courses.
Some patients were classified as “probable or possible autoimmune.” Data for these patients were not used in the main analysis, but inclusion would not have changed the results. Two patients categorized as “probable autoimmune” had anti‐GlyR. Clinical characteristics of these 2 patients showed no notable similarities. Testing serum of these patients consistently showed a positive result on live CBA, but we were unable to analyze CSF or to confirm our findings with additional techniques. Except for these 2 epilepsy samples, we have not identified any positive sample in 1,206 patients outside published clinical phenotypes. 19 , 25 Although anti‐GlyR can be directly pathogenic, 18 we prefer to be careful in describing unconfirmed findings. The “possible autoimmune” category contained 3 patients with similar clinical characteristics and drug‐resistant epilepsy. Both serum and CSF showed an unknown pattern on IHC. We are currently analyzing these samples using immunoprecipitation and mass spectrometry. In 6 other patients, IHC showed a diffuse neuropil staining However, live hippocampal neuron staining was negative, CSF was not available for confirmation, and patients had different clinical phenotypes, all questioning the clinical relevance.
Our study has limitations. Due to the small number of antibody‐positive patients, there is a risk of overfitting. Therefore, we also assessed a penalized model. In addition, external validation in a second, independent, foreign cohort showed comparable test characteristics. Concerning data extraction, this was performed thoroughly; however, it is possible that recall bias occurred, especially in the variables that are not discussed in the standard intake. An example is “family history of autoimmune diseases.” We therefore decided to exclude this variable from the analysis. Another limitation was the use of only serum in most patients. Although highly sensitive to test for anti‐LGI1, 12 anti‐CASPR2, 22 anti‐GAD65, and anti‐GABABR, 26 it is somewhat less sensitive to screen for anti‐NMDAR 27 or anti‐AMPAR. 28 Testing only serum poses the risk of missing a small proportion of patients. However, both anti‐NMDAR and anti‐AMPAR encephalitis tend to present more fulminantly, 2 and the syndromes would probably have revealed themselves during follow‐up, as exemplified by the only patient who was antibody negative (in serum) at inclusion, but who developed a panencephalitis 11 months after inclusion with anti‐NMDAR in CSF. In retrospect, it is unclear, because of the lack of CSF at inclusion, whether this patient had anti‐NMDAR, causing his seizures before deterioration, or if he coincidentally developed anti‐NMDAR encephalitis later on. The presence of esophageal carcinoma in this case underlines the importance of screening for tumors in these patients. 29 Samples were screened with immunohistochemistry, and questionable and positive samples were tested more thoroughly (CBAs, ELISA, live hippocampal neurons). This approach was chosen because of the high sensitivity of IHC. 12 , 17 , 22 , 26 , 27 , 28 , 30 , 31 Although we cannot guarantee that we have identified all patients, previous publications support our claim that this approach seems most reliable and sensitive to test large cohorts of patients with low prior chances, as also enforced by testing 186 samples negative by IHC with additional CBA and ELISA without revealing additional antibodies.
To conclude, patients with AES present differently and have specific, although not always discriminative characteristics. Recognition of these patients can be difficult, but is important for treatment decisions. An ACES score ≥ 2 can be used to identify patients with higher risk of having AES, and to select those who require antibody testing.
Author Contributions
M.A.A.M.d.B., R.D.T., M.J.M.M., and M.J.T. contributed to the conception and design of the study; all authors contributed to acquisition and analysis of data; M.A.A.M.d.B., A.E.M.B., H.M., R.D.T., P.A.E.S.S., and M.J.T. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Acknowledgments
This work was supported by the Dutch Epilepsy Foundation, project numbers 14‐19 and 19‐08. It is generated within the European Reference Network for Rare Immunodeficiency, Autoinflammation, and Autoimmune Diseases. H.M., and M. Elisak and P. Marusic of the ACES Study Group, were supported by the Charles University project GA UK No 746120.
We thank all patients participating in this study; the epileptologists from Kempenhaeghe and Stichting Epilepsie Instellingen Nederland for their cooperation; and A. Doets for her help with R.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
 Antibodies Contributing to Focal Epilepsy Signs and Symptoms Score
Antibodies Contributing to Focal Epilepsy Signs and Symptoms Score
