
- Altmetric
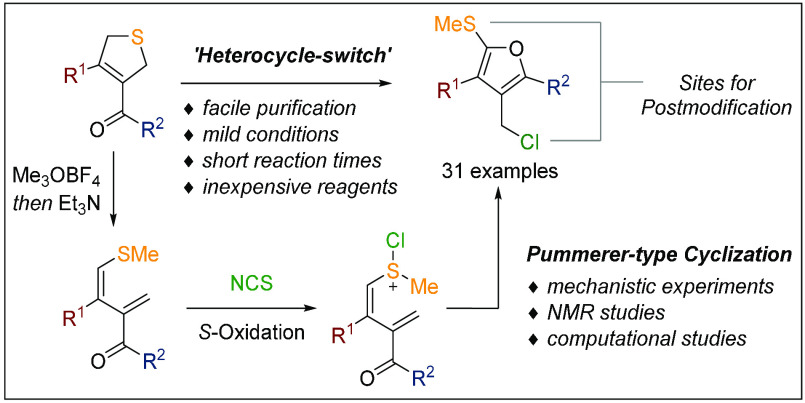
Despite the many methods available for the synthesis of furans, few methods remain that allow for the custom-made assembly of fully substituted furans. Here we report a powerful protocol to rapidly construct tetrasubstituted, orthogonally functionalized furans under mild reaction conditions. The developed method involves the regioselective ring-opening of readily available 2,5-dihydrothiophenes followed by an oxidative cyclization to provide the heterocycle. The selective oxidation at sulfur is promoted by N-chlorosuccinimide as an inexpensive reagent and proceeds at ambient temperature in high yield within 30 min. The obtained furans serve as exceptionally versatile intermediates and were shown to participate in a series of valuable postmodifications. The fate of the initial sulfonium intermediate was investigated by mechanistic experiments, and computational studies revealed the existence of an unprecedented Pummerer-type rearrangement. The potential for organic synthesis is highlighted by the total synthesis of bisabolene sesquiterpenoids (pleurotins A, B, and D).
Introduction
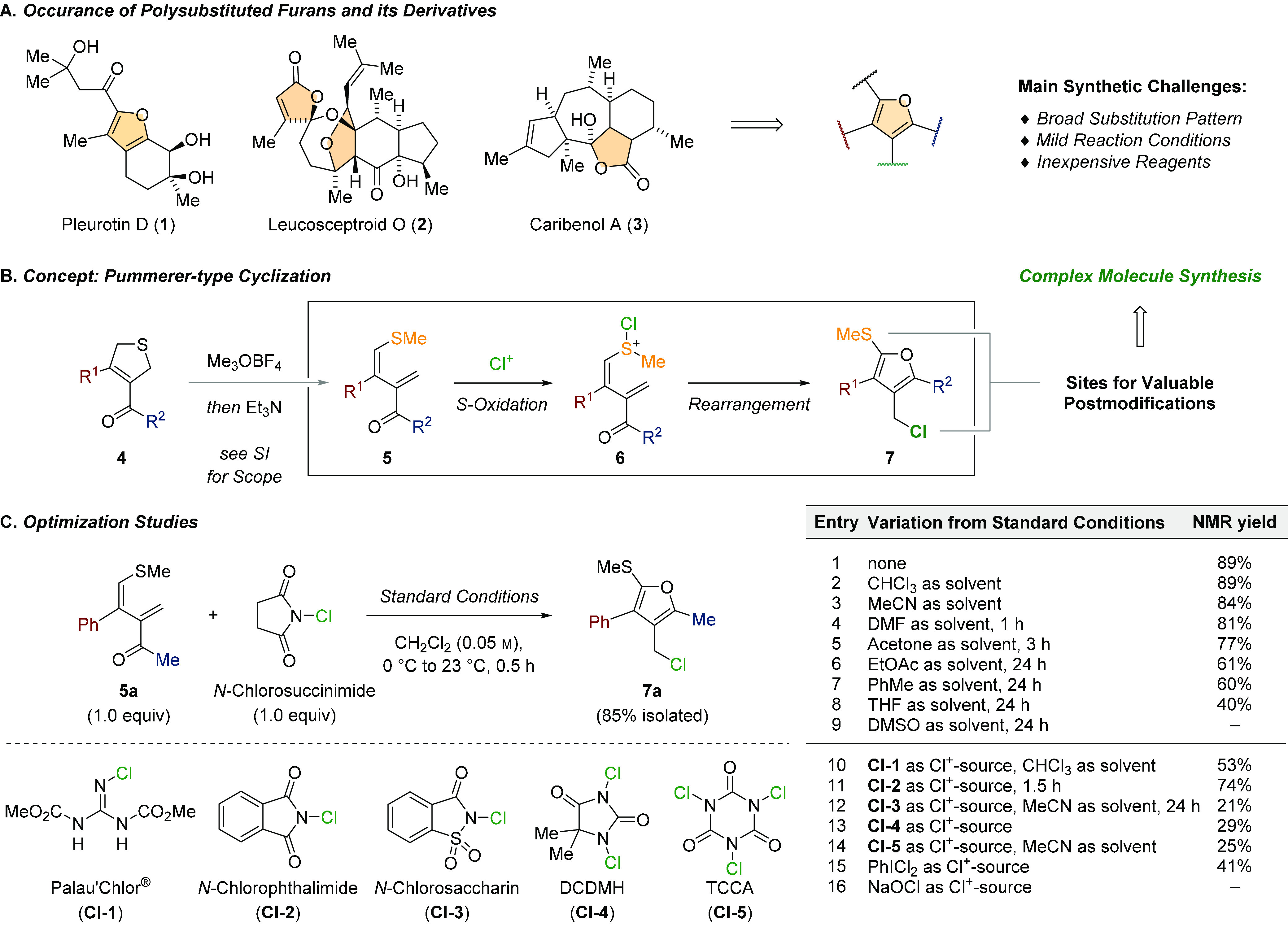
The custom-made synthesis of structurally diverse five-membered heterocycles represents a fundamental process of research and development in medicinal chemistry, crop science, and natural product synthesis.1 Despite the numerous synthetic methods available today, the synthesis of highly substituted and electron-rich furans has remained challenging for reasons that relate to their tendency to undergo hydrolysis to give, for instance, 1,4-dicarbonyls or to suffer from oxidation reactions (Scheme 1A).2 Therefore, fully intact furans as found in pleurotin D (1)3 are underrepresented in natural products and pharmaceuticals. Otherwise, furans are highly valuable functional units that allow for the late-stage unmasking of delicate carbonyl functions as demonstrated in numerous elegant natural product syntheses.4 For example, the butenolide motifs of leucosceptroid O (2)5 and caribenol A (3)6 were revealed via the late-stage oxidation of an advanced furan intermediate. The broad range of synthesis applications of these heterocycles requires highly modular approaches that allow for selective modification of the entire substitution pattern (12 regioisomers in total).7 While condensation chemistry has dominated classical furan syntheses in the past,2 modern synthesis strategies are primarily based on the cyclization of linear precursors employing transition-metal catalysts.8 We now demonstrate the simple and rapid conversion of readily available 2,5-dihydrothiophenes 4 into tetrasubstituted furans 7 at ambient temperatures under metal-free reaction conditions (Scheme 1B). The key transformation is promoted by selective S-chlorination of 1,3-diene 5 to generate oxidized intermediate 6 and spontaneous Pummerer-type rearrangement to release furan 7. The obtained furans were shown to be exceptionally valuable substrates for a series of selective postfunctionalizations and enabled synthesis access to the family of pleurotin natural products.

Assembly of Tetrasubstituted Furans via a Pummerer-Type Strategy
Results and Discussion
Reaction Development
During our recent studies on the [3 + 2]-cycloaddition of thiocarbonyl ylides with electron-deficient alkynes,9 we found that the exposure of 2,5-dihydrothiophene 4a (R1 = Ph, R2 = Me) to Meerwein’s salt (Me3OBF4) effected selective S-methylation and subsequent addition of triethylamine induced immediate ring-opening. Obtained 1,3-diene 5a turned out to be surprisingly stable and did not suffer from decomposition or dimerization even at elevated temperatures.10 A screen of different reaction conditions revealed that treating 5a with equimolar amounts of N-chlorosuccinimide (NCS) at 0 °C in dichloromethane (0.05 m) induced clean conversion to furan 7a within 30 min (Scheme 1C). Simple purification via filtration through a plug of Florisil afforded 7a in 85% yield (silica gel: 77%). A solvent screen demonstrated the robustness of this transformation providing 7a in high yields in chloroform (89%), acetonitrile (84%), N,N-dimethylformamide (81%, 1 h) and acetone (77%, 3 h). Furan 7a was also obtained by employing ethyl acetate, toluene, or tetrahydrofuran as the solvent, but despite extended reaction times, no full consumption of diene 5a was observed (40–61%). Dimethyl sulfoxide was not compatible with the oxidant, and no product was formed.11 Related chlorinating reagents such as Palau’Chlor (Cl-1 in chloroform, 56%)12 and N-chlorophthalimide (Cl-2, 74%) also initiated furan formation; however, 7a was consistently obtained in slightly lower yields. A significant decrease in yield became apparent when applying N-chlorosaccharin (Cl-3 in acetonitrile, 21%), 1,3-dichloro-5,5-dimethylhydantoin (Cl-4, 29%), trichloro-cyanuric acid (Cl-5 in acetonitrile, 25%), or iodobenzene dichloride (PhICl2, 41%). The use of sodium hypochlorite did not lead to any product formation in either dichloromethane or acetonitrile as the solvent.
Synthesis Scope
At the outset, a panel of highly functionalized 1,3-dienes was synthesized (34 examples, see Supporting Information Section 3 for details) and subjected to the optimized reaction conditions (Scheme 2). By varying the aromatic moiety in the 3-position (R1, highlighted in red), furans 7a–e were obtained in high yields (77–88%). The modular synthesis of 1,3-dienes also enabled rapid access to substrates that carry alkynes as well as aliphatic residues attached to the 3-position (7f–h, 53–90%). Because of the tendency of the diene precursor to undergo dimerization,10 a lower yield (51%) was obtained for trisubstituted furan 7i. Next, we investigated the variation of the ketone residue (R2, highlighted in blue). To our delight, aryl- and heteroaryl-derived ketones allowed for the isolation of 7j and 7k, respectively. The presence of a cyclopropane (7l) was also tolerated; however, decomposition of the furan occurred upon exposure to Florisil (36% yield). Therefore, the crude reaction mixture was diluted with pentane to induce the precipitation of succinimide. Subsequent filtration provided analytically pure furan 7l in almost quantitative yield (95%). Similar behavior was observed for styrene derivatives 7m–p (53–99%) during the purification process. Finally, the standard reactions conditions were used to access alkyne 7q (74%) and benzyl-protected furfuryl alcohol 7r (69%).

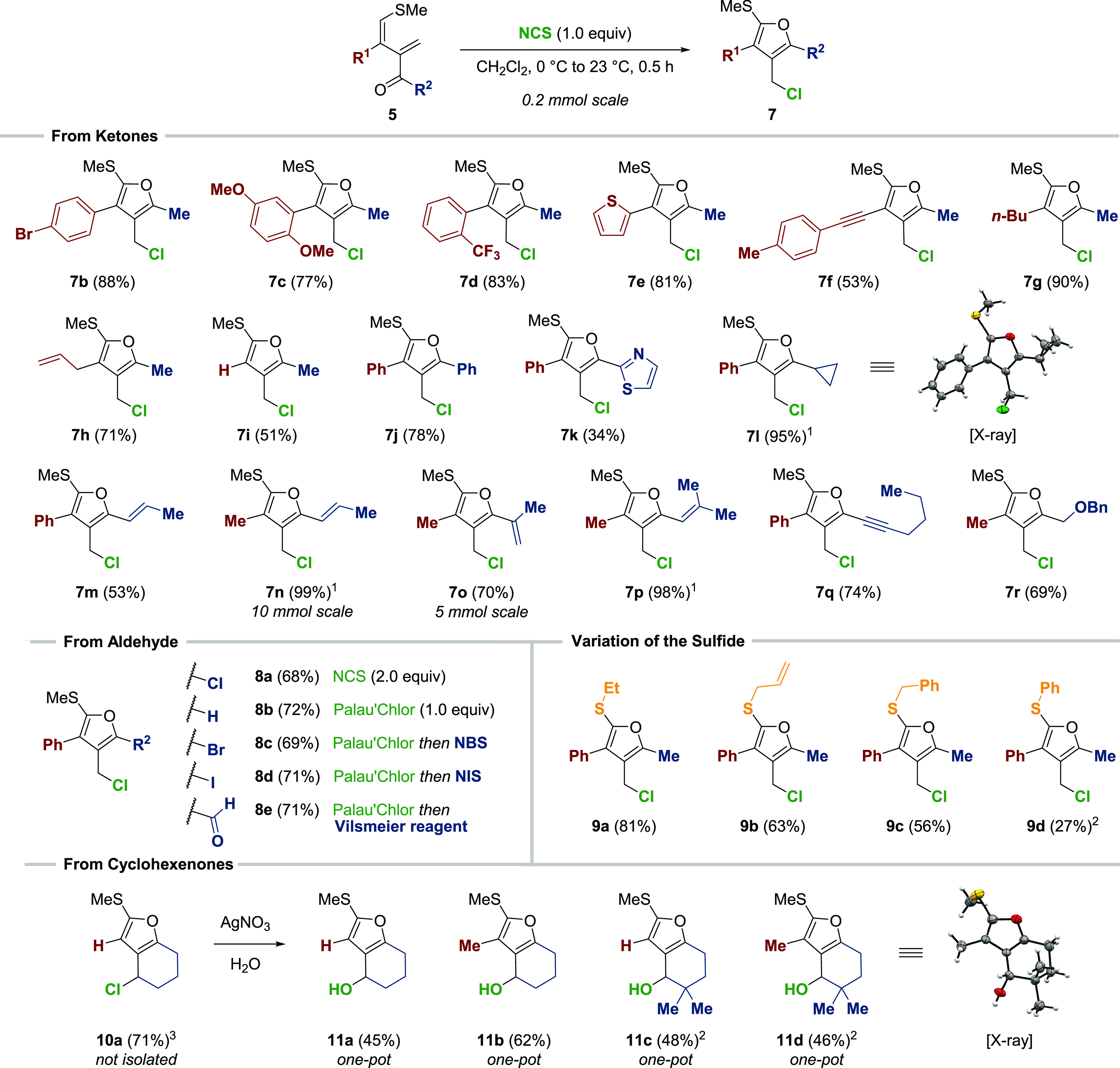
Scope of Tetrasubstituted Furans
Standard conditions: substrate (0.2 mmol), NCS (1.0 equiv), CH2Cl2 (0.05 m), 0–23 °C, 0.5 h. See Supporting Information Section 4 for experimental and substrate-specific details. 1Trituration with pentane followed by the removal of succinimide via filtration. 2Palau’Chlor (Cl-1) used as the Cl+-source. 3Yield determined by adding nitromethane as an internal standard to the crude reaction mixture.
By subjecting an aldehyde to our standard reaction conditions, a mixture of unreacted diene and 5-chlorofuran 8a was obtained. This observation revealed the high reactivity of furan 8b to undergo electrophilic aromatic substitution with remaining NCS. Consequently, the use of 2 equiv of chlorination reagent allowed for the rapid, clean formation of 8a (68%). It is noteworthy that the use of Palau’Chlor (Cl-1, 1.0 equiv) at a lower temperature (0 °C, 1 h) enabled the selective synthesis of trisubstituted furan 8b. On the basis of these results, the syntheses of bromide 8c (N-bromosuccinimide, 69%), iodide 8d (N-iodosuccinimide, 71%), and aldehyde 8e (Vilsmeier reagent, 71%) were realized by simply telescoping the reaction. Weinreb amides and (thio)esters (R2 = N(OMe)Me, SC12H25, OMe) were reluctant to form furans, and only complex product mixtures were observed. The exchange of the S-methyl unit for S-ethyl, S-benzyl, or S-allyl substituents led to furans 9a–c in good yields (56–81%). For the electron-poor S-phenyl derivative, only the use of Palau’Chlor instead of NCS enabled the formation of furan 9d (27%). We concluded our studies of furan formation by using dienes derived from cyclohexenones as substrates: while the clean formation of furan 10a was observed in the crude reaction mixture (71% NMR yield), purification led to instantaneous hydrolysis to give alcohol 11a. To simplify the purification process, silver(I) nitrate-mediated hydrolysis of the chlorides was performed after complete formation of the furan to afford 11a–d in synthetically useful yields (45–62%). For sterically more hindered substrates possessing a gem-dimethyl group (11c–d), only Palau’Chlor was effective at promoting furan formation.
Postmodifications
In our previous work on tetrahydro- and dihydrothiophene syntheses, we often relied on high-pressure conditions for the key [3 + 2]-cycloaddition steps.9 To make the current chemistry more accessible, we generated the substrates for the furan synthesis from triflate 12, which was synthesized in one step from commercially available starting material (Scheme 3A).13 The robustness and scalability of the furan formation was demonstrated by the synthesis of more than 1.5 g of furan 7a in a single run. Remarkably, the standard reaction conditions once more enabled quick separation of the desired product from succinimide (13) by simple trituration followed by filtration. Recovered 13 allows for recycling by conversion to NCS.14 With significant quantities of 7a in hand, we investigated its synthesis potential for the modification of both the benzylic chloride (4-position) and the methyl sulfide (2-position) to realize complete control of the furan substitution pattern (Scheme 3B). We first explored the ability of highly reactive chloride 7a to undergo C–C bond formation via nucleophilic displacement reactions. Furans 14a–c were formed in excellent yields (81–99%) employing copper catalysis. Exposure to sodium cyanide provided 14d (53%) and reductive displacement of the chloride with Superhydride gave 14e in nearly quantitative yield. In addition, malonate 14f was accessible in 71% yield. The formation of C–O bonds was accomplished by the addition of cesium acetate to deliver furan 14g (78%) or by oxidation under Kornblum conditions (DMSO, NaHCO3) to give aldehyde 14h (45%). The iron(III)-catalyzed cross-coupling of 7a with bis(pinacolato)diboron provided boronic ester 14i (33%),15 and the introduction of nitrogen was realized by the synthesis of azide 14j (95%). In addition, Wittig salt 14k was readily accessible in 87% yield upon exposure of 7a to triphenylphosphine, and furan 7j was smoothly converted to sulfone 14l in the presence of sodium p-toluenesulfinate (75%). To our surprise, 7a underwent an unprecedented palladium-catalyzed [4 + 4]-dimerization reaction (Pd(OAc)2, rac-BINAP) to release eight-membered carbocycle 15 whose structure was ultimately proven by single-crystal X-ray analysis.

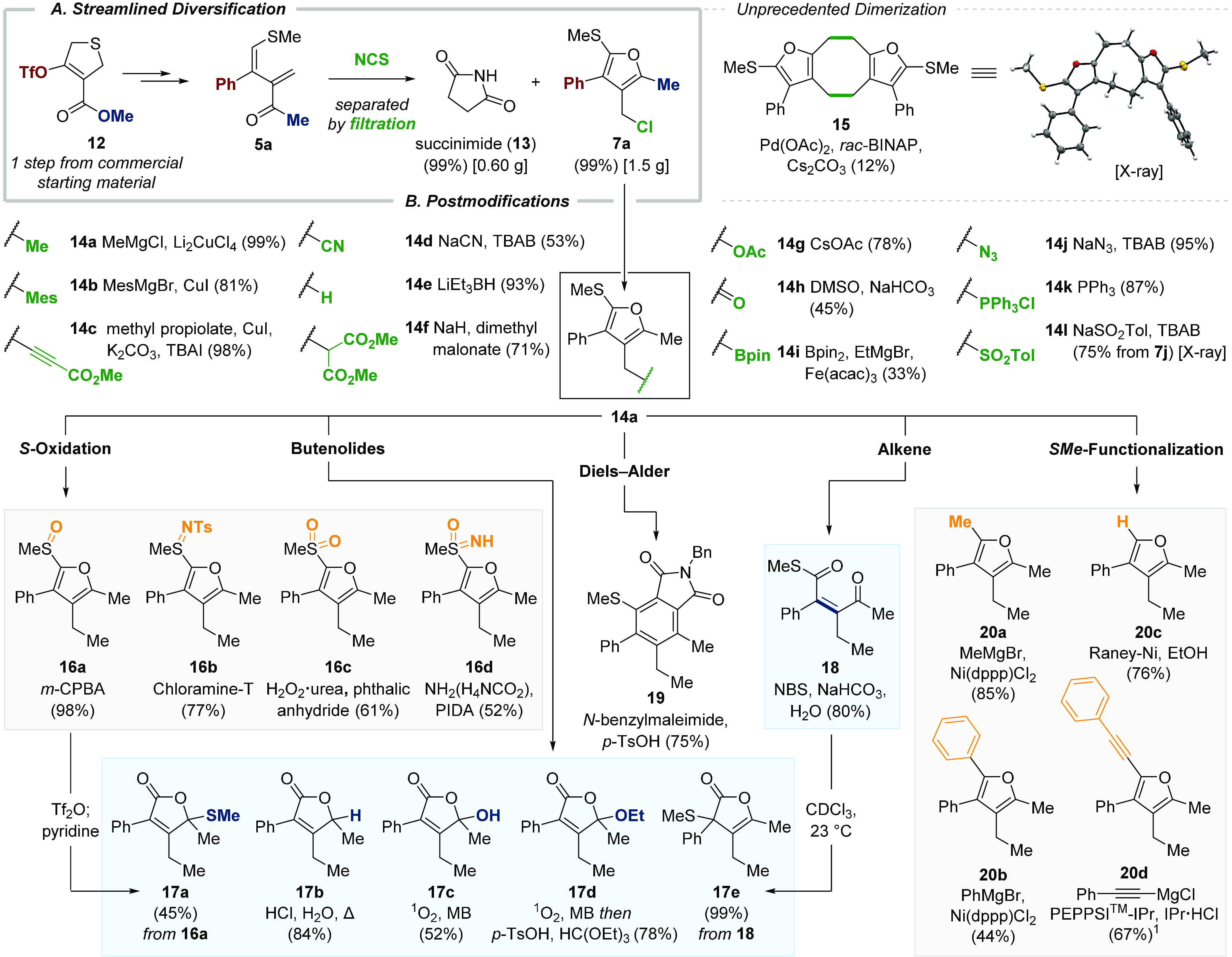
Postmodifications
See Supporting Information Section 6 for experimental details. 1Obtained after two cycles.
Having established the synthetic utility of the chloride by a broad range of substitution reactions, we turned our attention to the 2-position of furan 14a. Selective oxidation of the sulfide to sulfoxide 16a, keeping the electron-rich furan untouched, was realized in nearly quantitative yield (m-CPBA, 98%). Corresponding sulfilimine 16b (chloramine-T, 77%),16 sulfone 16c (H2O2·urea, 61%),17 and sulfoximine 16d (ammonium carbamate, PIDA, 52%)18 were accessible in good to moderate yields. Treating sulfoxide 16a with triflic anhydride initiated the conversion of the furan to butenolide 17a in 45% yield. The hydrolysis of 14a under acidic conditions (HCl (aq.), t-BuOH, reflux) gave 17b, and oxidation with singlet oxygen in ethanol led to the rapid formation of γ-hydroxybutenolide 17c and γ-ethoxybutenolide 17d (52–84%).19 When treating 14a with aqueous NBS, the desired tetrasubstituted alkene, 18, was formed. Upon standing in CDCl3 at 23 °C, slow rearrangement (4 days) triggered by initial lactone formation and the subsequent 1,2-alkylthio shift delivered α-butenolide 17e.20 As expected, 14a smoothly participated in Diels–Alder cycloaddition with N-benzylmaleimide accompanied by acid-promoted aromatization to give thioanisole 19 (75%).21 To conclude, we investigated the utility of heteroaryl sulfide when participating in cross-coupling reactions.22 The use of Ni(dppp)Cl2 (20 mol %) enabled successful cross-coupling with Grignard reagents, providing furans 20a (MeMgBr, 85%) and 20b (PhMgBr, 44%).23 In addition, the sulfide was also exploited as a traceless auxiliary group, and reductive desulfurization with Raney nickel provided trisubstituted furan 20c (76%).24 Finally, a palladium-NHC-catalyzed cross-coupling reaction (PEPPSI-IPr) gave alkynyl-substituted furan 20d in 67% yield.25
Total Synthesis of Pleurotin Natural Products
The presence of a tetrasubstituted furan as the core structure of bisabolene sesquiterpenoid pleurotins A–D (2, 26–28)3 revealed furan 7n as an ideal starting material (2.5 g prepared in a single batch, Scheme 4). Allylation of the chloride utilizing 2-methallylmagnesium chloride (21) followed by S-oxidation (m-CPBA, NaHCO3) furnished sulfoxide 22 in 70% overall yield. Sulfoxide–magnesium exchange employing ethylmagnesium bromide (EtMgBr) followed by exposure to chloride 23 gave 24 (64%).26 Ring-closing metathesis of 24 (4 mol % second generation Grubbs catalyst, 40 °C) forged the cyclohexene motif of 25 in 74% yield.

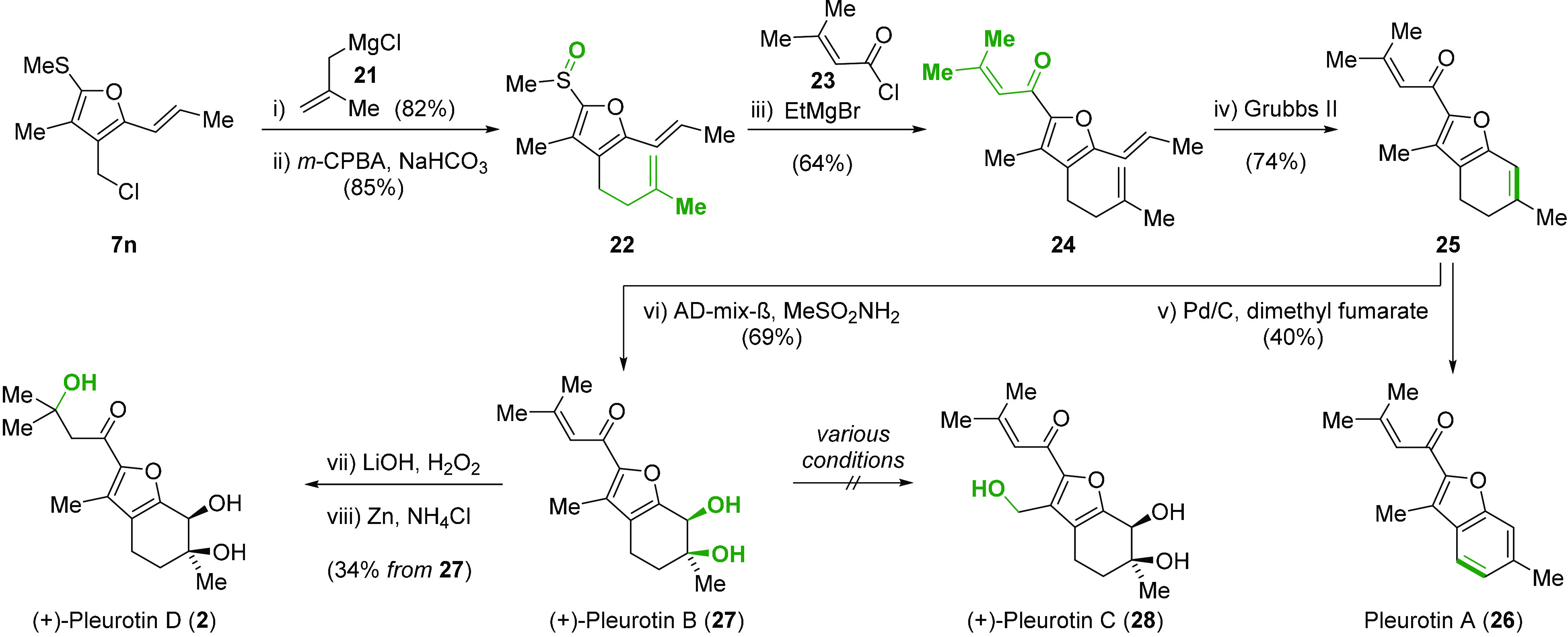
Total Synthesis of Pleurotins A, B, and D
Having secured ample quantities (200 mg) of common precursor 25, pleurotin A (26) was prepared by dehydrogenation of the benzylic position employing Pd/C (40%). The presence of dimethyl fumarate as a hydrogen scavenger prevented unwanted reduction of the enone.27 On the other hand, Sharpless dihydroxylation of 25 provided (+) pleurotin B (27) in 69% yield but only 32% ee.28 It is noteworthy that several attempts to accomplish the selective C–H oxidation of 27 to access pleurotin C (28) were unsuccessful. Finally, pleurotin D (2) was obtained from pleurotin B (27) in two steps involving epoxidation under Scheffer–Weitz conditions (LiOH, H2O2), followed by reductive ring-opening of the oxirane (Zn, NH4Cl).
Mechanistic Investigations
The high selectivity and efficiency of the oxidative cyclization attracted our attention to the study of the detailed mechanism of this transformation by combining experimental and quantum chemical methods (Scheme 5). By changing the solvent from dichloromethane to water, sulfoxide 29 was obtained in 85% yield from 1,3-diene 5a.29 Upon exposure to oxalyl chloride, 29 underwent rapid rearrangement to deliver furan 7a (77%). In addition, trans-1,3-diene 30 was successfully converted to 7a by employing the standard conditions (61% NMR yield). Notably, the use of alternative oxidant N-fluorobenzenesulfonimide (NFSI) or [bis(trifluoroacetoxy)iodo]benzene (PIFA) in ethanol allowed for the direct incorporation of nitrogen and oxygen as shown for furans 31 and 32.30 However, when diene 33 was subjected to the standard conditions, a classical Pummerer rearrangement31 to give 34 (73%) was observed.

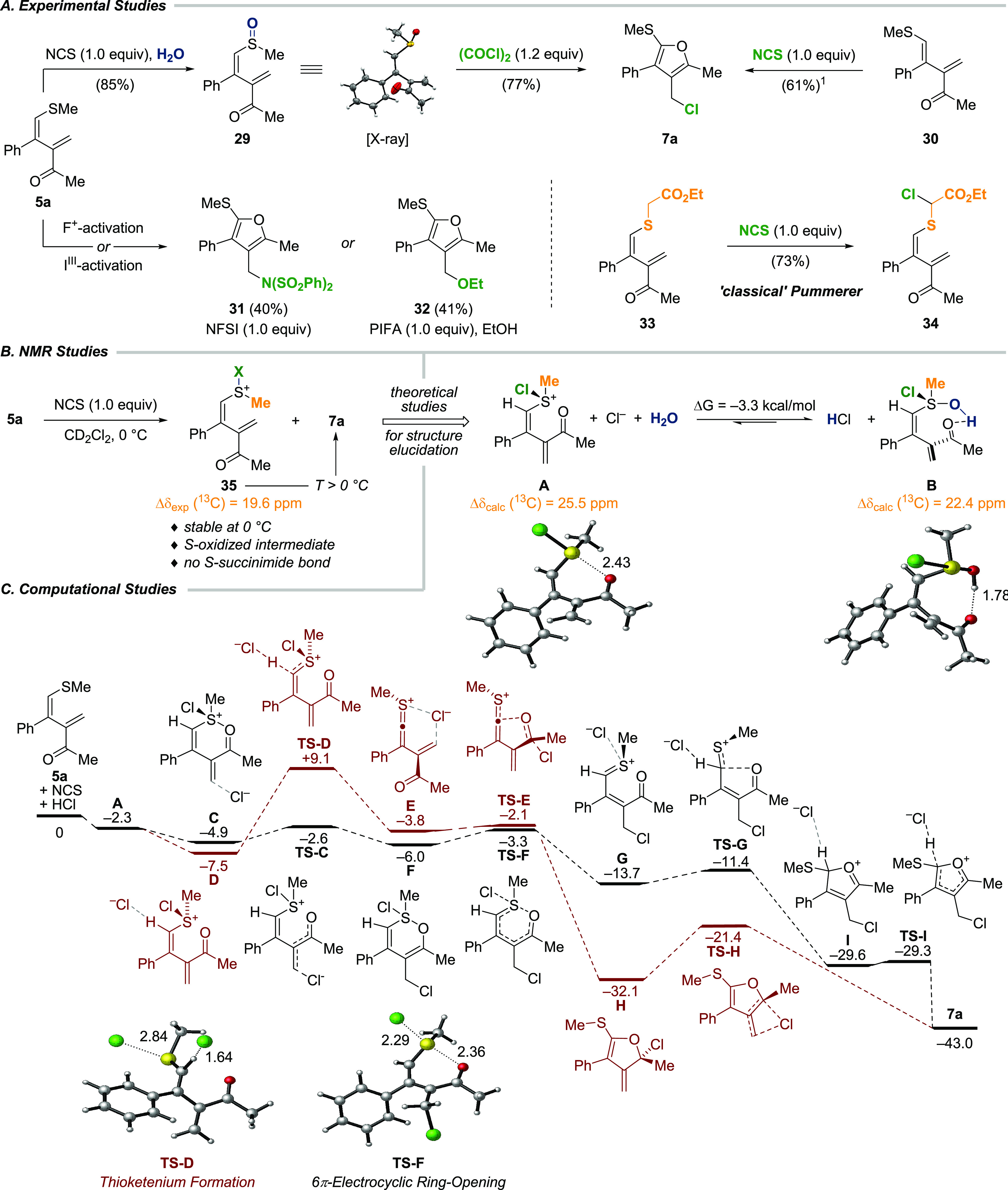
Mechanistic Investigations
Proposed reaction mechanism as calculated with B3LYP-D3(BJ)/6-311++G(2d,2p) in dichloromethane treated as an implicit solvent (details in Supporting Information Section 9.1). Relative Gibbs free energies at 298 K are given in kcal/mol, whereas the energy of 5a plus N-chlorosuccinimide and hydrochloride is arbitrarily set to zero. The additive Pummerer pathway is highlighted in black, and the elimination pathway is highlighted in red. Hydrochloride that is regenerated when forming TS-D and TS-I is omitted for clarity. 1Yield determined by adding nitromethane as an internal standard to the crude reaction mixture.
In addition, NMR monitoring of the reaction of 5a at 0 °C revealed the formation of intermediate 35 which rapidly converted to furan 7a upon warming to 23 °C (Scheme 5B, for NMR spectra see Supporting Information Section 8.2). Comparison of the 13C NMR shift of the S-methyl group in 35 and 5a (Δδexp = 19.6 ppm) further supported the hypothesis of an initial S-oxidation. The use of 15N-labled NCS excluded the formation of an intermediate in which succinimide is covalently bound to sulfur as suggested for related systems (no 1H–15N HMBC coupling observed),32 and calculation confirmed this reaction to be endergonic (Supporting Information Section 9.2). Moreover, the addition of potassium hydroxide to 35 led to a mixture of sulfoxide 29 and furan 7a (ratio = 1.0:1.2). Since 29 readily undergoes furan formation upon exposure to hydrochloric acid, we speculated about the existence of potential intermediates A and B. The formation of tetravalent sulfur intermediate B is also in agreement with seminal work by Pummerer.31a Indeed, quantum chemical calculation confirmed a stabilization of B compared to A by ΔG = −3.3 kcal/mol. The comparison of experimental and calculated 13C NMR shifts provided further support for the formation of B (Δδexp = 19.6 ppm and Δδcalc = 22.4 ppm compared to Δδcalc = 25.5 ppm for A, see Supporting Information Section 9.2).
The equilibrium between A and B is considered to be slow at 0 °C; otherwise, B would not be detectable on the NMR time scale. The possibility of B being the reactive species that releases chloride was excluded due to a thermodynamically unfavorable reaction pathway (Supporting Information Figure S9e). According to these results, 5a is first oxidized by NCS to the sulfonium chloride A (ΔG = −2.3 kcal/mol).33 The presence of acid turned out to be crucial since no reaction was observed under basic conditions (K2CO3), which was further confirmed by calculations in which a reaction Gibbs free energy of ΔG = +23.9 kcal/mol in the absence of HCl was found (Supporting Information Section 9.2). From there, key intermediate A can enter either an additive Pummerer pathway (Michael-type addition of chloride, highlighted in black) or a classical Pummerer elimination pathway (highlighted in red). According to the Curtin–Hammett principle, the Michael addition (TS-C) is strongly favored by ΔΔG⧧ = −11.7 kcal/mol. Hence, intermediate C is formed (ΔG = −4.9 kcal/mol), where a second chloride is already in loose interaction with the Michael acceptor. Consequently, a 1,4-addition of chloride leads to F, which undergoes 6π electrocyclic ring-opening (ΔG⧧ = 2.7 kcal/mol, TS-F). The obtained thionium intermediate G, is energetically strongly favored (ΔG = −13.7 kcal/mol) and resembles the reactive species generated from additive Pummerer reaction pathways.34 All attempts to find a transition state for a direct chloride displacement connecting intermediates C and G were unsuccessful and identified F as part of the reaction pathway. Ring closure in G (ΔG⧧ = 2.3 kcal/mol, TS-G) furnishes I, and furan 7a is formed under the deprotonation and regeneration of HCl. The low reaction barriers of this pathway are consistent with the rapid conversion of 1,3-diene 5a to furan 7a at ambient temperature. In the Pummerer elimination pathway (depicted in red), intermediate D is formed, where a second chloride is in loose contact with the vinylic proton (ΔG = −7.5 kcal/mol). Deprotonation by chloride proceeds via an activation barrier of ΔG⧧ = 16.6 kcal/mol (TS-D) to deliver unprecedented thioketenium complex E (ΔG = −3.8 kcal/mol). Consecutive rotation around the central σ-bond closes dihydrofuran H (ΔG⧧ = 1.7 kcal/mol, TS-E), and a 1,3-chloride shift (ΔG⧧ = 10.7 kcal/mol, TS-H) then furnishes furan 7a.
It is noteworthy that direct ring closure of E to 7a is also feasible, requiring a similar activation energy (ΔG⧧ = 1.7 kcal/mol). However, because of the high reaction barrier to form E, this pathway can be excluded. Similarly, mechanisms involving vinylogous chlorination or intramolecular 1,5-chloride transfer from 5a to form an intermediate strongly resembling G can also be ruled out (Supporting Information Section 9.2).
Conclusions
The developed protocol enables the rapid assembly of a variety of tetrasubstituted furans under mild conditions. The required 2,5-dihydrothiophenes are readily available and amenable to broad diversification. The inherent orthogonal substitution pattern allows for the selective functionalization along the periphery of the heterocyclic core structure. The Pummerer-type rearrangement represents a powerful alternative to conventional furan syntheses based on condensation chemistry or transition-metal catalysis. The potential of this method was showcased by a series of postmodifications that culminated in the total synthesis of pleurotins A, B, and D. The underlying mechanism of the rearrangement was studied in detail by combining experimental and DFT investigations considering various pathways. The efficiency and selectivity observed under these mild reaction conditions are in agreement with the low energy barriers obtained for the additive Pummerer pathway. An extension of this methodology to other heterocycles is underway in our laboratories and will be reported in due course.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c12194.
Experimental details and spectroscopic data (PDF)
X-ray crystallographic data for 5b (Supporting Information Table 2), 7l, 11d, 14l, 15, and 29 (CIF)
Author Contributions
§ F.-L.H. and C.H. contributed equally.
Notes
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the Austrian Science Fund FWF (P31023-NBL to T.M. and M2005 to M.P.), the Center for Molecular Biosciences CMBI, and the Tyrolean Science Fund TWF (UNI-0404/2340 to F.-L.H. and F.16642/5-2019 to L.A.W). We are grateful to Prof. Christoph Kreutz, Prof. Thomas Müller, and Dr. Christina Meisenbichler (University of Innsbruck) for help with NMR and HRMS studies. The computational results presented here have been achieved using the LEO HPC infrastructure at the University of Innsbruck.
References
Starting material methyl 4-hydroxy-2,5-dihydrothiophene-3-carboxylate was purchased from Aaron Chemicals or prepared via the reaction of methyl 2-mercaptoacetate with methyl acrylate on the decagram scale.
 Rapid
Assembly of Tetrasubstituted Furans via Pummerer-Type
Rearrangement
Rapid
Assembly of Tetrasubstituted Furans via Pummerer-Type
Rearrangement