
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Dissemination of carbapenem-resistant Klebsiella pneumoniae poses a threat to the successful treatment of bacterial diseases and increases the need for new antibacterial agents development. The objective of this study was to determine the antimicrobial activity of carvacrol against multidrug-resistant K. pneumoniae. Carbapenemase production was detected by MALDI-TOF. The PCR and sequencing showed that the blaKPC-2, blaOXA-48, blaNDM-1, blaCTX-M-8 genes were present in carbapenem-resistant K. pneumoniae strains. The polymyxin-resistant K. pneumoniae strain exhibited alterations in mgrB gene. The antimicrobial activity of carvacrol was evaluated in vitro using broth microdilution and time-kill methods. For this, carbapenem-resistant K. pneumoniae and polymyxin-resistant strains, were evaluated. The in vitro results showed that carvacrol had antimicrobial activity against all isolates evaluated. The survival curves showed that carvacrol eradicated all of the bacterial cells within 4 h. The antimicrobial effect of carvacrol in vivo was determined using a mouse model of infection with Klebsiella pneumoniae carbapenemase (KPC). The treatment with carvacrol was associated with increased survival, and significantly reduced bacterial load in peritoneal lavage. In addition, groups treated with carvacrol, had a significant reduction in the total numbers of white cell and significantly increased of platelets when compared to the untreated group. In vivo and in vitro studies showed that carvacrol regimens exhibited significant antimicrobial activity against KPC-producing K. pneumoniae, making it an interesting candidate for development of alternative treatments.
Introduction
Multidrug-resistant (MDR) infections are considered a major public health problem [1, 2]. The emergence of MDR bacteria and the lack of new antibiotics is a worrying prospect [3]. A recent report suggests that failing to control drug-resistant infections may cause an excess of 10 million deaths per year and cost up to US$100 trillion by 2050 [4]. Carbapenem-resistant Klebsiella pneumoniae strains are frequent cause of healthcare-associated infections and hospital-associated outbreaks [5]. Carbapenem resistance in these pathogens is one of the main causes of morbidity and mortality, and represents a serious health problem worldwide, since it limits therapeutic options for treating infections [1, 6]. Thus, the global spread of MDR has resulted in increased use of polymyxin with the inevitable risk of emerging resistance [7].
According to the World Health Organization (WHO), the control of the spread of antibiotic resistance remain a priority, as well as, development of new therapies against these bacteria [8]. Therefore, the increase in antibiotic-resistant bacteria has revived interest in the study of plant materials as sources of new compounds as alternative therapeutic agents to control pathogenic microorganisms [9, 10]. A major group of plant antimicrobial compounds is represented by essential oils, which consist of complex mixtures of volatile secondary metabolites [11]. Therefore, bioactive compounds extracted from essential oils are promising antimicrobials [12].
The phenolic monoterpene carvacrol [2-Methyl-5-(1-methylethyl) phenol, isomeric with thymol] is a essential component of the essential oils of plants of the Labiatae family, including Origanum and Thymus and has emerged for its wide spectrum of activity [10]. Some studies have reported their pharmacological activities, such as anti-inflammatory effects, antioxidant, antitumor, analgesic, anti-hepatotoxic, insecticidal and antimicrobial properties [9, 13–17]. This study evaluated the antimicrobial potential of carvacrol in vitro and in vivo against multidrug-resistant K. pneumoniae strains.
Material and methods
Chemicals
Carvacrol (2-methyl-5-[1-methylethyl] phenol); lot: W224502, purity ≥ 98%) were purchased from Sigma (St. Louis, USA). Tween 80 (0.5%) was used as the solvent for the carvacrol.
Bacterial strains
Multidrug-resistant K. pneumoniae strains were obtained from urine culture of hospitalized patients in a tertiary teaching hospital. Bacteria were grown overnight in Mueller Hinton (MH) broth and submitted to phenotypic and molecular assay as previously described [18, 19]. This study was conducted with the approval of the Research Ethics Committee from the Universidade Federal da Grande Dourados (no. 877.292/2014 and 4.014.325/2020). This ethics protocol contemplates informed consent from the patients from whom the strains were isolated.
Bacterial identification and phenotypic assays
Bacterial species were identified using the Phoenix 100® automated system (BD Diagnostic Systems) and confirmed by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF) using the Microflex LT spectrometer (Bruker Daltonics, Massachusetts, USA). The minimal inhibitory concentrations (MICs) were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) standards [20]. Preliminary screening for the presence of carbapenemases was performed by the modified Hodge test (MHT) according to CLSI guidelines. Positive results were confirmed by ertapenem hydrolysis using mass spectrometry [21].
PCR amplification of resistance genes
The presence of resistance genes (blaTEM-like, blaSHV-like, blaCTX-M-1-like, blaCTX–M-2-like, blaCTX-M-8-like, blaCTX-M-14-like, blaGES-like, blaKPC-like, blaSME-like, blaNDM-1-like, blaIMP-like, blaSPM-like, blaVIM-like, blaSIM-like, blaGIM-like, blaOXA-48-like, mgrB and mcr-1) was evaluated using Polymerase Chain Reaction (PCR) amplification, followed by sequencing as previously described [18, 19].
Antibacterial activity of carvacrol
Multidrug-resistant K. pneumoniae strain bacteria were grown overnight in 3 mL Mueller–Hinton (MH) broth at 37°C with constant shaking at 200 rpm. Optical density was measured at 600 nm on the following day, and the cultures were then diluted to ∼5.0 × 105 CFU/mL in a low-binding 96 well microtiter plate containing increasing concentrations of carvacrol (72–0.03 mg/mL). The microtiter plates were incubated at 37°C and the Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC) of carvacrol were determined as previously described [20, 22]. Polymyxin B (4 mg/L) and amikacin (16 mg/L) (Sigma-Aldrich) were used as controls for the assays with carbapenemase-producing and polymyxin-resistant K. pneumoniae strains, respectively. Polymyxin B and meropenem sensitive control (Escherichia coli 25922) was used as a control to validate antimicrobial susceptibility tests.
Time-kill test
The time-kill kinetics of the carvacrol at 1 × MBC was performed using the broth macrodilution (MH broth) technique following CLSI guidelines [20, 22]. Time-kill assays were performed using a final inoculum concentration of approximately 5.0 × 105 CFU/mL [23] incubated at 37°C. Samples were collected at 0, 4, 8, 12, 24 h and 100 μL of inoculum was spread out on to MacConkey agar plates. The plates were incubated for 24 h at 37°C and viable cell counts were performed by inspection of colony-forming units (CFUs) to determine the inhibitory effects of carvacrol. The values of the bacterial counts were transformed into CFU/mL and expressed in log to ensure normal data distribution [24]. To confirm the absence of antimicrobial activity of solvent, the negative (water, culture medium and 0.5% Tween 80) and positive (water, culture medium, 0.5% Tween 80 and bacterial suspension) controls were assessed.
Animals
Seventy-eight female Swiss mice (Mus musculus), 8–10 weeks old, weighing approximately 20–30 g (n = 6 in each group) were obtained from the Central Animal Facility of the Universidade Federal da Grande Dourados. Forty-two were used in the lethal dose test and thirty-six in the antibacterial activity assay of carvacrol in vivo. The mice were maintained in polypropylene boxes with beddings of wood shaving and provided with commercial feed (Nutival®) and filtered water ad libitum throughout the experiment. Light and temperature were controlled using a 12 h photoperiod (12:12 h DL) at 22 ± 2°C and 55 ± 10% humidity on a ventilated shelf (ALESCO®, Monte Mor, Brazil). All the animal care or handling out following the recommendations in the Guide National Council to Control Animal Experimentation (CONCEA). In this study, we assessment of survival, lethal dose, and longevity of infected animals, for that reason humane endpoints were not used, but all efforts were made to minimize suffering. The experiment was only maintained for 24 hours, and the behavior was monitored every hour. After evaluating the infection survival curve, animals that remained alive were euthanized after 24 hours of experimentation. The study was conducted with the approval of the Research Ethics Committee on Animal Use of the Universidade Federal da Grande Dourados (no. 010/2017). The institutional animal ethics committee reviewed and specifically approved the mortality predicted in the study design.
Lethal dose test
The lethal dose (LD100) and mean lethal dose (LD50) of KPC-producing K. pneumoniae in mice was assessed as previous described [25]. The mice were injected with a 0.1 mL intraperitoneal aliquot of the following concentrations of KPC-producing K. pneumoniae: 1.5 ×108, 3.0 ×108, 4.0 ×108, 4.8 ×108, 6.0 ×108 and 9.0 ×108 CFU/mL. The animals were observed for 24 h, the numbers of dead mice in each group were counted and the percentage mortality was calculated. The acute toxicity of carvacrol in mice has previously been described and concentration below of 250 mg/kg showed no mortality in mice [26].
In vivo antibacterial activity
To evaluate carvacrol’s in vivo activity, a murine infection model induced by KPC-producing K. pneumoniae (Fig 1) was performed, as previously described [25], with the following modifications. In brief, female Swiss mice were randomly divided into treatment groups (n = 6). All animals were injected with a 0.1 ml intraperitoneal (i.p.) aliquot of 4.0 × 108 CFU/mL (LD50). Six groups of 6 mice were treated with the following regimens: polymyxin B (2 mg/kg, intraperitoneal (i.p.), 12/12 h), carvacrol (50 mg/kg, oral gavage (o.g.), 8/8 h), carvacrol (25 mg/kg, o.g., 8/8 h), carvacrol (10 mg/kg, o.g., 8/8 h), infected control group (untreated) and a naïve group. Length of survival was observed in mice surviving at 24 h. All surviving animals were anesthetized with a combination of xylazine, and ketamine (10 and 60 mg/kg, i.p., respectively). Animals were euthanized by exsanguination and organs were collected for analysis. Blood samples were collected for hematological studies using an automated hematology analyzer (Sysmex XE-3000 Hematology Analyzer, Sysmex, Japan). The white cell count (WBC) and platelet abundance (PLT) were determined [27]. Peritoneal fluid samples were obtained through incision and lavage with Milli-Q water by an aseptic technique. Peritoneal lavage fluid was incubated on agar Mueller-Hinton medium supplemented with meropenem (4 mg/L) to verify the presence of KPC-producing K. pneumoniae and for quantitative cultures, respectively.

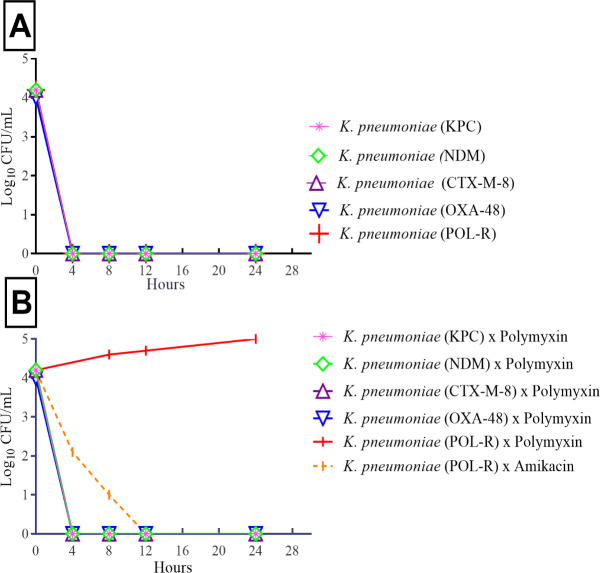
Time-kill curves of multidrug-resistant K. pneumoniae strains.
A) Carvacrol activity against carbapenem-resistant (KPC, NDM, CTX-M-8 and OXA-48) and polymyxin-resistant K. pneumoniae strains (POL-R). B) Carbapenem-resistant K. pneumoniae strains (KPC, NDM, CTX-M-8 and OXA-48) tested against polymyxin B and polymyxin-resistant K. pneumoniae strain (POL-R) tested against polymyxin B and amikacin.
After collecting blood, the organs (spleen, liver, lung and kidney) were collected, and weighed. The tissues were buffered formalin-fixed, embedded in paraffin and, sectioned at 5 μm. The sections were stained with hematoxylin and eosin and observed by light microscopy for histopathological evaluation.
Statistical analysis
Means ± the standard error of the mean (SEM) were calculated and ANOVA/Newman-Keuls post-hoc tests were performed using GraphPad Prism software (version 6.01; Graph-Pad Software Inc., San Diego, CA, USA). Results with P value < 0.05 was considered significant.
Results
Four clinical carbapenem-resistant K. pneumoniae strains, and one polymyxin-resistant were included in this study (biorepository accession numbers: KP01, KP02, KP03, KP04 and KP05). Strains showed resistance to the antibiotics tested by broth microdilution as follows: meropenem (MIC >32 mg/L), imipenem (MIC >32 mg/L), ertapenem (MIC >32 mg/L) (Table 1). Carbapenemase production was detected by MHT and MALDI-TOF. PCR amplification and sequencing showed that blaKPC-2, blaOXA-48, blaNDM-1, blaCTX-M-8 genes were present in carbapenem-resistance K. pneumoniae strains. The polymyxin-resistance K. pneumoniae exhibited alterations in the mgrB coding sequence. The other genes evaluated (blaTEM-like, blaSHV-like, blaCTX-M-1-like, blaCTX–M-2-like, blaCTX-M-14-like, blaGES-like, blaSME-like, blaIMP-like, blaSPM-like, blaVIM-like, blaSIM-like, blaGIM-like and mcr-1) were not detected in these strains.

| Strains | Genes | MIC (mg/L) | MIC and MBC (mg/L) | ||
|---|---|---|---|---|---|
| Carbap | Pol | Ami | Carv | ||
| KP01 | blaKPC-2 | >32 (R) | < 2 (S) | <8 (S) | 130 |
| KP02 | blaOXA-48 | >32 (R) | < 2 (S) | <8 (S) | 130 |
| KP03 | blaNDM-1 | >32 (R) | < 2 (S) | <8 (S) | 260 |
| KP04 | blaCTX-M-8 | >32 (R) | < 2 (S) | <8 (S) | 130 |
| KP05 | altered mgrB | >32 (R) | 8 (R) | <8 (S) | 130 |
S: susceptibility; R: resistance; Carbap: meropenem, imipenem and ertapenem; Pol: polymyxin B; Carv: carvacrol; Ami: amikacin.
Carvacrol exhibited significant inhibitory effects, with MICs/MBCs of 130 mg/L for CTX-M-8, OXA-48, KPC, and polymyxin-resistant K. pneumoniae strains. For NDM-1 producing K. pneumoniae, the MICs/MBCs were 260 mg/L. MIC and MBC values were equal in the strains evaluated. No inhibitory effects were observed in the positive control, with 0.5% of Tween 80. The survival curves of the strains among 0 and 4th hour suggest a linear drop in viable cell counts (Fig 1A). All strains treated with carvacrol showed decreases in cell counts of approximately two log10 CFU/mL. Considering the time of cell death, the results showed total inhibition of carbapenemases-producing K. pneumoniae strains after 4 h of treatment with carvacrol. Polymyxin B (4 mg/L) was used as a positive control and successfully inhibited the carbapenemase-producing strains within 4 h (Fig 1B). Amikacin (16 mg/L) was used as a positive control and successfully inhibited the polymyxin-resistant strain within 12 h.
KPC-producing K. pneumoniae strain was selected for the infection in animals. A dose-dependent survival curve was generated using female Swiss mice that received intraperitoneal injections of KPC-producing K. pneumoniae in different concentrations. To determine the LD100 and LD50 of KPC-producing K. pneumoniae, percent survival was observed for 24 h after infection (Fig 2). All animals in the control group (untreated) and in the groups infected with concentrations of 1.5 ×108 and 3.0 ×108 CFU/mL of KPC-producing K. pneumoniae, survived for 24 h. Concentrations of 4.0 ×108 and 4.8 ×108 CFU/mL promoted 50% (LD50) and 60% mortality, respectively. All animals infected with concentrations of 6.0 ×108 and 9.0 ×108 CFU/mL died in 24 h (LD100).

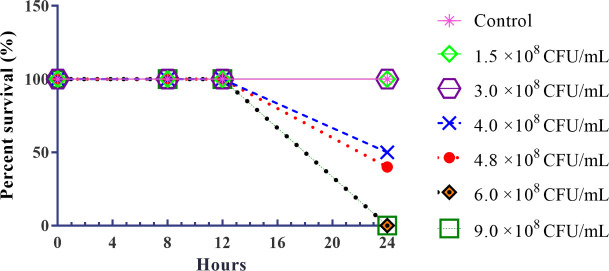
Survival curves mice model infected with different concentrations of KPC-producing K. pneumoniae.
Control: untreated group; concentrations of KPC-producing K. pneumoniae inoculum (1.5 ×108; 3.0 × 108; 4.0 × 108, 4.8 × 108, 6.0 × 108 and 9.0 × 108 CFU/mL).
The antimicrobial activity of carvacrol in vivo was evaluated for 24 h, using a model of infection with by KPC-producing K. pneumoniae. Half of the control group (untreated) died within 24 h after infection (50% mortality). However, all mice of the group treated with polymyxin B (2 mg/kg), and carvacrol (10, 25 and 50 mg/kg) remained alive (0% mortality) (Fig 3).

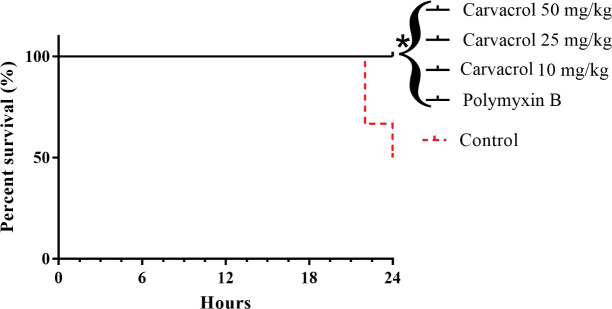
Survival curves of mice infected with KPC-producing K. pneumoniae and treated with carvacrol.
Polymyxin B and untreated (Control) were used as a positive and negative controls, respectively. *P<0.05 compared with the control group.
In order to characterize the immune response of mice infected and treated with carvacrol, white cell count (WBC) and platelet were determined. Statistical analysis revealed that the white series of cells demonstrated significant alterations. Groups treated with carvacrol (10, 25 and 50 mg/kg) and polymyxin B (2 mg/kg) showed significant reductions in the number of WBC (p < 0.01) (Fig 4A). All groups treated with carvacrol (10, 25 and 50 mg/kg) showed significantly increased platelet counts (p < 0.05), a result not observed in the group treated with polymyxin B (Fig 4B).

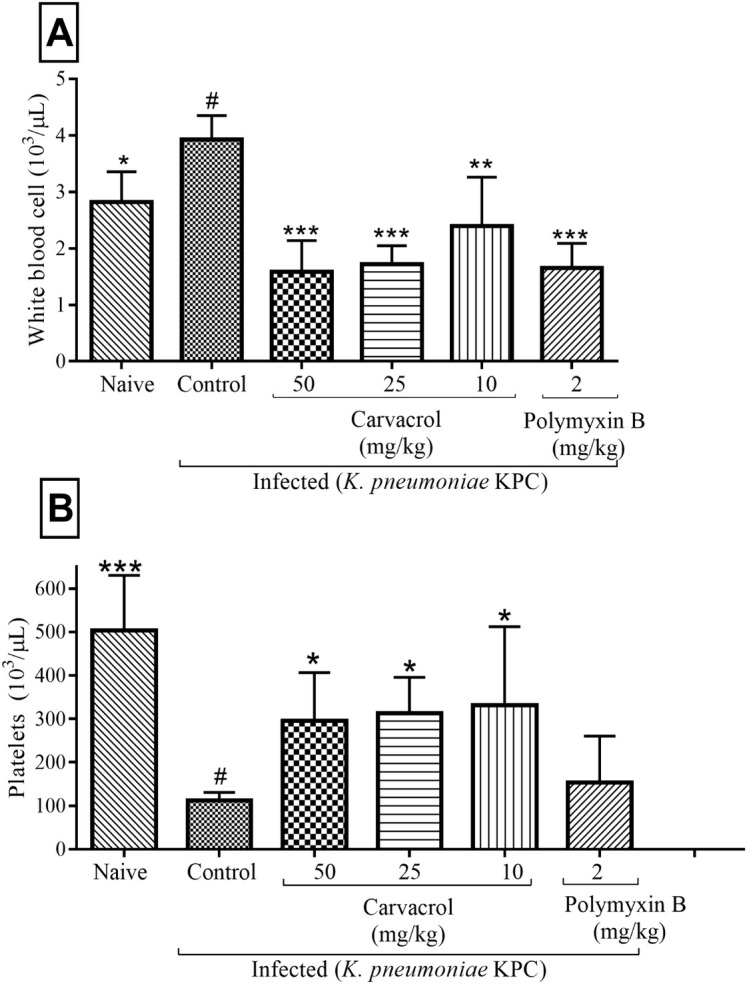
Effects of carvacrol on hematological parameters in mice infected with KPC-producing K. pneumoniae after 24 hours.
WBC (A) and platelets (B). ***P<0.001, **P<0.01 and *P<0.05 compared with the control group (#). Differences among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test.
The induction of sepsis was confirmed by bacterial culture of murine blood samples. This procedure demonstrated the presence of KPC-producing K pneumoniae strains in all infected mice (100%). To better characterize the difference observed for the mortality rates between the control and treated groups, we determined the number of CFUs in peritoneal lavage fluid. Bacteria was recovered from peritoneal lavage fluid of all animals, and the difference in the number of CFUs was significant for carvacrol (10, 25 and 50 mg/kg; p < 0.001) and polymyxin B (p < 0.001) (Fig 5). There was no significant difference in organ weight. Histological analysis showed no alterations in the organs.

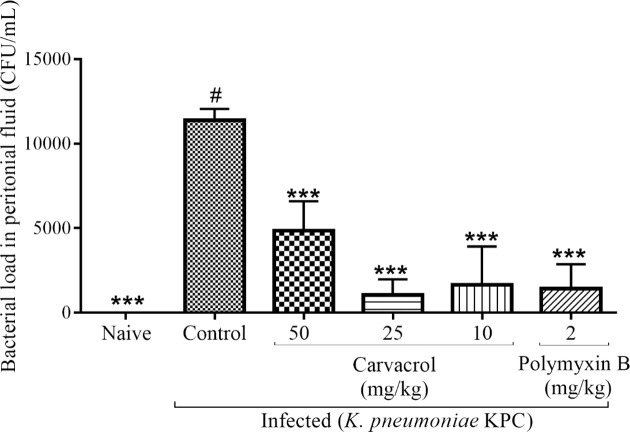
Effects of carvacrol on the number of CFUs in peritoneal lavage fluid from infected mice after 24 hours.
***P<0.001, **P<0.01 and *P<0.05 compared with the control group (#). Differences among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test.
Discussion
K. pneumoniae is one of the most common and clinically important pathogens, causing a wide spectrum of infectious diseases [28]. The emergence and rapid spread of KPC-producing K. pneumoniae continue to pose serious threats for the treatment of healthcare-associated infections, with high mortality, especially in immunocompromised patients and neonates [29, 30]. Carbapenem-resistant K. pneumoniae isolates are particularly difficult to treat due to multifactorial resistance mechanisms that limit therapeutic options [31]. In this study, the antimicrobial activity of carvacrol against multidrug-resistant K. pneumoniae strains was assessed. For this, the antimicrobial resistance mechanisms of carbapenem-resistant K. pneumoniae isolates were analyzed, and the strains were found to acquire resistance through genes encoding carbapenemases, including blaKPC-2, blaOXA-48, blaNDM-1, and blaCTX-M-8. The spread of carbapenemase-encoding genes among these pathogens is a cause of great concern, drastically compromising the therapeutic options available for treatment [19]. Thus, developing new therapies against these bacteria is a priority.
Carvacrol was investigated as a potential novel therapeutic agent and showed encouraging inhibitory effects against carbapenem and polymyxin-resistant K. pneumoniae strains. Carvacrol showed a low MIC and MBC value (ranging from 130 to 260 mg/L) for carbapenem and polymyxin-resistant K. pneumoniae strains. This is a promising result, as there are limited antibiotics available for treating MDR Gram-negative bacteria [32]. Carvacrol exhibited inhibitory effects (MIC = 130 mg/L) in vitro against KPC-producing K. pneumoniae, eradicating all bacterial cells, similar to polymyxin B and amikacin, both commercial antibiotics. Carvacrol is described as a potential antimicrobial agent against Gram-positive and Gram-negative bacteria [33]. However, to our knowledge, there is no description of the antimicrobial activity of carvacrol against MDR bacteria, as shown in our study.
The antimicrobial action of carvacrol and its time-kill curves provided evidence of its rapid action. The inhibitory effects of carvacrol could be attributed to the interactions between the structural and functional properties of the cytoplasmatic membrane, where carvacrol interacts with the lipid bilayer and aligns itself between fatty acid chains, leading to the expansion and destabilization of the cytoplasmic membrane [10, 13, 34]. Several mechanisms have been proposed to explain the antimicrobial activity of carvacrol against bacteria [10, 35–37]. However, further studies are required to elucidate the mechanisms of action and cell death caused by carvacrol in carbapenem-resistant K. pneumoniae.
In vitro results showed that carvacrol had similar antimicrobial activity against all isolates evaluated, indicated that the activity is not restricted to resistance genes or chromosomal polymyxin-resistant mechanisms. Thus, considering the similar results in vitro, antimicrobial activity of carvacrol in a murine model was evaluated with KPC-producing K. pneumoniae-induced infection. Treatment with carvacrol significantly increased the survival of infected mice compared to the control group (untreated). In addition, mice treated with carvacrol showed a significant decrease in the number of CFU in the collected peritoneal lavage, similar to the group treated with polymyxin B.
An elevated WBC count was observed in untreated mice (control group) compared to the naïve and treated groups, suggesting infectious or inflammatory processes. Moreover, carvacrol decreased the number of total leukocytes in the untreated group, similar to polymyxin B, indicating that carvacrol may be efficient in the treatment of infections since leukocytes are the first line of defense against invading pathogens. Several studies have used in vitro and in vivo assays to demonstrate that carvacrol exerts its anti-inflammatory properties by reducing the production of inflammatory mediators such as leucocytes, possibly through the induction of IL-10 release [17, 35, 36, 38, 39]. In addition, a significant increase was observed in the number of platelets in mice treated with carvacrol when compared to the untreated (control) group, suggesting that treatment with carvacrol decreased the severity of the infection. On the other hand, the group with polymyxin B did not show any differences in platelet numbers compared to the control group. This may be attributed to the fact that polymyxin B has no effect on platelet activation and can selectively inhibit platelet aggregation [40]. In the diagnosis of sepsis, the number of platelets is an important laboratory finding [41]. Platelets play a role in maintaining hemostasis, modulate innate and adaptive immune responses, and low platelet count is a marker for poor prognosis in septic patients [42]. Low platelet counts were correlated with an increased risk of infection in patients [43]. Thus, in our study, the increase in platelet count may be related to the reduced severity of the infection. The antiplatelet properties of carvacrol showed that carvacrol has a moderate antiplatelet effect, inhibiting platelet aggregation [44, 45].
In addition, carvacrol has been classified as a generally recognized safe compound and is approved for use in food items [9, 26]. Data regarding the acute and short-term in vivo effects in different animal species are available and suggest that carvacrol does not pose a risk to human health [36]. Nevertheless, the results of this study indicate that the use of carvacrol as a therapeutic agent can exert significant in vitro and in vivo antimicrobial effects against KPC-producing K. pneumoniae, increasing animal survival and significantly decreasing bacterial loads. However, the absence of cytokine dosage is a limitation of this study. So, further studies are needed to elucidate the role of cytokines in the antimicrobial properties of carvacrol. Also, the linear dose-response of carvacrol was not applicable to our study. Carvacrol shows a biphasic dose-response relationship, in which the low dose causes stimulation, and the dose increases an inhibition. This seems to be similar to a hormetic effect. However, the hormetic effect mechanism is extremely limited, mainly in the context of antimicrobial activities [46, 47]. Additional studies are required to elucidate the dose-response of carvacrol.
In conclusion, preliminary results in mice are hopeful and indicate that carvacrol has potential as an antimicrobial agent against KPC-producing K. pneumoniae. However, more studies of carvacrol activity and its action mechanisms in animal models are necessary to enhance our understanding and establish its efficacy.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
 In vitro and in vivo antibacterial activity assays of carvacrol: A candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae
In vitro and in vivo antibacterial activity assays of carvacrol: A candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae
