
- Altmetric
Supplemental Digital Content is available in the text.
Previous studies have reported associations between ambient fine particle concentrations and preeclampsia; however, the impact of particulate pollution on early- and late-onset preeclampsia is understudied. Furthermore, few studies have examined the association between source-specific particles such as markers of traffic pollution or wood combustion on adverse pregnancy outcomes. Electronic medical records and birth certificate data were linked with land-use regression models in Monroe County, New York for 2009 to 2013 to predict monthly pollutant concentrations for each pregnancy until the date of clinical diagnosis during winter (November–April) for 16 116 births. Up to 30% of ambient wintertime fine particle concentrations in Monroe County, New York is from wood combustion. Multivariable logistic regression was used to separately estimate the odds of preeclampsia (all, early-, and late-onset) associated with each interquartile range increase in fine particles, traffic pollution, and woodsmoke concentrations during each gestational month, adjusting for maternal characteristics, birth hospital, temperature, and relative humidity. Each 3.64 µg/m3 increase in fine particle concentration was associated with an increased odds of early-onset preeclampsia during the first (odds ratio, 1.35 [95% CI, 1.08–1.68]), second (odds ratio, 1.51 [95% CI, 1.23–1.86]), and third (odds ratio, 1.25 [95% CI, 1.06–1.46]) gestational months. Increases in traffic pollution and woodsmoke during the first gestational month were also associated with increased odds of early-onset preeclampsia. Increased odds of late-onset preeclampsia were not observed. Our findings suggest that exposure to wintertime particulate pollution may have the greatest effect on maternal cardiovascular health during early pregnancy.
See Editorial, pp [Related article:] 618–619
Pregnancy can be regarded as a cardiometabolic stress test,1 with failure of maternal adaptation and preexisting factors converging to contribute to adverse pregnancy outcomes such as preeclampsia.2,3 Preeclampsia is a leading contributor to maternal-fetal morbidity and mortality worldwide and complicates 2% to 8% of all pregnancies.4 Furthermore, women with a history of preeclampsia have a 2- to 4-fold increased risk of future heart failure, coronary heart disease, cardiovascular disease death, or stroke.5 Preeclampsia can be defined as new-onset hypertension and either proteinuria or end-organ dysfunction after 20 weeks gestation. This heterogenous and multisystemic disorder can also be characterized by gestational age at onset, with early-onset (ie, placental disease) occurring at <34 weeks and late-onset (ie, maternal systemic disease) at ≥34 weeks. Early-onset preeclampsia accounts for ≈5% to 20% of all preeclampsia cases worldwide but includes more severe clinical cases than late-onset.6 Early-onset is thought to reflect poor placentation during early pregnancy whereas late-onset is thought to reflect maternal constitutional factors. Only one study has examined associations between particle pollution exposure during pregnancy and early- and late-onset preeclampsia as separate outcomes.7
Recent meta-analyses have reported increased odds of preeclampsia associated with increases in ambient particulate matter with aerodynamic diameter <2.5 µm (PM2.5) during the entire pregnancy and first trimester.8,9 However, few studies have considered the impact of source-specific particles (eg, from wood smoke or traffic pollution) on adverse pregnancy outcomes.10,11 The inclusion of source-specific PM2.5 is particularly important because its chemical composition may substantially alter its toxicity. Wang et al12,13 estimated that, on average, 30% of ambient wintertime (November–April) fine particle concentrations in Monroe County, New York was from residential wood burning. Previously, we observed an 18% to 21% increased odds of a hypertensive disorder associated with each 0.52 µg/m3 increase in Delta-C (marker of wood smoke) concentration in the seventh and eighth gestational months, during wintertime.14
We linked electronic medical record data with birth certificate data and separately estimated the odds of preeclampsia (all, early-, and late-onset) associated with ambient fine particle concentrations during only winter months by using a land-use regression model (LUR)15 to estimate gestational month-specific pollutant concentrations until the date of clinical diagnosis for each member of a population of pregnant women in Monroe County, New York during 2009 to 2013. We hypothesized that ambient PM2.5, black carbon (BC; marker of traffic pollution), and Delta-C during gestational months 7 to 9 (ie, late pregnancy) would be associated with increased odds of preeclampsia and late-onset preeclampsia during wintertime. We also hypothesized that increases in the same pollutant concentrations during gestational months 1 to 3 (ie, early pregnancy) would be associated with increased odds of early-onset preeclampsia during winter.
Methods
Our data can be made available to other researchers. The data that support the findings of this study are available from the corresponding author on reasonable request.
Using a retrospective cohort study design and electronic medical record data linked to birth certificate data from the Finger Lakes Perinatal Database System, we retained records of 20 596 live births to female residents of Monroe County who delivered at either Strong Memorial Hospital or Highland Hospital in Rochester, NY from 2009 to 2013. For women with more than 1 birth during 2009 to 2013, the first birth was selected. We excluded women diagnosed with chronic hypertension (n=572) or gestational hypertension (n=607), infants with congenital anomalies (n=236), a birth weight <500 g (n=63) or >5000 g (n=28), and out of range clinical values including gestational weeks at delivery recorded as <12 or >45 weeks or birth history/number of conceptions >25 (n=378). We also excluded those missing covariate information (n=1835). To avoid the fixed cohort bias, a type of selection bias where shorter pregnancies are missed in the beginning of the study period and longer pregnancies are missed at the end, we excluded births (n=761) with an estimated date of conception before July 17, 2008 (ie, 24 weeks before January 1, 2009) or after March 12, 2013 (ie, 42 weeks before December 31, 2013), leaving 16 116 births available for analysis. Using the date of the last menstrual period, we calculated the start and end dates of each gestational month for each pregnancy. If the last menstrual period date was missing (n=778), it was estimated using the infant date of birth and clinical estimate of gestational weeks. We then included only those women with complete gestational exposure data until the date of clinical diagnosis during winters (November 1 to April 30) of 2008 to 2013. The study was approved by the University of Rochester Research Subjects Review Board (RSRB #00057468). No informed consent was required as this study did not involve direct contact with study participants. This study involved access to electronic medical records and birth certificate data.
Air Pollution and Weather Data
PM2.5, BC, and Delta-C concentrations, temperature, and relative humidity were measured during winters (November 1 to April 30) of 2008 to 2013 at the New York State Department of Environmental Conservation site located in Rochester, New York (43°08’46’’ N, 77°32’54’’ W, Elevation=137 m, U.S. EPA site code 36-055-1007). PM2.5 concentrations were measured hourly using a Tapered Element Oscillating Microbalance (TEOM; Thermo Fisher, Franklin, MA). BC, a marker of traffic pollution,16 was measured using a 2-wavelength (370 and 880 nanometers) aethalometer (AE-22 aethalometer, Magee Scientific, Berkeley, CA). Delta-C, a marker of wood smoke, was reported as the difference between BC measured at 370 and 880 nanometers.17 Delta-C has been identified as surrogate for wood smoke because it is strongly correlated with levoglucosan and potassium, 2 species found in residential wood combustion.12,17 Temperature and relative humidity were measured at 5-minute intervals and provided as hourly averages. Gestational month-specific concentrations of BC, Delta-C, and PM2.5 at each mother’s residential address were estimated using a previously developed LUR model for the winter (November 1 to April 30) in Monroe County, NY.15 Exposures were calculated during the wintertime by averaging LUR-predicted daily concentrations for up to 9 gestational months for each pregnancy until the date of clinical diagnosis.
Outcome Assessment
The primary outcome was preeclampsia (yes/no), defined in the electronic medical records using the International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnoses of mild or unspecified preeclampsia (ICD-9 codes: 642.40-642.44), severe preeclampsia (ICD-9 codes: 642.50-642.54); or eclampsia (ICD-9 codes: 642.60-642.64). For those women with preeclampsia (n=823), we used the date of clinical diagnosis and gestational age at time of diagnosis to define early- and late-onset preeclampsia. Electronic medical record use began at these hospitals in March 2011; however, not all women with a preeclampsia diagnosis and a delivery before March 2011 had detailed clinical information scanned into the electronic medical records. Therefore, the date of clinical diagnosis, gestational age at time of diagnosis, and subsequent preeclampsia subtype could not be confirmed in nearly 58% (n=478), 40% (n=328), and 24% (n=194) of women with preeclampsia, respectively. For these participants, we either back-calculated the diagnosis date for those with gestational age at time of diagnosis (n=312) or assigned the delivery date as the diagnosis date for those missing both gestational age at diagnosis and date of clinical diagnosis (n=420). We could not verify the preeclampsia subtype for the remaining observations and thereby excluded them from the analysis (n=91).
Statistical Analyses
We used multivariable logistic regression to separately estimate the odds of preeclampsia (all, early-, and late-onset) associated with each unit increase in each pollutant concentration during each exposure window in winter (up to 9 regression models). We did not include exposure after disease onset for any of the analyses. In each analysis, we only included those women with complete gestational exposure data until the date of clinical diagnosis during winters (November 1–April 30) of 2008 to 2013. All pollutant concentrations were modeled as continuous variables and reported as an interquartile range (IQR) to make valid comparisons among pollutant effects across pregnancy. The IQR for the first month of pregnancy for each pollutant concentration was multiplied by each gestational month-specific regression coefficient to obtain a scaled odds ratio.
We included the following covariates as potential confounders and predictors of the outcome based on previously published work,7,14,18–23 primiparity (primiparous or multiparous), multifetal gestation (yes or no), prepregnancy diabetes mellitus (yes or no), gestational diabetes mellitus (yes or no), maternal age (<20, 20–24, 25–29, 30–34, 35–39, or ≥40 years), maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, or Other), maternal education (high school or less, high school grad/GED, some college, Associate’s degree, Bachelor’s degree, Master’s degree or higher), prepregnancy body mass index (underweight, normal, overweight, class I obesity, class II obesity, or class III obesity), year of conception (2008, 2009, 2010, 2011, 2012, or 2013), birth hospital (Highland or Strong Memorial), average relative humidity, and average temperature. We did not have information on self-reported exposure to secondhand smoke or diet. To reduce potential bias that may result from limiting our analysis to wintertime, we further adjusted for season of conception in a sensitivity analysis. From each model described above, we report the adjusted odds ratio and 95% CIs for each IQR increase for each pollutant concentration. We used SAS version 9.4 (SAS Institute, Inc, Cary, NC) to construct all datasets and perform all statistical analyses.
Results
Study Population
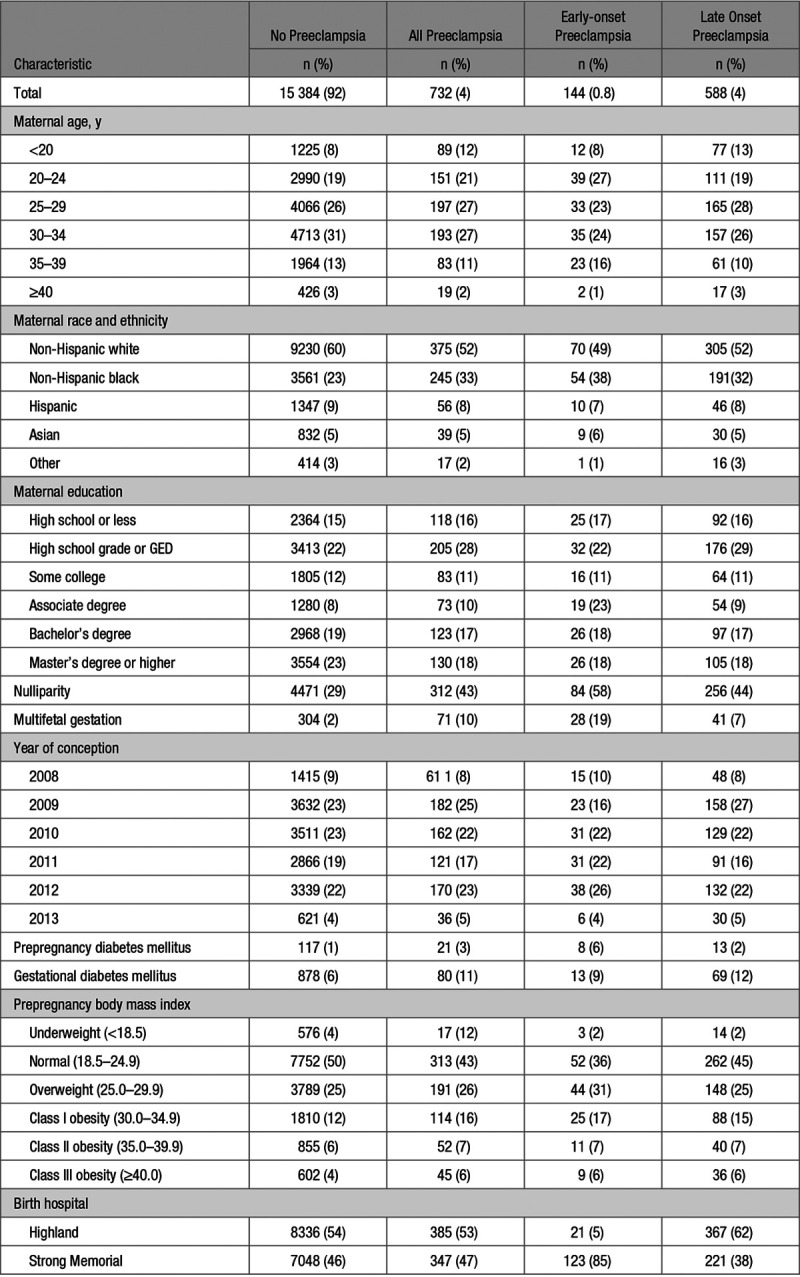
Compared with women without preeclampsia, a greater proportion of those with preeclampsia were non-Hispanic black (33% versus 23%), nulliparous (43% versus 29%), had a multifetal gestation (10% versus 2%), had gestational diabetes mellitus (11% versus 6%), and were more often obese (29% versus 22%; Table 1). Compared with women without preeclampsia, a greater proportion of women with early-onset preeclampsia were 20 to 24 years of age (27% versus 19%), non-Hispanic black (38% versus 23%), nulliparous (58% versus 29%), had a multifetal gestation (19% versus 2%), had prepregnancy or gestational diabetes (15% versus 7%), were more obese (30% versus 22%), and delivered at Strong Memorial (85% versus 46%). Compared with women without preeclampsia, women with late-onset preeclampsia were generally non-Hispanic black (32% versus 23%), nulliparous (44% versus 29%), had a multifetal gestation (7% versus 2%), had gestational diabetes mellitus (12% versus 6%), and delivered at Highland (62% versus 54%).

Exposure Assessment
Mean monthly pollutant concentrations for the first gestational month, estimated using LUR are presented in Table S1 in the online-only Data Supplement. The correlation coefficients between LUR modeled mean pollutant concentrations, relative humidity, and temperature during the first month of pregnancy during the winter months are presented in Table S2. PM2.5 was weakly correlated with BC (r=0.31), Delta-C (r=0.26), and relative humidity (r=0.26), but inversely correlated with temperature (r=−0.32). Relative humidity and temperature were inversely correlated (r=−0.45; Table S2).
Particulate Concentrations and Odds of Preeclampsia
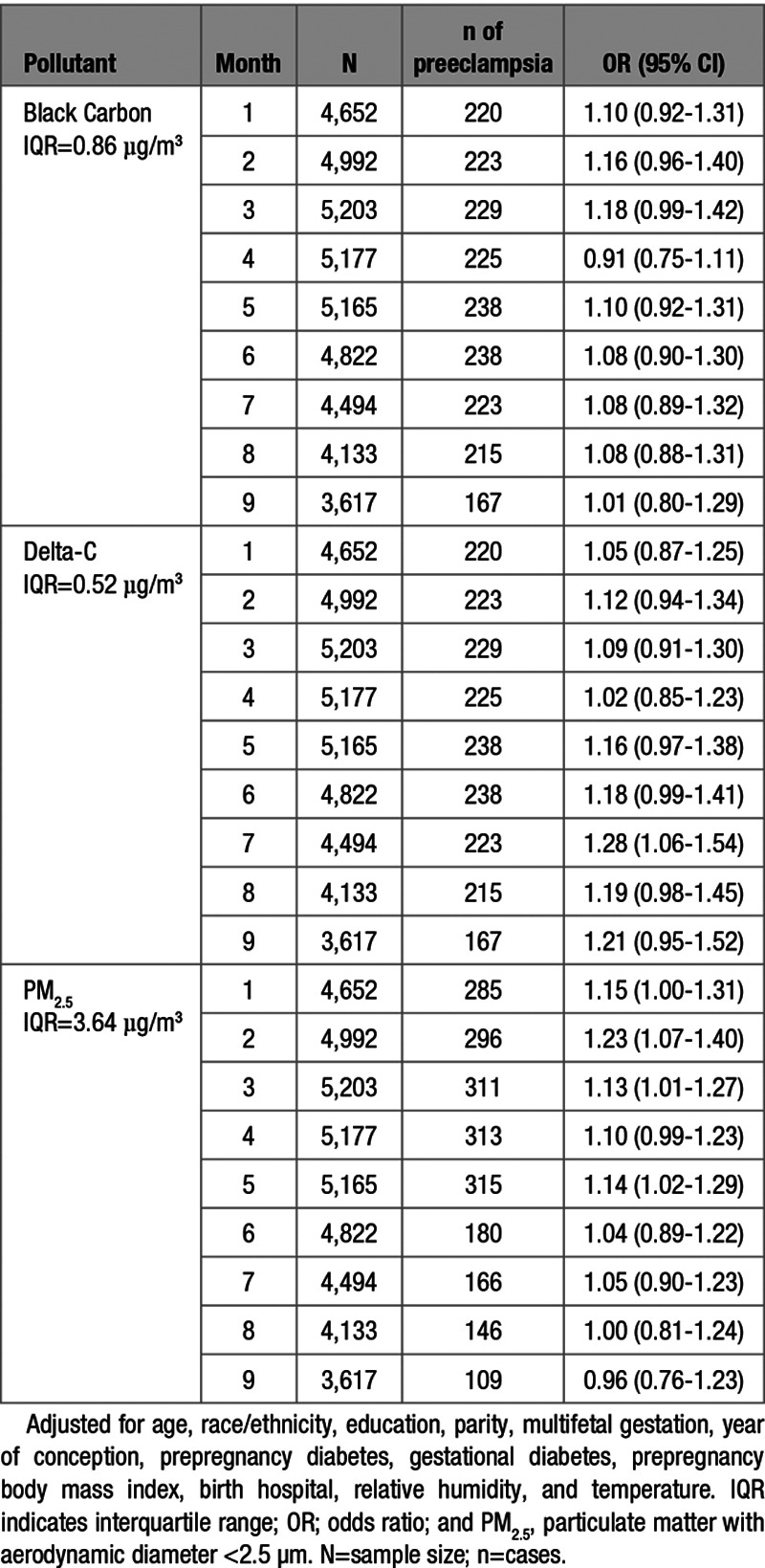
The odds of preeclampsia associated with each IQR increase in concentrations of BC, Delta-C, and PM2.5, in each gestational month are presented in Table 2. Increased Delta-C concentrations were generally associated with increases in preeclampsia, with the larger effect estimates occurring in late pregnancy after adjusting for maternal characteristics, birth hospital, relative humidity, and temperature. For example, each 0.52 µg/m3 increase in Delta-C concentration during the seventh gestational month was associated with an increased odds of preeclampsia (odds ratio [OR], 1.28 [95% CI, 1.06–1.54]), with similar sized effects in months 8 (OR, 1.19 [95% CI, 0.98–1.45]) and 9 (OR, 1.21 [95% CI, 0.95–1.52]). Increased PM2.5 concentrations were generally associated with increases in preeclampsia during months 1 to 5, with the largest effect estimate in the second month (OR, 1.23 [95% CI, 1.07–1.40]; IQR, 3.64 µg/m3). Similar patterns were not observed for BC. Further, we observed no association between BC or PM2.5 concentrations during gestational months 7 to 9 and the odds of preeclampsia (Table 2).

Particulate Concentrations and Odds of Preeclampsia Subtypes
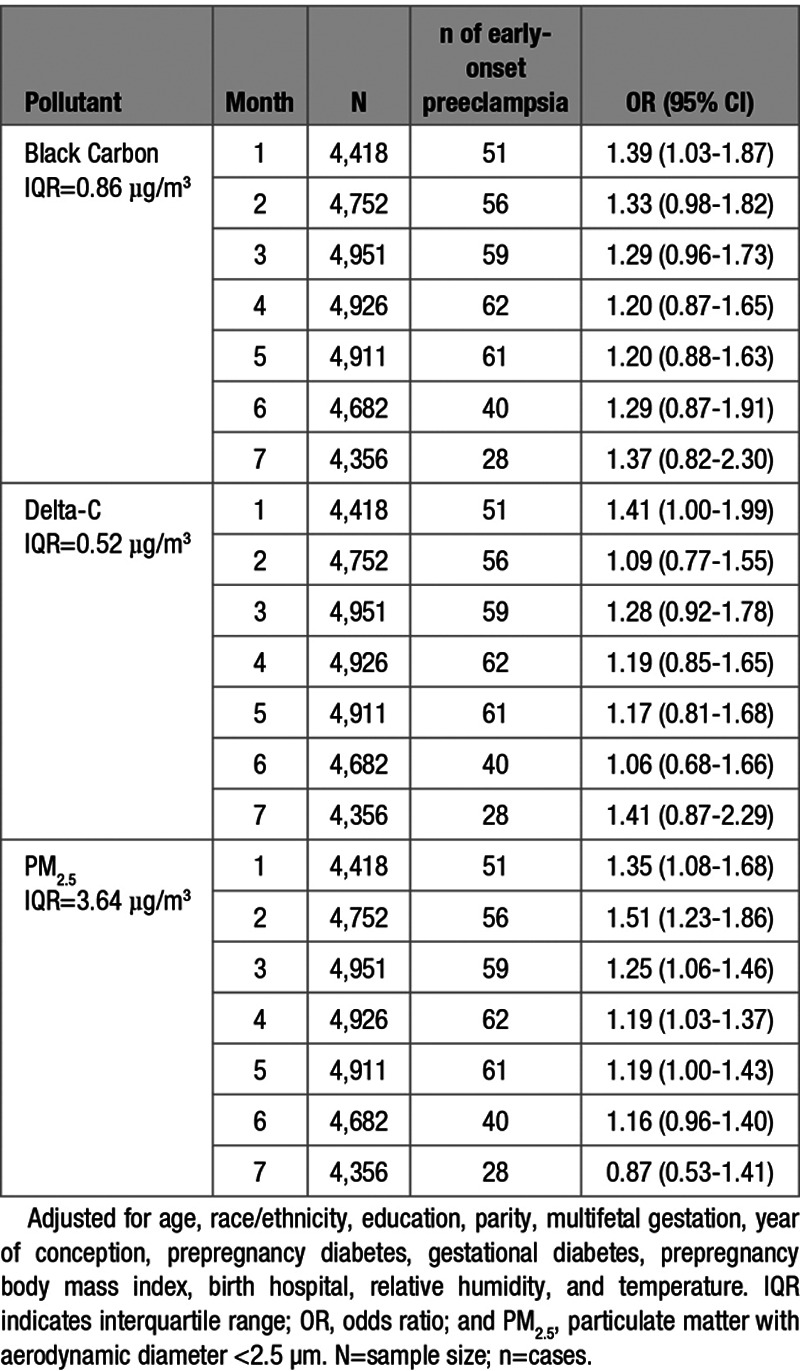
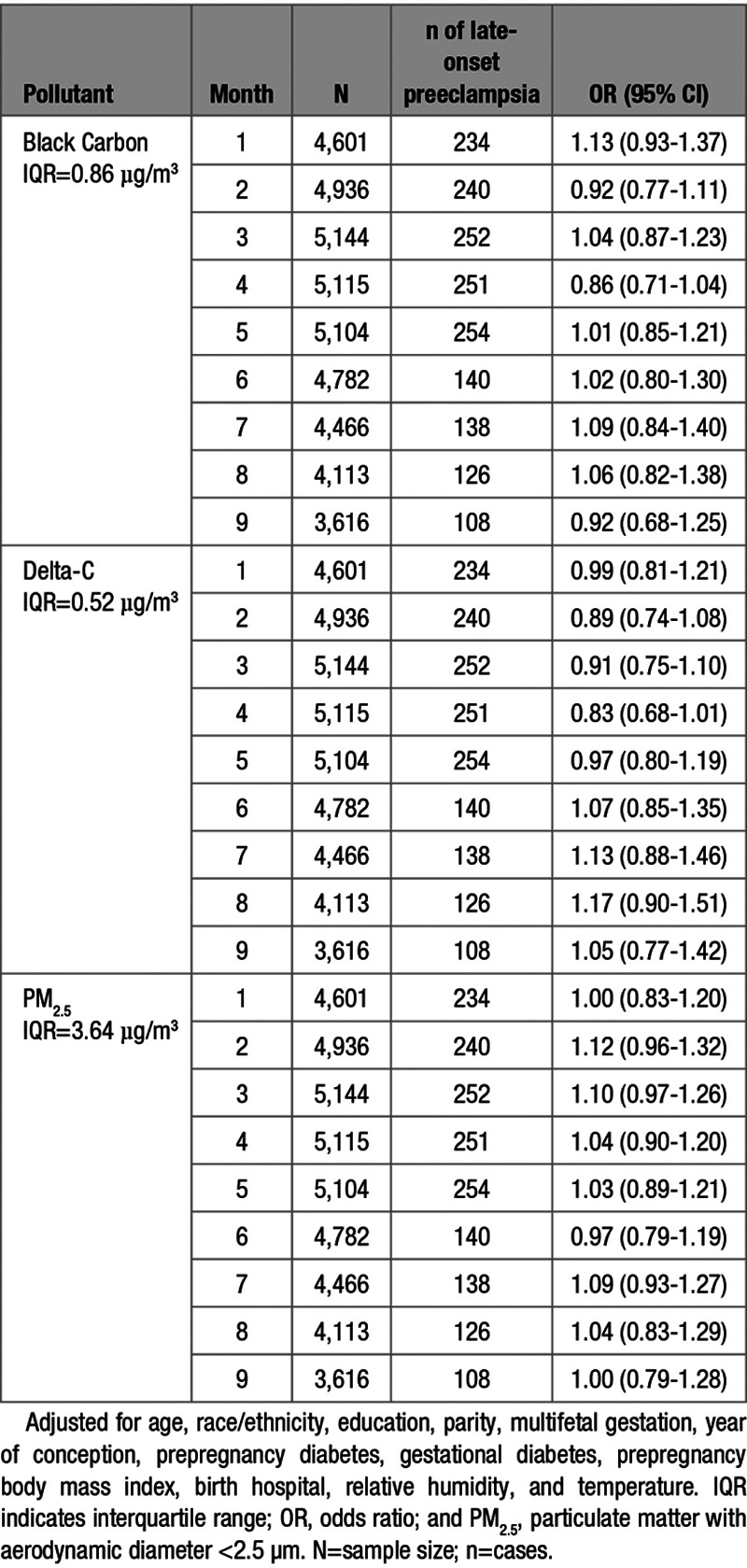
IQR increases in BC, Delta-C, and PM2.5 concentrations during the first gestational month were associated with 39%, 41%, and 35% increases in the odds of early-onset preeclampsia, respectively. Additionally, each 3.64 µg/m3 increase in PM2.5 concentration was associated with an increased odds of early-onset preeclampsia during the second (OR, 1.51 [95% CI, 1.23–1.86]), and third (OR, 1.25 [95% CI, 1.06–1.46]) gestational months. We also observed increased odds of early-onset preeclampsia associated with IQR increases in PM2.5 concentration in the fourth (OR, 1.19 [95% CI, 1.03–1.37]) and fifth gestational months (OR, 1.19 [95% CI, 1.00–1.43]; Table 3). No associations were observed between any pollutant concentration during gestational months 7 to 9 and the odds of late-onset preeclampsia (Table 4).


Sensitivity Analyses
Similar to our main analyses, we observed no association between BC or PM2.5 concentrations during gestational months 7 to 9 and the odds of preeclampsia (Table S3). IQR increases in BC, Delta-C, and PM2.5 concentrations during the first gestational month were associated with 54%, 47%, and 42% increases in the odds of early-onset preeclampsia. Each 3.64 µg/m3 increase in PM2.5 concentration was associated with 14% to 68% increases in the odds of early-onset preeclampsia during gestational months 2 to 6 (Table S4). No associations were observed between any pollutant concentration during gestational months 7 to 9 and the odds of late-onset preeclampsia (Table S5).
Discussion
We observed 13% to 51% increased odds of preeclampsia, specifically, early-onset preeclampsia was associated with IQR increases in PM2.5 concentrations during gestational months 1 to 5, with the largest relative odds in the second month, after adjusting for maternal characteristics, birth hospital, relative humidity, and temperature. Increased concentrations of BC and Delta-C during the first gestational month were also associated with increased odds of early-onset preeclampsia. However, there was no increased odds of late-onset preeclampsia associated with any pollutant concentration during any gestational month. Similar patterns were generally observed after adjusting for season of conception, with effect estimates of greater magnitude for the associations between BC, Delta-C, and PM2.5 concentrations and early-onset preeclampsia during early pregnancy.
Other studies have also reported increased odds of preeclampsia associated with PM2.5 concentrations during gestational months 1 to 5. Using a hospital-based cohort of 34 705 singleton births in Allegheny County, Pennsylvania, the authors observed an elevated, albeit imprecise and nonstatistically significant, odds of preeclampsia associated with each IQR increase in PM2.5 concentration in the first trimester (OR, 1.15 [95% CI, 0.96–1.39]; IQR, 4 µg/m3).23 Previously, in a retrospective cohort of 81 186 singleton births at 4 hospitals in Los Angeles and Orange Counties, CA, the authors reported an increased odds of preeclampsia associated with 1.35 µg/m3 increases in PM2.5 concentration during the first trimester (OR, 1.10 [95% CI, 1.06–1.15]), second trimester (OR, 1.12 [95% CI, 1.07–1.16]), third trimester (OR, 1.10 [95% CI, 1.06–1.15]), and the entire pregnancy (OR, 1.11 [95% CI, 1.06–1.15]).22 However, our findings are inconsistent with our a priori hypothesis and previous studies that reported increased odds of preeclampsia during late pregnancy.7,22 Dadvand et al7 reported an increased odds of preeclampsia associated with each 7.3 µg/m3 increase in PM2.5 concentrations during the third trimester (OR, 1.51 [95% CI, 1.13–2.01]) in a retrospective cohort of 8398 pregnant women in Barcelona, Spain. They also examined the association between PM2.5 and the odds of early- and late-onset preeclampsia (defined in the same manner as our study) and reported an increased odds of early-onset preeclampsia (OR, 1.69 [95% CI, 0.93–3.05]) associated with each 7.6 µg/m3 increase in PM2.5 concentration during the first trimester. However, these findings were based on a small number of cases of early-onset preeclampsia (n=26). Additionally, they reported an increased odds of late-onset preeclampsia associated with each 7.3 µg/m3 increase in PM2.5 concentration during the third trimester (OR, 1.42 [95% CI, 1.01–2.00]), with smaller nonsignificant relative odds for the first and second trimester.
The inconsistency of our finding with previous studies may be due to heterogeneity in PM sources and composition across geographic locations (Los Angeles County, CA; Barcelona, Spain). Additionally, in Monroe County, the median concentration (6.8 µg/m3) was lower than the United States EPA national ambient air quality standard (12.0 μg/m3) and median concentrations in Los Angeles (17.0 µg/m3) and Barcelona (17.3 µg/m3). Therefore, it is possible that concentrations of PM2.5 in Monroe County may have been too low to affect the maternal vascular system during late pregnancy in our study population.
Ibrahimou et al10 examined the associated between maternal exposure to elemental carbon, a source of traffic emission, and risk of preeclampsia in a cohort of 100 490 pregnant women in Hillsborough and Pinellas counties, FL. They reported an increased odds of preeclampsia associated with each IQR increase in elemental carbon concentrations during firstt trimester, after adjusting for aluminum, sociodemographic characteristics, pregnancy complications, prepregnancy body mass index, and gestational weeks (OR, 1.08 [95% CI, 1.01–1.16]), which is consistent with our finding of an increased odds of early-onset preeclampsia associated with increased BC concentrations in the first gestational month.
The underlying biological mechanism by which particulate pollution may contribute to preeclampsia remains unknown, but systemic inflammation, oxidative stress, and endothelial dysfunction24 have been suggested as potential pathways. Given that preeclampsia is characterized as a placenta-mediated pregnancy complication, particles may have their greatest impact during maternal vascular remodeling processes during early pregnancy. It has been posited that inhalation of these particles may trigger a pulmonary inflammatory response that releases cytokines into the maternal systemic circulation.24 These cytokines could contribute to placental inflammation, systemic oxidative stress,24,25 and failed remodeling of the maternal spiral arteries leading to poor placentation during months 1 to 3 (ie, early pregnancy).
Strengths of our study include using LUR-estimated particle pollution concentrations rather than central site measured values (presumably resulting in less exposure misclassification and downward bias) and use of clinical diagnoses of preeclampsia from electronic medical records instead of birth certificate data (resulting in less outcome misclassification and downward bias). The use of electronic medical records also allowed us to obtain gestational age at time of preeclampsia diagnosis and date of clinical diagnosis, classify preeclampsia as early- or late-onset, and truncate exposure before the date of diagnosis. Our ability to ensure that exposure preceded the outcome and did not include exposure periods after the diagnosis of preeclampsia reduced the potential for nondifferential exposure misclassification.
There are also several limitations that should be considered when making inference, including that LUR models were not developed for the entire calendar year or for pollutants like ozone, and thus, we were unable to examine the association between ambient pollutant concentrations during pregnancy and preeclampsia for time periods outside of November to April. We also did not have information on residential mobility and assumed that each woman’s residential address was the same throughout pregnancy, or if she did move, her level of exposure remained the same throughout each month of pregnancy26 resulting in Berkson and classical error, with classical error resulting in downward bias, and underestimates of risk.27,28 We did not include data on median household income, which is highly correlated with place of residence near sources of pollution, therefore, residual confounding cannot be ruled out. Future studies should additionally include indicators of socioeconomic status in their regression models to rule out potential unmeasured confounding. Finally, we did not adjust for multiple comparisons, instead, inference was primarily made by considering the pattern of association across these 7 to 9 gestational month mean pollutant concentrations, rather than only whether each was statistically significant.
Future studies using data-driven statistical methods such as distributed lag models are needed to estimate the association between maternal exposure to air pollution and pregnancy outcomes29–32 and to identify unbiased estimates of critical windows of exposure that may not correspond to clinically defined trimesters or gestational months (ie, gestational weeks).33 Given that maternal exposure to air pollution during pregnancy is ubiquitous, knowing the critical windows of exposure will be beneficial in developing and implementing targeted interventions.
Perspectives
The underlying pathophysiology of preeclampsia is not clearly understood. However, our study provides some evidence that low level exposure to PM2.5 during early to mid-pregnancy in winter is associated with an increased odds of preeclampsia, specifically, early-onset preeclampsia in Monroe County, NY. Our results also highlight the importance of understanding whether individual sources (here traffic and wood smoke) are individually toxic and thereby better inform polices to protect public health. Future studies are needed to replicate these findings and explore the effect of wood smoke and traffic pollution on placental malperfusion, an underlying clinical feature that contributes to preeclampsia.
Sources of Funding
This work was supported by the National Institutes of Health (Grant #’s T32-HL007937 and P30-ES001247) and the New York State Energy Research and Development Authority (Contract # 32971).
Disclosures
None.
References
2.
3.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
Novelty and Significance
What Is New?
Low level exposure to PM2.5 concentrations during gestational months 1 to 5 is associated with 13% to 23% and 19% to 51% increased odds of preeclampsia and early-onset preeclampsia, respectively.
Exposure to wood smoke and traffic pollution during the first month of pregnancy in Monroe County is associated with an increased odds of early-onset preeclampsia.
What Is Relevant?
Our findings extend the previous body of literature on PM2.5 and preeclampsia and highlight the importance of understanding the toxicity of individual sources which may contribute to the incidence of this condition.
Our findings have public health implications given the health and economic burden of preeclampsia and the ubiquity of air pollution.
Early gestational exposure to PM2.5, wood smoke, and traffic pollution during winter is associated with increased odds of preeclampsia, specifically, early-onset preeclampsia.
 Wintertime Wood Smoke, Traffic Particle Pollution, and Preeclampsia
Wintertime Wood Smoke, Traffic Particle Pollution, and Preeclampsia