
- Altmetric
To demonstrate feasibility of acute peritoneal dialysis (PD) for acute kidney injury during the coronavirus disease 2019 (COVID-19) pandemic, we performed a multicenter, retrospective, observational study of 94 patients who received acute PD in New York City in the spring of 2020. Patient comorbidities, severity of disease, laboratory values, kidney replacement therapy, and patient outcomes were recorded. The mean age was 61 ± 11 years; 34% were women; 94% had confirmed COVID-19; 32% required mechanical ventilation on admission. Compared to the levels prior to initiation of kidney replacement therapy, the mean serum potassium level decreased from 5.1 ± 0.9 to 4.5 ± 0.7 mEq/L on PD day 3 and 4.2 ± 0.6 mEq/L on day 7 (P < 0.001 for both); mean serum bicarbonate increased from 20 ± 4 to 21 ± 4 mEq/L on PD day 3 (P = 0.002) and 24 ± 4 mEq/L on day 7 (P < 0.001). After a median follow-up of 30 days, 46% of patients died and 22% had renal recovery. Male sex and mechanical ventilation on admission were significant predictors of mortality. The rapid implementation of an acute PD program was feasible despite resource constraints and can be lifesaving during crises such as the COVID-19 pandemic.
Worldwide, the incidence of acute kidney injury (AKI) in patients with coronavirus disease 2019 (COVID-19) is high. New York City was at the epicenter of the COVID-19 outbreak in the United States during the spring of 2020. In New York City, the incidence of AKI in hospitalized patients with COVID-19 was 36%–57%, and 14%–19% of patients with AKI required kidney replacement therapy (KRT).1 , 2 The abrupt rise of COVID-19 cases led to a critical and unpredicted shortage of resources and personnel in providing hemodialysis (HD) and continuous KRT (CKRT). To offset these shortages, nephrologists in New York City swiftly incorporated acute peritoneal dialysis (PD) for the treatment of AKI.
As nephrologists from 4 major academic medical centers in New York City—Montefiore Medical Center, Mount Sinai Hospital, New York University Medical Center, and Weill Cornell Medicine, we formed the New York City–PD consortium and conducted a multicenter observational study of patients with AKI who received acute PD in these centers during the COVID-19 pandemic surge from March 14 to May 6, 2020. Each medical center independently developed protocols to provide acute PD and had previously reported their center-specific experience.3, 4, 5, 6 The objectives of this study were to demonstrate the regional feasibility of providing acute PD during the pandemic surge, and to report clinical outcomes and examine risk factors of mortality.
We collected data retrospectively, via chart review of electronic medical records, on patient characteristics, hospitalization, KRT prescription and delivery, and clinical outcomes; compared laboratory volumes before and after initiation of KRT; and examined predictors of mortality. Detailed methods are provided as Supplementary Materials. A total of 94 patients with AKI received acute PD (Supplementary Table S1). The mean age was 61 ± 11 years; 34% were female; and 39% were Black. The mean body mass index was 32 ± 8 kg/m2, and 49% had a body mass index ≥30 kg/m2. Prior to admission, 46% had diabetes, 65% had hypertension, and 30% had chronic kidney disease. The median modified Charlson comorbidity index was 2 (interquartile range [IQR]: 1–4), and the index ranged from 0 to 9 (Supplementary Table S2). On admission, 20% had a temperature of ≥102 °F (Supplementary Table S3); 32% had a respiratory rate ≥25 per minute; 20% had an arterial pH < 7.25; 24% required either non-breather or high flow oxygen; and 32% required mechanical ventilation. The mean modified acute physiology and chronic health evaluation (APACHE) II score was 13 ± 6 (Supplementary Table S4). COVID-19 nucleic acid–based tests were positive in 94% of patients. The median levels of peak C-reactive protein, D-dimer, and fibrinogen were 51 mg/dL (IQR: 30–300), 10 μg/ml (IQR: 5–20), and 782 mg/dL (IQR: 689–934), respectively. During their hospitalization, 87% required mechanical ventilation, and 47% required prone positioning to improve oxygenation.
Indications for KRT initiation were many, with some patients having more than one. These included hyperkalemia (n = 38), fluid overload (n = 44), uremia (n = 39), metabolic acidosis (n = 33), and anuria or oliguria (n = 8; Supplementary Table S5). Prior to KRT initiation, the serum potassium level was 5.1 ± 0.9 mEq/l (ranged from 3.1 to 7.5), and serum bicarbonate level was 20 ± 4 mEq/L (ranged from 11 to 32; Supplementary Table S6). PD was the initial modality of KRT in 56 patients (Supplementary Table S5; Supplementary Figure S1). The remaining patients were started on intermittent HD (n = 32) or CKRT (n = 5) and switched to PD. Typically, the patients were dry overnight if PD was interrupted. One center used icodextrin for overnight dwells. All centers had PD teams setting up manual or automated PD. Reasons for switching to PD included lack of a functional vascular access or clotting (n = 8), shortage in HD/CKRT supplies (n = 18), and a nursing shortage (n = 15). Most patients were started on acute PD in standard intensive care units (ICUs; n = 55); one was started in the emergency room, and 16 in overflow ICUs, with limited access to water and electricity. PD catheters were placed by general surgery (66%), interventional radiology (16%), and transplant surgery (19%). Despite no prior exposure, the ICU staff members in all centers were accepting of PD; expectations of ultrafiltration rate and changes in the lab values, such as serum creatinine, were quelled with good communication.
On PD day 3 (n = 80), the most common form of PD fluid used was 2.5% dextrose (Supplementary Table S7). The median exchange volume prescribed and delivered was 1.5 L (IQR: 1.0–2.0). Most patients received 5 exchanges per day, and 1 to 2 hours per exchange. On average, 86% of the prescribed PD volume was delivered, and the volume of ultrafiltration achieved in a 24-hour period was 0.7 L (IQR: 0.3–1.7). Compared to the levels prior to the initiation of KRT, the serum potassium level decreased to 4.5 ± 0.7 mEq/L on PD day 3 (P < 0.001) and to 4.2 ± 0.6 mEq/L on day 7 (P < 0.001), and the serum bicarbonate level increased to 21 ± 4 mEq/L on PD day 3 (P = 0.002) and to 24 ± 4 mEq/L on day 7 (P < 0.001; Figure 1 a and b and Supplementary Table S6). Sensitivity analyses limited to patients who received acute PD as the initial modality, and to patients who required prone positioning, showed similar results in serum potassium and bicarbonate levels (Supplementary Figure S2). On PD day 3, a total of 84% of patients had a serum potassium level ≤5 mEq/L, and 71% had a serum bicarbonate level ≥20 mEq/L. The most common complication was catheter malfunction (n = 17), followed by peritoneal fluid leakage (n = 11) and peritonitis (n = 5; Supplementary Table S5). There were no exit-site infections. Twenty patients switched to HD/CKRT (n = 13) or were supplemented with HD/CKRT (n = 7). The most common reasons for switching to or supplementing with HD/CKRT were PD catheter malfunction (n = 5), persistent hyperkalemia or metabolic acidosis (n = 4), and persistent volume overload (n = 4).

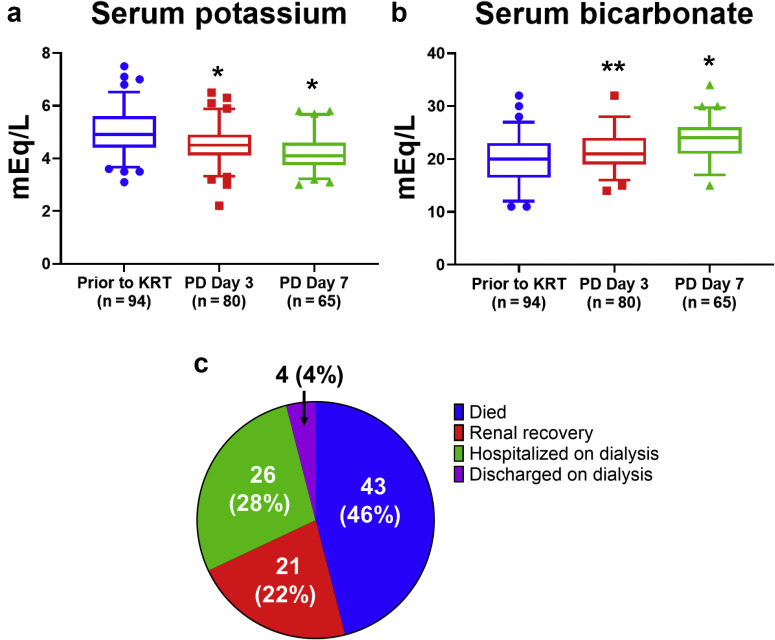
(a,b) Changes in serum potassium and bicarbonate levels. Data are presented in box plots as median, 5th percentile, and 95th percentile. Data points beyond the percentile range are plotted as individual points. Paired t tests were used for comparisons. Reference group was values prior to kidney replacement therapy (KRT). ∗P < 0.001; ∗∗P = 0.002. (c) Patient outcome after a median follow-up of 30 days. PD, peritoneal dialysis.
After a median follow-up of 30 days (range: 6 to 67 days), 46% of patients had died, 22% had had renal recovery (defined as KRT independence), 28% remained hospitalized on dialysis, and 4% had been discharged on dialysis (Figure 1c). Of those who were discharged on dialysis, 4 were discharged on PD, and 1 was discharged on HD. Of those who remained hospitalized on dialysis, 11 remained on PD. Among those with renal recovery, the median serum creatinine level was 1.6 mg/dL (IQR 1–3.4; Supplementary Table S6). In the Cox multiple regression model that adjusted for sex and mechanical ventilation on admission, compared to women, men had a hazard ratio of 2.68 with a 95% confidence interval of 1.21 to 5.94 (P = 0.02); compared to those who were not on a mechanical ventilator on admission, patients who were on a mechanical ventilator had a hazard ratio of 2.26 (95% confidence interval: 1.18–4.33, P = 0.01). The Kaplan-Meier survival curves by sex and the status of mechanical ventilation on admission are shown in Supplementary Figure S3 (P for log-rank test was 0.04 for both).
We demonstrate that the rapid deployment of acute PD is feasible, and outcomes are acceptable in a crisis with restricted resources. The International Society for Peritoneal Dialysis recommends that PD should be considered a suitable modality for treatment of AKI.7 Prior to the COVID-19 pandemic, the use of acute PD in the United States was uncommon, and recent experience in delivering acute PD was lacking. We rapidly implemented acute PD programs, with limited resources, and overcame obstacles. Challenges to implementing acute PD included rationing supplies, staff shortages, PD catheter placement, and the need for prone positioning. Acute PD teams led by nephrologists coordinated with surgeons or interventional radiologists, ordered PD supplies, and trained staff to provide PD. We delivered ∼86% of the PD volume prescribed, which is comparable to typical CKRT therapy delivered. Serum potassium and bicarbonate levels improved; 4% of patients were switched to or supplemented with HD/CKRT due to persistent hyperkalemia or metabolic acidosis. Despite the high severity of illness in our cohort and challenges in delivering PD, the rates of death and renal recovery were similar to those of patients with AKI requiring KRT in other cohorts.8
Our study also provides insights to providing acute PD to patients who required prone positioning and were obese. Prone positioning prior to the COVID pandemic in conjunction with PD had rarely been described. In this study, 47% required prone positioning at some time during the hospitalization. Sensitivity analyses limited to patients who required prone positioning showed improvements in serum potassium and bicarbonate levels with acute PD. With team effort and modifications, such as adjusting the PD volume and positioning the catheter exit site laterally, challenges with prone positioning can be overcome.6 Obesity has been considered a relative contraindication for PD. Despite having a mean body mass index of 32 kg/m2 in our cohort, most patients achieved treatment goals, and our rate of complications was low.
The advantages of PD over HD/CKRT were demonstrated in our cohort. Thirty-seven (39%) patients switched from HD/CKRT to PD, and the major reasons were access issues, depleted supplies, and staff shortages. Compared to HD/CKRT, it is easier to train staff to implement PD manually or with cyclers. The high rate of thrombosis associated with COVID-19 was bypassed by treating AKI with acute PD. Lastly, 17% of patients were started on acute PD in overflow ICUs with curtailed access to water and electricity, and where HD/CKRT was limited or not possible.
To the best of our knowledge, this is the largest cohort describing patients who received acute PD during the COVID-19 pandemic. We provided life-sustaining KRT despite severe shortages, with no rationing. Our data supplement our previous single-center reports3, 4, 5, 6 and demonstrate the feasibility of implementing acute PD in patients who require prone positioning and are obese. Although future studies are needed to compare the long-term outcomes between patients with AKI who received PD vs. HD/CKRT, our experiences may serve to create a template to implement acute PD as a successful dialysis modality in future catastrophic situations. It is during times of hardship that we become the most creative and resourceful. We hope that this study paves the way for a future in which acute PD is seen as a valid alternative form of KRT for acutely ill patients.
Disclosure
VS has received speaker fees from Baxter. All the other authors declared no competing interests.
References
1
2
3
4
5
6
7
8
Acknowledgments
The NYC-PD Consortium acknowledges the nurses and other hospital staff for providing excellent care of patients and implementing the acute PD program during the COVID-19 pandemic. Due to limits in words and references, we were unable to acknowledge all articles that have made significant contributions in the field.
WC is supported by the
Author Contributions
WC, MJR, VS, MYS, JU, NC, and SS researched the idea and designed the study; DS, OES, SA, LS-R, KP, MHS, MYS, RD, JU, NC, and SS acquired data; WC, MJR, RD, JU, NC, SS, VS, and OES performed data processing, analysis, and interpretation; WC and RD performed statistical analysis; and MJR, LG, and JU mentored. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Supplementary Methods.:
Table S1.: Patient characteristics.
Table S2.: Modified Charlson comorbidity index.
Table S3.: Admission and in-hospital information.
Table S4.: Modified APACHE II score, based on admission data.
Table S5.: Information on kidney replacement therapy.
Table S6.: Laboratory values.
Table S7.: PD information on PD day 3.
Table S8.: Cox regression for survival analysis, unadjusted.
Figure S1.: Patient flowchart.
Figure S2.: Changes in serum potassium and bicarbonate levels among patients who were started on acute PD as the initial modality (A,B), and among patients who required prone position during the hospitalization (C,D). Data are presented in box plots as median, the 5th and 95th percentiles. Data point beyond the percentile range are plotted as individual points. Paired t tests were used for comparisons. Reference group was values prior to kidney replacement therapy. a P < 0.001; b P < 0.01; c P < 0.05.
Figure S3.: Predictors of mortality. Kaplan-Meir curves by sex (A), status of mechanical ventilation on admission (B).
Supplementary References.:
 Use of peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City: a multicenter observational study
Use of peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City: a multicenter observational study