
- Altmetric
Regulatory B cells (Bregs) ameliorate autoimmune disease and prevent allograft rejection. Conversely, they hinder effective clearance of pathogens and malignancies. Breg activity is mainly attributed to IL‐10 expression, but also utilizes additional regulatory mechanisms such as TGF‐β, FasL, IL‐35, and TIGIT. Although Bregs are present in various subsets defined by phenotypic markers (including canonical B cell subsets), our understanding of Bregs has been limited by the lack of a broadly inclusive and specific phenotypic or transcriptional marker. TIM‐1, a broad marker for Bregs first identified in transplant models, plays a major role in Breg maintenance and induction. Here, we expand on the role of TIM‐1+ Bregs in immune tolerance and propose TIM‐1 as a unifying marker for Bregs that utilize various inhibitory mechanisms in addition to IL‐10. Further, this review provides an in‐depth assessment of our understanding of Bregs in transplantation as elucidated in murine models and clinical studies. These studies highlight the major contribution of Bregs in preventing allograft rejection, and their ability to serve as highly predictive biomarkers for clinical transplant outcomes.
INTRODUCTION
B lineage cells uniquely produce antibodies — the sine qua non of humoral immunity. However, B (lineage) cells also play non‐antibody‐mediated roles that significantly impact the immune response through antigen presentation, T cell co‐stimulation/co‐inhibition, and cytokine secretion. B cells produce an array of pro‐ and anti‐inflammatory cytokines (including TNFα, IFNγ, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17, and IL‐35) that can profoundly influence both innate and adaptive immune responses. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Regulatory B cells (Bregs) expressing anti‐inflammatory cytokines such as IL‐10, potently downregulate the immune response, ameliorating autoimmune disease and allograft rejection, while limiting anti‐tumor and infectious immunity. 2 , 3 , 7 , 9 , 10 , 13 , 14 In contrast, effector B cells (Beffs), which express pro‐inflammatory cytokines, have the opposite effect. 15 , 16 , 17 , 18 , 19 , 20 , 21 While this review focuses on Bregs, the net modulatory effect of B cells on the immune response likely results from the balance of the opposing activities of both Bregs and Beffs. A better understanding of these subsets could lead to novel therapeutic approaches to either enhance or dampen the immune system.
Despite significant advances, a number of aspects of Breg biology remain poorly understood. These include: the lack of a specific phenotypic or transcriptional marker; poor insight into their development (stochastic vs distinct lineage); effector function in vivo; and the relationship between various Breg subsets identified in the literature. For example, Bregs are still primarily identified by their expression of IL‐10, their best studied suppressive cytokine, leading to a number of different phenotypic "subsets” whose function and lineal relationship to one another are unclear. While a variety of other suppressive regulatory mechanisms have been identified, including PD‐L1, FasL, TIGIT, granzyme B, TGF‐β, and IL‐35, 8 , 22 , 23 , 24 , 25 , 26 the relationship of these cells to IL‐10+ Bregs remains unclear.
In the first part of this review, we provide a detailed assessment of these salient issues in murine models and suggest that TIM‐1, first identified in transplantation, may represent a functional marker that helps unify Bregs with different phenotypes and mechanisms of action. Since most Bregs have phenotypes resembling those of B cells belonging to various canonical B cell subsets, we address the potential localization and in vivo intercellular interactions of Bregs with other immune cells. Murine transplantation represents a good model for dissecting the immune response, immunoregulation, and tolerogenic mechanisms, since it parallels the human clinical setting where otherwise naïve animals receive a potent immune stimulus in the form of an allograft.
In the second portion of this review, we shift the focus to Bregs in clinical transplantation. Similar to murine models, we discuss attempts to identify human Bregs despite lack of a specific marker. However, the clinical setting raises issues not generally considered in animal models. Thus, we not only review the evidence for a role for Bregs in transplantation, but also whether Breg numbers or activity can predict allograft outcomes. This would allow physicians to proactively monitor and individualize immunosuppressive therapy, for example, by identifying transplant recipients at low risk for rejection whose immunosuppression can be safely reduced or pre‐emptively increasing immunosuppression in high‐risk recipients to reduce future rejection episodes and premature allograft loss. Further, we address the effects of currently used immunosuppressive regimens on Bregs with the prospect of therapeutic manipulation of Bregs to promote immunological tolerance.
REGUALTORY B CELLS IN MICE
The problem in defining Breg subsets and phenotype without a specific marker
There are no specific markers that definitively identify Bregs. As a result, many studies have utilized IL‐10 expression as a surrogate marker since IL‐10 was the first mechanism of Breg activity described and remains dominant in many models. 11 , 27 However, defining Bregs by their expression of IL‐10 alone is also a “narrow” definition since it may ignore Bregs that utilize other mechanisms of action. The spleen is the largest reservoir for Bregs and is the focus of most murine Breg studies. 28 IL‐10+ Bregs are rare, comprising approximately 1% of all B cells in naïve spleen. 11 , 28 However, they can expand up to 3%‐5% of the total splenic B cell population after antigen challenge. 13 IL‐10 expression has been identified in most canonical B cell subsets, including follicular (FO), marginal zone (MZ), marginal zone precursor (MZP or T2‐MZP), Transitional (Tr), and plasma cells (PCs) in varying frequency. 20 , 29 , 30 , 31 , 32 , 33 , 34 However, the exact role and relative activity of these different Breg "subsets” remains completely unclear. Until recently, B cell IL‐10 expression was only identified by intracellular staining after in vitro mitogenic stimulation. 11 Since IL‐10+ B cells could not be identified without prior in vitro stimulation and permeabilization, most studies assessing Breg function depended on adoptive transfer of freshly isolated B cell subsets found to be relatively enriched for IL‐10 expression. For example, the CD1d+ (MZ), CD21hiCD23hiCD24hi (T2‐MZP), and the unusual CD1dhi CD5+ (“B10”) subsets are all enriched for IL‐10 expression and can transfer IL‐10‐dependent amelioration of murine colitis, EAE, and SLE. 11 , 12 , 35 However, this approach confuses the frequency of Bregs within transferred populations, with the actual activity of IL‐10+ B cells within a given subset. Although enriched, IL‐10+ B cells still comprise a minority of cells (eg, 10%‐15%) within each of these subsets. Moreover, the IL‐10+ B cells in each of these subsets comprise only 10%‐20% of the total number of IL‐10+ B cells in secondary lymphoid organs (SLO). Thus, most IL‐10+ B cells (potential Bregs) are not included in studies of these individual subsets, and most transferred B cells are in fact, not Bregs. As a specific example, ~15% of CD5+CD1dhi B cells express IL‐10. However, this subset makes up only ~2% of splenic B cells and therefore encompasses only ~20% of all IL‐10+ B cells. 2 , 13 While the majority of the IL‐10+ B cells are contained within the remaining 98% of splenic B cells, their frequency is too low (~1%) to demonstrate Breg activity upon adoptive transfer.
We systematically addressed both the relative frequency of IL‐10+ B cells within each B cell subset and identified the relative contribution of each subset to total B cell IL‐10. 29 We found that IL‐10 expression, albeit at lower levels, can consistently be identified without in vitro stimulation both by intracellular staining, and more easily, using IL‐10‐GFP reporter mice. 29 This allowed us to directly examine the influence of in vitro stimulation on the apparent distribution of IL‐10+ B cells. In the absence of in vitro stimulation, on a protein level, we found that MZ and FO B cells and PCs each comprised ~25%‐30% of all IL‐10+ B cells in spleen— totaling ~85% (Table 1). The remaining ~15% were distributed amongst Tr B cells and splenic B1a subsets. However, the frequency of IL‐10 expression within each subset varied markedly. PCs were found to be highly enriched, with ~60% expressing IL‐10. However, since PCs comprise 0.4%‐1% of all splenocytes, numerically they account for only ~30% of all B cell IL‐10 in spleen. 29 In contrast, while only ~0.30% of FO B cells express IL‐10, FO B cells comprise ~55% of splenic B cells, and thus contain ~30% of all IL‐10+ B cells. As implied above, transfer of FO B cells is unlikely to show regulatory activity if over 99% of transferred cells are not Bregs. Thus, FO cells have been largely overlooked as potential Bregs despite representing a significant proportion of all IL‐10+ B cells. MZ B cells lie in between these extremes. ~6% of MZ B cells express IL‐10 and they make up ~8% of all B cells. Thus, this population, as well as the somewhat smaller subset of MZ precursors (MZP), are frequent enough to isolate, and enriched sufficiently for IL‐10+ cells to transfer Breg activity. 32 , 34

| B cell subset | % IL‐10+ cells within each subset | Fold increase with stimulation IL‐10 | % of total B cell IL‐10 | ||
|---|---|---|---|---|---|
| Unstimulated | Stimulated | Unstimulated | Stimulated | ||
| Marginal Zone (MZ) | 5.6 | 23 | 4.1 | 24 | 33 |
| Follicular (FO I/II) | 0.3 | 1.7 | 5.7 | 33 | 44 |
| Plasma cell (PC) | 58 | 74 | 1.3 | 28 | 5.0 |
| Marginal zone precursor (MZP) | 1.0 | 6.2 | 6.2 | 3.0 | 4.0 |
| Transitional 1 | 3.0 | 11 | 3.7 | 7.0 | 12 |
| Transitional 2 | 0.7 | 5.2 | 7.2 | 1.0 | 1.0 |
| Splenic B1a | 3.4 | 7.6 | 2.2 | 4.0 | 1 |
Not surprisingly, the frequency of IL‐10 expression increased in every subset after the commonly used approach of in vitro simulation with LPS, PMA, and ionomycin (LPIM) for 5 hours (Table 1). 29 In most B cell subsets, the frequency of IL‐10 expression increased 3‐ to 5–fold; however, the rank order of IL‐10 expression amongst the subsets was unchanged. 29 For example, the frequency of IL‐10+ MZ and FO cells increased approximately 4‐ and 6‐fold, respectively. However, PCs, starting at 60% IL‐10 expression, had little room to increase further (1.3‐fold from 60% to 75%). 29 The ~5‐fold increase of IL‐10 expression amongst B cells dwarved the increase in the small PC population, and as a result, the percentage of IL‐10 expressed by PCs dropped from 30% to 5% of all B lineage IL‐10. This likely explains why PCs were missed as a major source of B lineage IL‐10/Breg activity until relatively recently. 18 , 31 The biologic importance of B cell IL‐10 observed only after potent in vitro stimulation is a valid argument favoring the Breg activity of PCs. Moreover, the IL‐10 expression level of PCs is higher than B cells at both the transcriptional and protein levels (fluorescence intensity). 18 , 31 Nonetheless, in the absence of in vitro stimulation, we find PCs comprise only 30% of all B lineage IL‐10, suggesting that B cells themselves might contribute directly to Breg activity.
An alternative approach for studying Bregs is to identify broad markers that encompass IL‐10+ Bregs regardless of subset markers. We identified TIM‐1 (T cell immunoglobulin and mucin domain 1), and subsequently Sun and colleagues identified CD9, as markers that encompass a majority (~75%‐85%) of all splenic IL‐10+ Bregs. 30 , 36 While broad, these markers still lack specificity, in that only a portion of CD9+ or TIM‐1+ B cells express IL‐10. However, potent suppressive activity of B cells identified by these markers suggests that Bregs belonging to each of the various canonical subsets encompassed, contribute to the Breg activity observed. TIM‐1 also has important functional aspects and may more broadly identify Bregs utilizing other mechanisms of action, as detailed below. Since freshly isolated IL‐10‐GFP reporter cells can be used to directly identify IL‐10‐expressing B cells within each subset by flow cytometry, a direct comparison of IL‐10+ Bregs belonging to different canonical subsets can be made. We believe this would represent an important advance in clarifying “Breg subsets” and understanding the purpose of IL‐10+ B cells being dispersed amongst every B cell subset.
Bregs in Murine Models of Transplantation and the discovery of TIM‐1 as a broad functional Breg marker
Transplantation across a full MHC mismatch represents a formidable immunological challenge due to the magnitude of the T cell response. It has been estimated that 1%‐10% of all T cells are alloreactive, orders of magnitude above that for nominal antigen. 37 , 38 , 39 A major focus in transplant research aims to identify tolerogenic pathways that might lead to development of new therapeutic targets. Importantly, animal models of transplantation closely parallel the human clinical setting. Accordingly, we set out to re‐examine a tolerogenic anti‐TIM‐1 mAb, RMT1‐10, that was thought to inhibit EAE and allograft rejection in mice by decreasing inflammatory Th17 and Th1 responses while augmenting Th2 cells and Foxp3+ Tregs. 40 In general agreement with other studies, 3 doses of anti‐TIM‐1 resulted in 60% long‐term acceptance of fully MHC mismatched islet allografts in WT recipients. 2 However, in trying to identify the in vivo cellular target of anti‐TIM‐1 antibody, we found that CD4+ T cells expressing TIM‐1 were rare. However, 10%‐15% of B cells in transplanted mice expressed TIM‐1. Surprisingly, when allograft recipients lacked B cells or were B cell depleted, treatment with anti‐TIM‐1 mAb actually accelerated acute rejection, and all of the salutary T cell responses were absent. This suggested that B cells not only express TIM‐1 but are actually the tolerogenic target of anti‐TIM‐1. Examination of TIM‐1+ B cells revealed a 20‐ to 25‐fold enrichment for IL‐10 compared to TIM‐1‐ B cells. Importantly, anti‐TIM‐1 treatment increased the number of TIM‐1+ B cells and their IL‐10 expression, resulting in an overall 5‐fold increase in both the frequency and number of IL‐10+ B cells. 2 Thus, TIM‐1 signals play a functional role in Breg induction.
Adoptive transfer of TIM‐1+, but not TIM‐1‐, B cells from allo‐immunized mice promoted transplant tolerance in B‐deficient hosts that was specific to the immunizing antigen. 2 , 15 , 36 , 41 Thus, TIM‐1+ B cells are regulatory and are antigen‐specific. Such antigen‐specificity has been corroborated in autoimmune models like EAE and CIA. 42 , 43 This also suggests that BCR‐signaling is required to activate and/or expand relevant Breg clones. This notion is supported by the finding that TIM‐1+ B cell expansion in response to anti‐TIM‐1 requires immunization. 2 As noted above, anti‐TIM‐1 treatment increased Th2 cells and IL‐10+ and Foxp3+ Tregs, and decreased Th1 and Th17 cells, but these effects were all B cell‐dependent. 2 , 44 Additionally, transfer of TIM‐1+ Bregs increased Tregs, and inhibited expression of IL‐17 and IFNγ by CD4+ T cells, further supporting a direct effect of B cells on Th differentiation. Transplant tolerance induced by either anti‐TIM‐1, or transfer of TIM‐1+ B cells was dependent on B cell IL‐10 expression. 2 , 29 As alluded to above, TIM‐1+ B cells contain ~75% of all IL‐10+ B cells, and IL‐10 expression is highly enriched across all canonical B cell subsets compared to TIM‐1‐ B cells. Since the number of TIM‐1+ B cells transferred in each individual subset is small, this supports the notion that B cells belonging to many of these subsets contribute to regulatory activity.
TIM‐1 contributes to Breg induction and suppressor function
TIM‐1 may be distinct amongst phenotypic markers, in that it plays an important functional role in Breg expansion and suppressor function. As noted above, TIM‐1 ligation with anti‐TIM‐1 (RMT1‐10) induces Breg expansion in vivo. 2 , 36 Moreover, knock‐in mice that express a mutant form of TIM‐1 with a deletion of the mucin domain (TIM‐1Δmucin), exhibit defects in both basal and induced IL‐10+ Bregs. 44 , 45 Specifically, anti‐TIM‐1 binds to mutant TIM‐1 but fails to induce IL‐10+ Bregs. Gray and colleagues previously showed that apoptotic cells induce IL‐10+ Bregs. 46 Phosphatidyl serine (PtdS), exposed on the surface of apoptotic cells is a natural ligand for TIM‐1. PtdS binds to WT TIM‐1+ B cells and induces IL‐10 expression, but does not bind to, or induce IL‐10, in WT TIM‐1‐ B cells or TIM‐1+ B cells from TIM‐1Δmucin mice. 44 , 47 Thus, TIM‐1 is the major PtdS receptor on B cells and promotes IL‐10 expression (Figure 1). Further, these data indicate that TIM‐1Δmucin is a loss‐of‐function mutant. As a consequence, these mice exhibit progressive spontaneous splenomegaly with age and more severe SLE when crossed onto a MRL‐Faslpr background. 45 Even young TIM‐1Δmucin mice exhibit more aggressive alloimmune responses. For example, TIM‐1Δmucin mice acutely reject single Class II MHC mismatched BM12 cardiac allografts that survive > 100 days in WT recipients. 44 Acute rejection is ameliorated by transfer of WT TIM‐1+ but not TIM‐1‐ B cells. Thus, TIM‐1 is not only a marker for IL‐10+ Bregs but plays a key role in their development and induction. This further suggests that Bregs may normally be maintained and expanded by sensing levels of apoptotic cells through TIM‐1.

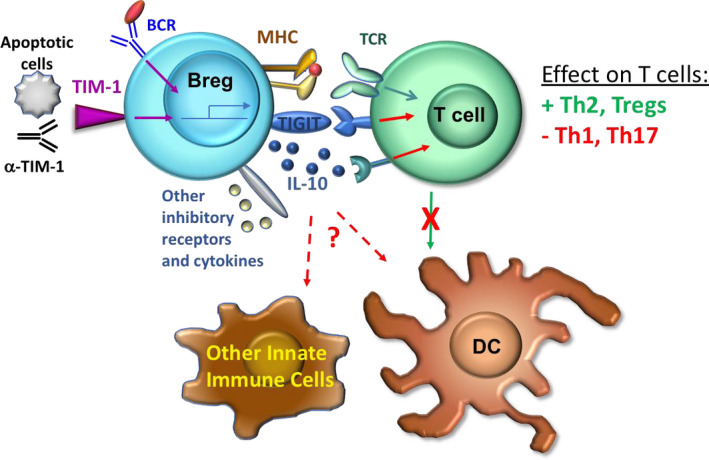
Tim‐1+ Breg induction and mechanism of action: Signals through TIM‐1 and the BCR promote expansion of Tim‐1+ Bregs and expression of suppressive molecules such as IL‐10, TIGIT and other cytokines and inhibitory receptors. Cognate Breg interactions with T cells suppress inflammatory Th1 and Th17 cell responses, enhance Th2 and Treg (Foxp3 and IL‐10) responses, and reduce subsequent T cell interactions with dendritic cells (DCs). Bregs may also directly interact with DCs and other types of innate cells and suppress their expression of pro‐inflammatory mediators
TIM‐1 is a broad regulator of Breg activity and identifies Bregs as major regulators of immune homeostasis
While total TIM‐1‐KO and TIM‐1Δmucin mice develop age‐related splenomegaly and enhanced immune responsiveness, they do not develop spontaneous autoimmunity. However, in these mice, loss of WT TIM‐1 on B cells may be offset by loss of TIM‐1 on T cells where it plays a costimulatory role. 48 To address this issue, Xiao and colleagues specifically deleted TIM‐1 on B cells (TIM‐1‐BKO). 26 TIM‐1‐BKO mice older than 10 months developed spontaneous multi‐system autoimmunity characterized by weight loss, dermatitis, rectal prolapse, and EAE‐like paralysis. Most TIM‐1‐BKO mice had inflammatory immune cell infiltrates in multiple organs including kidneys, liver, and lungs. Based on the role of TIM‐1 in regulating the induction of IL‐10+ Bregs, Xiao and colleagues directly compared the transcriptomes of TIM‐1+ and TIM‐1‐ B cells from WT and TIM‐1Δmucin mice. This revealed that TIM‐1 signaling not only regulated IL‐10, but a host of other inhibitory cytokines and co‐inhibitory molecules including, Ebi3, GITRL, Fgl2, CLTLA‐4, Lag3, and TIGIT (Figure 1). Thus, TIM‐1 regulates an array of potentially inhibitory molecules and may link different “types” of Bregs that utilize distinct mechanisms. Indeed, specific deletion of TIGIT in B cells resulted in spontaneous EAE‐like paralysis and multi‐organ inflammatory cell infiltration, though less severe than that seen in TIM‐1‐BKO mice. 26 Notably, there was not a major overlap between IL‐10 and TIGIT, which were expressed by distinct populations of TIM‐1+ B cells. TIM‐1 was also found to negatively regulate B cell expression of inflammatory cytokines such as IL‐6, IL‐12, and IL‐23, perhaps enforcing the regulatory balance between Bregs and Beffs. 26 Taken together, these data suggest that while TIM‐1 may not be specific for IL‐10 per se, it may be specific for Bregs that use a variety of mechanisms of action. This may also explain why loss of B cell TIM‐1 leads to a more severe inflammatory phenotype than loss of either B cell TIGIT or IL‐10. 26 , 29 In summary, these data firmly establish the role of TIM‐1+ Bregs cells in immune homeostasis through IL‐10, TIGIT, and presumably other inhibitory molecules under the control of TIM‐1 signaling. To this extent, TIM‐1 may unify various Breg subsets. Whether or not all Breg functions are controlled by TIM‐1, or distinct families of Bregs under control of other signaling pathways exist, remains to be elucidated.
Breg in vivo mechanisms of action, localization, and intercellular interactions
In both allograft and other models, Bregs skew Th differentiation, inhibiting Th1 and Th17 cells, while promoting Th2 cells and Foxp3+ Tregs. 2 , 3 , 15 , 43 , 49 Acute deletion of B cell IL‐10, using a Tamoxifen‐inducible B cell‐specific conditional knock out mouse, augmented IL‐17 and IFNγ expression by CD4+ T cells and increased IFNγ expression and proliferation by CD8+ T cells. 29 Bregs have also been shown to modulate the function of innate immune cells including monocytes, dendritic cells (DCs), neutrophils, and NK T cells. Bregs predominantly inhibit innate cell secretion of inflammatory mediators such IL‐12, IFNγ, TNFα, and nitric oxide that contribute to priming and skewing of the adaptive immune response. 5 , 6 , 7 , 10 Despite these effects on the immune response, the nature and localization of Breg interactions with other immune cells in vivo remains unclear. Antigen specificity and the requirement for MHCII and CD40 suggest that Bregs act through direct cognate interactions, at least with CD4+ T cells. 2 , 3 , 9 , 11 , 35 , 42 However, Tr B cell maturation in the absence of conventional (MHCII and CD40‐dependent) T:B interactions may not be entirely normal. 50 , 51 Further, Bregs could indirectly suppress T cell function by inhibiting antigen‐presenting cell function, similar to Tregs. 52 The apparent antigen specificity of Bregs might result from a requirement for cell activation in close proximity to DCs involved in presenting antigen to responding T cells.
To directly examine the interactions between Bregs and other immune cells and identify their anatomical localization in spleen, we utilized 2‐photon intravital microscopy. 29 IL‐10‐GFP reporter mice were immunized with nitrophenyl‐chicken gamma globulin (NP‐CGG) to expand hapten‐specific NP‐reactive B cells. IL‐10+ (Bregs) or IL‐10‐ (non‐Breg) B cells were isolated and pulsed in vitro with NP‐Ovalbumin (Ova) and adoptively transferred into WT hosts along with NP‐Ova‐pulsed DCs and naïve TCR‐transgenic Ova‐specific OT‐I CD8+ or OT‐II CD4+ T cells. (Transferred cells were labeled with different fluorescent tracking dyes). Both T cells and antigen‐pulsed DCs clustered in the T cell zone. Amongst B cells localized to the T:B border, Bregs made more frequent and more prolonged contacts with both CD4+ and CD8+ T cells than non‐Bregs, and Bregs specifically made forays into the T cell zone. These Breg‐T cell interactions were specific‐antigen dependent since Bregs pulsed with irrelevant antigen (NP‐CGG) did not interact with T cells. Of note, Bregs did not make sustained interactions with DCs even when both were pulsed with the same antigen. However, T cell contacts with DCs were reduced in both frequency and duration in the presence of Bregs in a specific‐antigen dependent manner. 29 These findings suggest that Bregs make cognate interactions with naïve T cells and this hinders subsequent T cell:DC interactions (Figure 1). Thus, Bregs act in a manner opposite to Tregs. 52 , 53 Since our analysis was limited to DCs that localized to the splenic T:B: border, we cannot exclude the possibility that direct Breg:DC interactions might occur in other splenic niches and under different circumstances.
These findings indicate that Breg:T cell interactions can occur at the T:B border—implicating FO B cells as Bregs. Consistent with this, we found that IL‐10+ FO B cells expressed more CCR7 than other IL‐10+ B cells, which should facilitate their migration to the T:B border and into the T cell zone. 29 Moreover, upon transfer, IL‐10+ FO B cells primarily migrated to the follicles, while IL‐10+ MZ B cells migrated mainly to the marginal zone. These results suggest that the sizeable fraction of IL‐10+ B cells with a FO B cell phenotype can function as Bregs, by upregulating CCR7 and migrating to the T:B border where they modulate T cell responses.
REGULATORY B CELLS IN HUMAN TRANSPLANTATION
Organ transplantation is the treatment of choice for patients with end‐stage kidney disease, and the only life‐sustaining approach for heart, liver, and lung failure. Solid‐organ transplant patients require life‐long immunosuppression to prevent allograft rejection. Current immunosuppressive regimens are empirically dosed and result in excellent early allograft survival. One year kidney allograft survival approaches 95%. 54 However, ~35% of patients lose their kidney allografts by 10 years, and the rate of this chronic fall‐off has changed little with time. 54 Chronic allograft loss is thought to primarily result from persistent low grade (clinically silent) rejection and remains transplantation's “Achilles heel.” Patients with failed allografts return to the transplant waiting list where they have 3‐fold increased mortality and worsen the organ shortage. 55 Having been immunized by their first transplant, these patients are more frequently sensitized to donor antigens, making a second organ harder to find. Thus, there is a major need to identify new immunosuppression strategies that will enhance allograft survival without increasing associated malignancy and infection. Incorporation of endogenous immunoregulatory mechanisms might allow us to take advantage of the immune system's inherent specificity to limit allograft damage. Thus, there have been major efforts to identify regulatory cells most relevant to transplantation that might be targeted to promote organ‐specific tolerance. In this regard, mounting evidence detailed below suggests that Bregs play an important role in allograft survival. Moreover, examination of Bregs might provide insight into a patient's “immune set‐point,” allowing risk stratification and pre‐emptive modification of immunosuppression according to the risk of rejection. In this section, we will review Breg phenotypes in humans, discuss evidence for the role of Bregs in transplantation, and, finally, discuss the use of Bregs as a biomarker for immunoreactivity in transplant patients.
Identification of human regulatory and effector B cells remains elusive
There exists no specific marker for human Bregs, echoing the limitations of the Breg field in mice. Typically, human Bregs are identified by expression of IL‐10, their “signature cytokine” based on murine data. Several groups have examined human B cell subsets for enrichment in IL‐10 expression. Blair and colleagues found that Tr B cells (CD24hiCD38hi) were enriched for IL‐10 after in vitro stimulation with CD40L. 56 Further, in an in vitro functional Breg assay, Tr B cells inhibited IFNγ, TNFα, and IL‐17 expression and increased FoxP3 expression by anti‐CD3 stimulated autologous CD4+CD25‐ conventional T cells. 56 , 57 In contrast, neither naïve nor memory B cells had any effect. Importantly, the effect of Tr B cells was abrogated by the addition of anti‐IL‐10 to the cultures, suggesting that modulation of T cells by Tr B cells in these assays is IL‐10 dependent. However, since anti‐IL‐10 will neutralize IL‐10 produced by either T or B cells in the cultures, the actual role of B cell IL‐10 cannot be strictly inferred. Addition of either anti‐CD80 or anti‐CD86 mAbs to the co‐cultures also blocked suppression of T cell inflammatory cytokines, raising the possibility that suppression by Tr B cells might also involve costimulatory signaling. Interestingly, compared to healthy volunteers, patients with systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) had a reduced number on their peripheral blood that also lacked in vitro Breg activity. 56 , 57 In contrast, Iwata and colleagues showed that CD24hiCD27+ memory B cells stimulated with CpG and anti‐CD40 expressed the most IL‐10. Further, no defect in IL‐10 expression was observed in patients with various autoimmune diseases including SLE and RA. 7 Subsequently, B cells with various other phenotypes were also reported to be enriched for IL‐10 expression (eg, CD25hiCD71hiCD73‐, TNFR2+, and CD271+CD431+CD11b+ B cells). 58 , 59 , 60 However, the same concerns that apply to murine models apply to these human studies in terms of representing “Bregs” as a whole. None of these markers are inclusive, only a small proportion of B cells within each of these subpopulations actually expresses IL‐10, and IL‐10 is expressed by multiple B cell subsets most of which are small. Moreover, while relatively brief (5‐hour) in vitro stimulation is now typically utilized in murine studies, human studies stimulated B cells for up to 5 days, which alters their phenotype and was not always accounted for. Readers should be wary of manuscripts that define Bregs as any B cells belonging to these subsets without examining their IL‐10 expression or their in vitro functional activity.
The IL‐10/TNFα ratio as an indicator of Breg/Beff balance
Given conflicting reports, we directly compared IL‐10 expression in peripheral blood B cells from healthy individuals, and found that all major B cell subsets (eg, Tr, Naïve and Memory) express IL‐10 at roughly similar frequencies (10%–15%). 61 However, we found that B cells within these same subsets co‐express pro‐inflammatory cytokines like TNFα, emphasizing the lack of a true Breg phenotype. Importantly, the frequency of TNFα expression differed in each subset, and the ratio of B cell IL‐10/TNFα expression correlated with in vitro regulatory activity. Thus, Tr B cells, which have a high IL‐10/TNFα ratio, were able to suppress expression of IFNγ and TNFα by anti‐CD3‐stimulated autologous T cells. There was a stepwise decrease in suppressive activity of memory and naïve B cells, correlating with their lower IL‐10/TNFα ratios. Addition of neutralizing anti‐IL‐10 mAb to the co‐cultures nullified Tr B cell suppressive activity, while addition of a blocking anti‐TNFR1 mAb uncovered the suppressive activity of both naïve and memory B cells. These data suggest that the relative expression of IL‐10 and TNFα within individual B cell subsets correlates with their Breg/Beff balance and thus with their ability to modulate T cell responses in vitro. By neutralizing TNF, the activity of Bregs in memory and naive subsets was uncovered. As further detailed below, the TrB cell IL‐10/TNFα ratio, but not IL‐10 expression alone, decreased in renal allograft patients with rejection.
These findings suggest that Bregs and Beff cells might counterbalance one another and contribute to clinical outcomes. This notion is supported in studies of patients with multiple sclerosis (MS). 62 , 63 While the phenotype was not further examined, B cells from patients with MS expressed less IL‐10 and more pro‐inflammatory cytokines such as TNFα, lymphotoxin, and GM‐CSF than B cells from healthy controls. Further, such B cells failed to suppress T cell proliferation in vitro. B cell depletion with Rituximab (humanized anti‐CD20) is now first‐line therapy for patients with remitting relapsing MS. 64 Importantly, in MS patients who responded clinically to B cell depletion, the reconstituting B cells had a normalized IL‐10/TNFα ratio, and now effectively suppressed T cell proliferation and inflammatory cytokine expression in vitro. 62
In summary, these data highlight some of the limitations of current markers for human Bregs and demonstrate the importance of measuring cytokines rather than just phenotype. The finding that IL‐10+ Bregs in many subsets can be suppressive obviates concerns about how Tr B cells themselves (which are short‐lived and either differentiate into mature naïve B cells or undergo cell death) would be sufficient to have a major suppressive impact on immune responses.
Human Bregs and TIM‐1
While TIM‐1 is an important functional marker for murine Bregs, it has not been extensively studied in human Bregs. Aravena and colleagues reported that ~5% of human peripheral blood B cells and 15%‐35% of the Tr B cell population constitutively expresses TIM‐1. 65 After 48‐hr stimulation, TIM‐1 expression was increased 1.5‐fold in all the canonical B cell subsets. Reportedly, ~40% of all Tr B cells expressed both TIM‐1 and IL‐10, and 90% of all TIM‐1+ TrB cells expressed IL‐10. TIM1+ but not TIM1‐ B cells suppressed pro‐inflammatory cytokine expression by autologous CD4+ T cells stimulated with anti‐CD3 and anti‐CD28. Compared to healthy controls, patients with systemic sclerosis had less TIM‐1+ B cells in their peripheral blood. Furthermore, these TIM‐1+ B cells expressed less IL‐10 and were unable to suppress autologous T cell pro‐inflammatory cytokines in vitro. These findings were corroborated in another small study where TIM‐1+ B cells from the peripheral blood of patients with atherosclerotic coronary artery disease had lower IL‐10 and in vitro suppressive activity compared to healthy controls. 66 Importantly, TIM‐1 may identify IL‐10+ B cells within the TrB cell subset in humans, and findings with TIM‐1+ Bregs parallel those of Tr B cells noted above. Of note, this contrasts with murine TIM‐1, which is more broadly distributed amongst B cell subsets. 2 Thus, the role of TIM‐1 as an inclusive marker for human Bregs is unclear. Further, unpublished data from our lab found that only ~1% of human B cells weakly expressed TIM‐1 with no selective enrichment within Tr B cells. Therefore, additional studies will be required to establish the role of TIM‐1 in human Bregs.
The role of Bregs in clinical transplantation
Antibodies targeting the allograft can significantly impact allograft survival by mediating an acute form of rejection (antibody‐mediated rejection) and contributing to chronic allograft rejection. 67 , 68 With the advent of Rituximab (humanized anti‐CD20), attempts were made to reduce antibody‐mediated components of rejection by pre‐emptively depleting B cells in the peri‐transplant period. Surprisingly, this led to a marked increase in acute T cell–mediated rejection (83%) in kidney transplant patients within the first three months post‐transplantation. 69 In a second study, cardiac allograft recipients were randomized to receive Rituximab in an attempt to reduce cardiac allograft vasculopathy (CAV), which is thought to be antibody‐mediated. 70 Patients who received Rituximab had significantly increased rather than reduced CAV at one year. Both studies are consistent with the notion that the depletion of Bregs in the peri‐transplant period enhances alloimmune responses. In this regard, kidney transplant patients who experienced rejection after Rituximab treatment had significant increase in serum pro‐inflammatory cytokines, including TNFα, within the first post‐transplant month. 69
As noted above, Rituximab is now first‐line therapy for relapsing or progressive MS. 71 How might the differential responses to B cell depletion in MS versus transplantation be explained? At baseline, B cells in patients with active MS express increased TNFα and decreased IL‐10 compared to healthy counterparts. 72 By depleting all B cells, the predominance of inflammatory B cells is disrupted, and the B cell compartment is reconstituted with B cells expressing normal TNFα and IL‐10, restoring homeostasis. Renal transplant recipients generally lack active autoimmunity, and we hypothesize their balance of Bregs and Beff cells is less skewed. Thus, early Breg depletion promotes the alloimmune response despite empiric immunosuppressive agents. A number of centers have utilized Rituximab to deplete B cells later in the post‐transplant course in attempts to treat antibody‐mediated rejection. 73 , 74 While the efficacy of B cell depletion in this setting is unclear, no obvious increases in subsequent T cell–mediated rejection have been reported. This difference between early and late B cell depletion might be explained by murine data suggesting that Bregs primarily act early in the immune response. For example, depletion of B cells at the time of EAE induction worsens disease, whereas depletion of B cells later in the disease course, when inflammation and an effector role for B cells is firmly established, ameliorates disease. 42 Taken together, these studies suggest that the immunologic status of any given patient is reflected by the relative proportion of Bregs and Beffs. The relative loss of these two subpopulations after B cell depletion affects the resultant clinical response. Ultimately, definitive identification and targeting of Breg or Beff populations might allow us to selectively alter immune balance to enhance or inhibit immune responsiveness.
Bregs as biomarkers for allograft rejection and prediction of clinical outcomes
Based on the idea that Bregs/Beffs might provide a window into a patient's “immune set‐point” and allograft outcome, we first analyzed B cell subsets in the peripheral blood of patients 2 years after kidney transplantation. When patients were divided into tertiles based on the absolute number of Tr B cells in their peripheral blood, those within the lowest tertile had the worst renal function and the highest incidence of donor‐specific antibodies (DSA) at the time of the assessment. 75
Based on our studies showing that Breg activity correlated best with the ratio of IL‐10/TNFα (which is highest in the Tr B cell subset), we determined whether this marker correlated with transplant outcomes. 61 We examined the IL‐10/TNFα ratio of peripheral blood Tr B cells in 41 renal transplant patients undergoing renal transplant biopsy for allograft dysfunction 2 to 20 years after transplantation. Patients who had rejection on their biopsies exhibited a decrease in Tr B cell frequency and IL‐10/TNFα ratio compared to patients with allograft dysfunction who did not have rejection, and compared to renal transplant patients with stable renal function or healthy control subjects. In contrast, neither TrB cell absolute number nor their IL‐10 expression alone could differentiate between these groups. Moreover, Tr B cells from patients experiencing rejection lost their ability to suppress autologous T cell pro‐inflammatory cytokines, confirming the relationship between the IL‐10/TNFα ratio and Breg activity (or Breg/Beff balance). This loss of Breg activity in patients with renal allograft rejection has been corroborated by others. 76 , 77 , 78 , 79 Further, the Tr B IL‐10/TNFα ratio strongly predicted rejection in biopsies obtained at the time of biomarker determination (ROC AUC, 0.82). 61 Finally, in patients exhibiting rejection, a Tr B IL‐10/TNFα ratio below the median was associated with 50% allograft loss in the ensuing 3 years, while no allografts were lost in patients with a Tr B IL‐10/TNFα ratio above the median. Thus, in rejecting patients, the Tr B cytokine ratio appeared to identify patients with worse rejection or rejection that was less responsive to therapy. These data suggested that Tr B cells or their cytokine ratio might have value as biomarkers to predict rejection or subsequent clinical course.
Upon further examination of the Tr B cells, we found that the most immature or T1 Tr B cell subset has a significantly higher IL‐10/TNFα ratio than the remaining T2 subset of Tr B cells. 80 Moreover, the ratio of T1/T2 Tr B cells generally parallels the Tr B IL‐10/TNFα cytokine ratio and might serve as a simpler marker of Breg/Beff activity. 80 Therefore, we examined the T1/T2 ratio in stable patients 2 years after kidney transplantation. We found that a low ratio was associated with significantly worse outcomes with decreased renal function (GFR) and 25% graft loss over the next 5 years, compared to stable GFR and no graft loss in patients with a higher ratio. Specifically, a low T1/T2 ratio at 2 years predicted graft loss with ROC AUC 0.82, whereas clinical parameters (such as GFR or the presence DSA) in the same patient cohort were not predictive (ROC AUC 0.56‐0.66, p = NS).
In general agreement with the above findings, Shabir and colleagues showed that the frequency of peripheral blood Tr B cells measured 2 weeks after kidney transplantation was inversely correlated with allograft rejection over the ensuing 4 years. While no patients with a Tr B cell frequency of ≥ 3% (amongst B cells) had rejection, 50% of patients with a Tr B cell frequency of < 1% had rejection. 81 In multivariate analysis, the association between Tr B cell frequency and rejection was independent of baseline clinical characteristics and commonly measured clinical variables. 81 These findings were corroborated by several other small single center studies in kidney and lung transplantation. 82 , 83 For example, enumeration of Tr B cells 3 months post‐transplantation was shown to modestly predict kidney allograft rejection within the 1st post‐transplant year (ROC AUC ~0.65). 82 Furthermore, following stem cell transplantation, patients who developed graft versus host disease (GVHD) had significantly fewer Tr B cells and IL‐10+ B cells in their peripheral blood, and decreased in vitro Breg activity, compared to either healthy controls or stem cell transplant patients without GVHD. 84
Based on the predictive value of the T1/T2 ratio (an indirect measure of Tr B cell cytokines) 2 years post‐transplant, we prospectively examined B cell subsets and their cytokines as early predictors of rejection and subsequent transplant outcome. 85 In a preliminary analysis of 160 patients who underwent serial biopsies, we found that the T1 B cell IL‐10/TNFα ratio at 3 months outperformed T1 B cell frequency, T1/T2 ratio, or Tr B cell IL‐10 as a strong predictor of any acute rejection within the first post‐transplant year (ROC AUC ~0.9). Importantly, in patients with normal surveillance biopsies at 3 months, this marker predicted rejection later in the first year with an average lead time of ~8 months (ROC AUC 0.85‐0.9). Moreover, a low T1 B cell IL‐10/TNFα ratio at 3 months was associated with decreased long‐term allograft function (GFR) and allograft survival in patients whether or not they had early rejection on 3‐month surveillance biopsy. Addition of anti‐TNF to B cells during overnight stimulation increased their IL‐10 expression and IL‐10/TNFα ratio, and augmented their in vitro Breg function. Thus, the T1 B cell IL‐10/TNFα ratio not only provides prognostic information but may provide insights into an immunoregulatory imbalance in high‐risk patients that can be restored using anti‐TNF, a drug that has not been typically used in the allograft setting.
While Bregs are enriched in Tr B cells, we do not believe that this subset is responsible for all Breg activity. In support of this argument, inhibition of Beff cells (eg, anti‐TNF) uncovers in vitro Breg activity in the much larger naïve and memory B cell populations. As in mice, it is likely that human IL‐10+ B cells present in various B cell populations all contribute to Breg activity. Why Tr B cells, which are short‐lived and either undergo cell death or maturation into naïve B cells, provide the most sensitive read‐out of a patient's immune set‐point, is not clear. Whether Tr B cells maintain their cytokine expression profile and suppressive function as they differentiate into mature B cells and plasmablasts in the SLO or parenchymal tissues is unknown. In this regard, Matsumoto and colleagues have shown that human plasmablasts differentiated from immature Tr B cells in vitro secrete significantly more IL‐10 and have a distinct phenotype from those derived from the differentiation of either mature naïve or memory B cells. 31 Regardless, assessment of Breg/Beff balance in transplant recipients may allow for personalized immunosuppression based on risk stratification and might pave the way for new immunosuppressive strategies that enhance Bregs to improve clinical outcomes.
Bregs as biomarkers for transplant tolerance and the confounding effects of immunosuppression
Outside of experimental protocols attempting to induce tolerance, solid‐organ transplantation remains dependent on life‐long immunosuppression. With the exception of liver transplantation (where up to 30% of patients might be able to be weaned off of immunosuppression), other solid‐organ transplants are eventually rejected once immunosuppression is stopped. However, worldwide, a small number of renal transplant recipients have maintained stable function despite discontinuation or withdrawal of immunosuppression. In attempts to gain insights into their spontaneous state of clinical tolerance, these patients have been the subject of several studies. 86 , 87 , 88 , 89 , 90 Companion studies of European and North American kidney transplant patients showed that those with tolerance had an increase in total B cells and Tr B cells, and increased Tr B cell IL‐10 expression compared with a group of transplant patients who remained on immunosuppression. 87 , 88 , 91 , 92
The comparison of tolerant transplant patients (off of immunosuppression) to transplant patients on chronic immunosuppression, raises immunosuppression as a potential confounder in these studies. Indeed, Hernandez‐Fuentes and colleagues showed that kidney transplant patients maintained on immunosuppression with either prednisone or azathioprine had a dose‐dependent reduction in B cells and Tr B cells in their peripheral blood. 93 , 94 Few differences were noted between tolerant patients and healthy controls. Amongst findings that did differ from healthy controls, tolerant patients had a greater frequency of granzyme B–expressing plasma cells that suppressed T cell proliferation in vitro. 23 Taken together, an increase in Bregs appears common to studies of spontaneously tolerant kidney transplant patients, however, the confounding effects of immunosuppression limit interpretation. As a further concern, in a prospective study, liver transplant patients who were or were not successfully weaned off of immunosuppression, had no differences in total or Tr B cells. 95 Thus, the “Breg profile” seen in spontaneously tolerant kidney transplant patients does not appear to be broadly applicable.
The effects of immunosuppressive agents on Bregs
Given evidence in both animal models and in humans that Breg/Beff balance may play an important role modulating the immune response, strategies to expand Bregs (or inhibit Beff cells) might promote allograft survival. As discussed above, murine studies show that anti‐TIM‐1 specifically expands Bregs in vivo. 2 Yoshizaki and colleagues showed that murine Bregs could be expanded in vitro using a cocktail of CD40L, Blys, IL‐4, and IL‐21, and such expanded cells maintained their suppressive activity. 42 However, extension of these findings to humans has thus far, been limited. Menon and colleagues showed that incubation of B cells from healthy donors with autologous plasmacytoid dendritic cells in the presence of TLR‐9 agonist CpG‐C over 3 days, resulted in a 1.5‐ to 2‐fold expansion of Tr B cells that were 4‐fold enriched for IL‐10. 14 Importantly, these expanded Tr B cells retained their T cell–suppressive activity in vitro. Unfortunately, in vitro Tr B cell proliferation and suppressive capacity was lost in patients with SLE. The response of B cells obtained from patients with end‐stage organ disease or those already on immunosuppression is not known. Further studies will be required to determine whether ex vivo expansion of Bregs for cell therapy is feasible.
Given their significant role in transplantation, it is important to consider the possible impact of therapeutic agents commonly used in clinical transplantation on Bregs (Table 2). Initial therapy typically utilizes intensive “induction” immunosuppression to block early acute rejection and ensure initial engraftment of a transplanted organ. Induction agents are typically antibody‐based lymphocyte depleting agents (such as anti‐thymocyte globulin or anti‐CD52) or inhibitors of growth signals (eg, anti‐CD25). After this initial therapy, less intensive chronic “maintenance” immunosuppression generally utilizes small molecules such as calcineurin inhibitors (CNIs), mTOR inhibitors, antimetabolites, and glucocorticoids. While the effects these agents on B cells or subsets have been examined, few studies have specifically addressed their effects on Bregs (summarized in Table 2). Amongst induction agents, Alemtuzumab (anti‐CD52) profoundly depletes peripheral B cells along with T cells. T cell reconstitution occurs slowly via homeostatic proliferation characterized by a persistent memory phenotype. In contrast, B cells return towards baseline by 3 months and rebound to 165% of baseline by 12 months. 96 B cell repopulation is characterized by predominance of Tr B cells through the first year. 75 , 96 , 97 Memory B cells are significantly decreased for several years. Initial predominance of Tr B cells during B cell repopulation is also seen after Thymoglobulin treatment. 98 Basiliximab (anti‐CD25) induction does not deplete peripheral B cells, but results in an increase in B memory cells. 99 , 100 , 101 Unfortunately, cytokine expression by these reconstituting B cells after induction therapy has not been examined closely enough to determine whether these therapies enhance or inhibit Breg activity.

| Immunosuppressive agent | Mechanism of action | Effect on B cells in vivo | Refs |
|---|---|---|---|
| Induction agents | |||
| Campath‐1H (Anti‐CD52) | Lymphocyte Depletion (B and T cells) | B cell repopulation with increased Tr B cells and reduced memory B cells | 75, 96, 97 |
| Thymoglobulin (polyclonal rabbit anti‐human thymocyte globulin) | Depletion of T cells as well as other leukocytes | B cell repopulation with increased Tr B cells | 98 |
| Basiliximab (anti‐CD25) | Blocks high affinity IL‐2R | Increase in total B and memory B cells. no effect on Tr B cells. | 100 |
| Maintenance immunosuppression | |||
| Cyclosporine A(CNI inhibitor) | inhibits calcineurin and blocks downstream NFAT signaling | Reduced Tr B cell number and IL‐10 expression | 102, 103 |
| Tacrolimus (CNI inhibitor) | inhibits calcineurin and blocks downstream NFAT signaling | No effect on B cells/subsets | 93, 94 |
| Prednisolone (corticosteroid) | Broad‐spectrum anti‐inflammatory. |
Reduces total, naïve, and Tr B cells in renal transplant patients Slows B cell reconstitution after bone marrow transplantation. | 93, 94, 111 |
| Mycophenolate (Antimetabolite) | Inhibits inosine monophosphate dehydrogenase (IMPDH), required for purine synthesis in lymphocytes | No effect on B cells/subsets | 93, 94 |
| Azathioprine (6‐mercaptopurine analog) | inhibits purine synthesis | Reduces total, naïve, and Tr B cells. | 93, 94 |
| Sirolimus and Everolimus (mTOR inhibitors) | lymphocyte inhibition |
de novo: reduction in Tr B cells r conversion: increases Tr B cells | 102, 104 |
|
Belatacept (high affinity CTLA‐4‐Ig) | selective T‐cell co‐stimulation blocker | Increases Tr B cells and B cell IL‐10 expression. Reduces B cell differentiation into plasmablasts, | 105, 106, 107 |
Many of the maintenance immunosuppressive agents affect Tr B cells. For example, prednisone and azathioprine cause a dose‐dependent reduction in the number of Tr B cells. 93 , 94 Azathioprine has been largely replaced by another antimetabolite, mycophenolic acid, which has no significant effect on total or Tr B cell number. The CNI, cyclosporine A, reduces both Tr B number and B cell IL‐10 expression. In contrast, the more widely used CNI, tacrolimus, has no demonstrable effect on these cells, suggesting that the effect of cyclosporine A on B cells is not due to calcineurin inhibition. 93 , 94 , 102 , 103 There are conflicting reports on the effects of the mTOR inhibitor, sirolimus, on Bregs. In one report, renal transplant recipients placed on sirolimus at the time of transplantation had reduced Tr B cells, while, a second report showed that liver allograft recipients converted from tacrolimus to sirolimus had increased Tr B cells and B cell expression of IL‐10 and TGF‐β. 102 , 104 Finally, the selective T cell co‐stimulation blocker, Belatacept (CTLA‐4‐Ig), increased the frequency of IL‐10+ B cells and Tr B cells, while reducing B cell differentiation into plasmablasts, when compared to patients treated with tacrolimus. 105 , 106 , 107 Thus, many immunosuppressive agents routinely used in the clinical management of transplant patients influence B cells and Tr B cells, but studies examining cytokine expression by these cells are insufficient. Identification of combinations of agents that might promote, rather than inhibit Bregs are needed. Future studies examining Bregs, as prognostic or diagnostic markers in transplantation, should consider the effects of different immunosuppressive agents on Breg phenotype and function.
Several immunomodulatory agents not routinely used in clinical transplantation have an apparent effect on Breg number and activity. For example, in a pilot Phase 2 randomized controlled trial, Belimumab (anti‐BAFF) was examined as an induction agent targeting B cell activation. Although Belimumab had no demonstrable effect on allograft outcomes in this small study, this agent was shown to enhance B cell IL‐10 and increase the ratio of their IL‐10/IL‐6 expression. 108 Furthermore, in patients with RA, anti‐TNF has been shown to increase the frequency of IL‐10+ B cells. 109 As noted above, preliminary data from our group demonstrate that anti‐TNF increases both B cell IL‐10/TNFα ratio and their suppressive activity in vitro. Together, these data suggest that anti‐TNF may specifically address the relative deficiency of Bregs in high‐risk transplant patients. While the effect of IL‐6R blockade on IL‐10+ B cells is not known, this agent increased Tr B cells while decreasing memory B cells in patients with RA. 110 These preliminary studies suggest that some immunotherapeutic agents might actually increase Bregs. Further study will be required to determine how these, or other experimental agents, might be used to optimally expand Bregs in vivo to promote allograft survival.
CONCLUSION
Bregs have a profound influence on alloimmune, as well as autoimmune, tumor, and antimicrobial immune responses. Their ability to inhibit both innate and adaptive immunity and promote Treg expansion underlies their key role in the regulation of immunity. Specific deletion of murine TIM‐1 results in spontaneous systemic autoimmunity ‐ highlighting the role of Bregs in self‐tolerance. The more dramatic effect observed following specific B cell deletion of TIM‐1 compared to IL‐10 or BLIMP‐1 is likely due to the fact that TIM‐1 signaling regulates expression of an array of inhibitory cytokines and co‐inhibitory molecules on B cells rather than a single inhibitory pathway or cell type. It remains possible that other signaling pathways or sets of transcription factors will identify other subsets of B cells with distinct or overlapping regulatory activity that are involved in other aspects of the immune response. The lack of a definitive marker for Bregs is even more problematic in humans where the role of inclusive markers like TIM‐1 and CD9 are not well established. In both humans and mice, Bregs are distributed across all canonical B cell subsets. The specific role played by Bregs identified in each of these subsets remains unclear. Identification of Bregs or Breg/Beff balance is best achieved by the relative expression of anti‐ vs. pro‐inflammatory cytokines like IL‐10 and TNFα. Accumulating evidence suggests that assessment of Bregs, and in particular, Breg/Beff balance, may identify patients at risk for rejection and poor transplant outcomes. Early identification of high‐risk individuals by the use of such biomarkers could pave the way for personalized immunosuppression in transplant recipients. Clinical protocols that can expand B cells (in vivo or in vitro) that are highly enriched for IL‐10 (or other anti‐inflammatory molecules), while reducing Beff cells/pro‐inflammatory cytokines, might improve clinical outcomes in allograft recipients at high immunological risk.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest related to the work presented in this article.
ACKNOWLEDGEMENTS
Supported by grants from NIH (NIH R01 AI114587, NIH P01 AI129880).
REFERENCES
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
 Regulatory B cells: TIM‐1, transplant tolerance, and rejection
Regulatory B cells: TIM‐1, transplant tolerance, and rejection
