
- Altmetric
Metal–halide perovskites transformed optoelectronics research and development during the past decade. They have also gained a foothold in photocatalytic and photoelectrochemical processes recently, but their sensitivity to the most commonly applied solvents and electrolytes together with their susceptibility to photocorrosion hinders such applications. Understanding the elementary steps of photocorrosion of these materials can aid the endeavor of realizing stable devices. In this Perspective, we discuss both thermodynamic and kinetic aspects of photocorrosion processes occurring at the interface of perovskite photocatalysts and photoelectrodes with different electrolytes. We show how combined in situ and operando electrochemical techniques can reveal the underlying mechanisms. Finally, we also discuss emerging strategies to mitigate photocorrosion (such as surface protection, materials and electrolyte engineering, etc.).
Introduction
Photovoltaic (PV), photocatalytic (PC), and photoelectrochemical (PEC) systems offer the promise to efficiently convert solar energy either directly to electricity or to industrially relevant chemicals and fuels.1 The active component is a semiconductor (SC) that can generate free charge carriers under illumination with higher energy than the bandgap (hv ≥ EBG). The major differentiator between PV and PC/PEC applications is the type of interfaces employed to extract and utilize these charge carriers. In PC/PEC devices, a SC/liquid interface is present that adds more functionality (and complexity) compared to the solid/solid interfaces in PV technologies. Even though the pioneering studies on illuminated SC/liquid interfaces date back to the 1970s, an industrially relevant photocatalyst/photoelectrode still remains elusive (apart from the use of TiO2-coated self-cleaning surfaces).2
Metal-halide perovskites (referred to as perovskites) possess optoelectronic properties that makes them ideal in solar energy harvesting, such as large extinction coefficient,3,4 large carrier diffusion length and lifetime,5,6 tunable bandgap,7,8 and mild synthesis conditions.9 Perovskite PVs have already reached a certified efficiency of 25.5%, which can be boosted to 29.1% when applied in tandem with Si.10 This performance, however, has not been translated to their photocatalyst/photoelectrode counterparts.11 Unfortunately, perovskites are extremely sensitive to most environmental factors (e.g., oxygen, moisture, heat, UV light, and especially the combination of these),12 and this instability still inhibits and complicates their practical use (even in the field of PVs). Degradation of perovskites is generally considered to be a surface- or interface-initiated process that propagates toward the bulk material.13,14 So far, the composition and morphology of the perovskite layer (grain boundaries),15 choice of device constituents (charge extraction, electrode, and encapsulation layers)16 and the passivation of various interfacial defects were scrutinized in this regard.17 Charge extraction layer/perovskite interfaces also influence the corrosion pathways.14,18 UV light activated surface trap states of TiO2,19 the surface hydroxyl groups of ZnO,20 or the interaction with the dopants from the organic hole-extraction layers are all relevant examples.21 The effect of these factors on the stability and performance of PC/PEC systems is yet to be understood, not even mentioning the role of liquid electrolyte present.
Decomposition pathways of perovskites are regarded as purely chemical in nature, involving at least one external reactant species (such as H2O or O2).18 Light can further accelerate the decomposition process (by photogenerated charge carriers) or even open additional degradation pathways (that involve redox reactions).22,23 Furthermore, under operating conditions, the presence of electric field and the accumulation of charge carriers at different interfaces cannot be neglected.14,24,25 Notably, significant ion traffic was observed even in the case of solid/solid interfaces. While the perovskite constituents often migrate deep into the charge extraction layers, reactive degradation products (such as I2) can corrode the back contacts, leading to device failure.26,27 Simultaneously, the organic cations (such as CH3NH3+ (MA+)) can be electrochemically reduced forming volatile products.25 In the case of PC/PEC devices, a further complication arises because of mass transfer through the SC/liquid junction. Specifically, migrating ions can leave the perovskite lattice and dissolve in the solution, making the light-driven processes irreversible.28−31 Combined electrochemical techniques are powerful tools to study these corrosion events in a fast and reliable manner. Once the elementary processes are uncovered, effective corrosion-mitigation strategies can be developed.
While photocorrosion studies on perovskites are still absent, much can be learned from precedent work on other SCs.32 During corrosion, either complete self-decomposition or the formation of active or inactive surface layers can occur. We can approach the underlying processes from a thermodynamics perspective (i.e., whether it can happen or not); however, the convoluted reaction kinetics (i.e., multiple steps, chemical transitions) must also be considered. Corrosion events can be divided into four categories: (i) chemical, where no net charge transfer occurs at the interface; (ii) electrochemical, where the selectively injected charge carriers induce corrosion; (iii) photoelectrochemical, where the photoexcited minority carriers are predominantly responsible for the corrosion; (iv) photochemical, where both the minority and majority charge carriers can induce chemical changes. In the case of photochemical corrosion there is no external driving force that removes the majority carriers (as opposed to photoelectrochemical corrosion). Therefore, a larger fraction of majority carriers can reach the photocatalyst surface and can induce corrosion processes.
In this Perspective, we discuss the last three corrosion processes from the above list, highlighting the peculiar features of perovskites. First, we present some general thermodynamic and kinetics aspects of photocorrosion. Selected examples of corrosion processes of perovskite photocatalysts and photoelectrodes form the main body of the article, where photo(electro)catalytic and photo(electro)synthetic examples are both scrutinized. This is followed by a compilation of in situ/operando methods, which provide mechanistic insights with spatial and temporal resolution. Finally, we discuss possible mitigation strategies, which also outline future research and development avenues.
General Considerations
Thermodynamics of Photocorrosion
During electrochemical charge carrier injection, the effect of one type of charge carrier (i.e., electron or hole) can be probed selectively. In stark contrast, under light illumination, both electrons and holes are generated in SCs simultaneously, and both can induce (photo)corrosion. By comparing the electrochemical potential of these charge carriers (conduction band (CB) position for electrons and valence band (VB) position for holes) with the redox potential of the respective decomposition reaction, the stability of the SC can be evaluated.33−35 During anodic (oxidative) corrosion reactions, the potential of holes should be more negative (on the vacuum scale), than the decomposition potential of the SC (eq 1). Meanwhile, for cathodic (reductive) corrosion reactions, the potential of electrons should be less negative than the decomposition potential (eq 2). Figure 1 illustrates the four different scenarios: (A) stable against overall photocorrosion, (B) susceptible to cathodic corrosion, (C) susceptible to anodic corrosion, (D) sensitive toward both cathodic and anodic corrosions. Based on this classification, the prototypical MAPbI3 likely falls into the B category, where the direct decomposition proceeds through cathodic photocorrosion.36
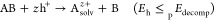
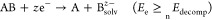

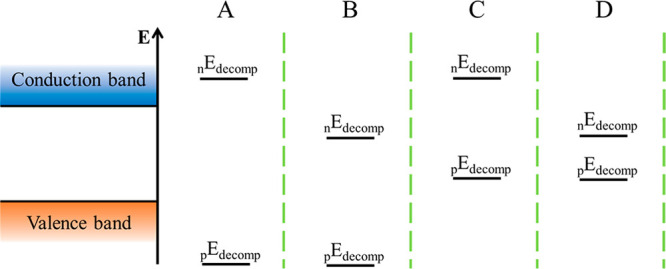
Photocorrosion stability of SCs based on the position of the decomposition potentials relative to the band edge energies. (A) Stable against photocorrosion, (B) stable against anodic but susceptible to cathodic corrosion, (C) stable against cathodic but sensitive to anodic corrosion, and (D) sensitive toward both cathodic and anodic corrosion. Adapted from ref (33) with permission from Elsevier, copyright 1977. nE and pE stands for negative and positive charge induced decomposition process, respectively.
The band diagram of a p-type SC electrode (category B, common case for perovskites) is shown in Figure 2. Under equilibrium conditions (without illumination), the Fermi level (EF) of the SC and the redox potential of the electrolyte (Eredox) equalizes (Figure 2A). Under illumination, the quasi-Fermi level of electrons deviates from the equilibrium value (Figure 2B). In the case of a slow redox reaction the surface concentration of electrons, and thus the quasi-Fermi levels are only slightly affected. In this situation, both the cathodic corrosion and the redox reaction can occur simultaneously. Therefore, stability can only be achieved if the corrosion kinetics is slow (scenario 1). If the redox reaction is fast, however, it can consume the surface electrons to such an extent, that the quasi-Fermi level drops below the electrochemical potential necessary to induce cathodic corrosion (scenario 2).

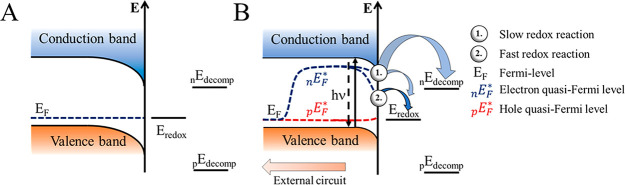
(A) p-type SC electrode in the dark in contact with an electrolyte with a redox active species (Eredox) present, showing a depletion layer with arbitrary depth. (B) p-type SC electrode under illumination and in contact with an electrolyte with a redox active species, where the redox reaction is (1) slow and (2) fast. Adapted from ref (35) with permission from Royal Society of Chemistry.
In realistic situations, perovskite surfaces are not perfectly flat on the atomic scale and contain various surface structures (e.g., grain boundaries, dislocations, terraces, and valleys).37 Such structural features are pronounced in nanostructured materials. As the coordination of the atoms occupying these surface imperfections is lower, compared to the ones situated in the bulk, they become thermodynamically more susceptible to corrosion. These sites can be the initiators of the corrosion processes, which proceed in an accelerated fashion as the surface becomes more defective in the process. It is difficult to predict the exact corrosion potential of these sites, as it requires the complete understanding of the corrosion mechanism. Furthermore, these states can also act as recombination centers, promote dark reactions, or even cause Fermi-level pinning.35 Finally, the species present in the electrolyte can coordinate to these surface atoms weakening their back-bonds to the bulk and ultimately changing their corrosion potential.38 At the same time, nanostructured perovskites can also offer beneficial properties that increase efficiency such as (i) short or directional charge carrier collection, (ii) tunable light distribution, (iii) quantum size effects, and (iv) increased surface area.39,40
Kinetics of Photocorrosion
Different kinetic models were developed to describe the mechanism of photocorrosion and to evaluate the photostability of SCs.41 These models exclusively focus on hole-induced decomposition (anodic decomposition) and also take into account the charge carrier generation and recombination (surface or bulk) processes. As multiple participating species42,43 and interdependent steps44 can be involved in the overall reaction scheme, it often becomes complex. Models rely on the description of the stepwise breaking of the back-bonds of surface atoms (following charge carrier generation). In the initial step, charge carriers generated in the bulk are captured by these back-bonds. In a subsequent chemical step, a component of the electrolyte stabilizes the radical-like intermediate. The capture of a second charge carrier results in the cleavage of the back bonds of the surface atoms, resulting in decomposition. Some sophisticated models consider these intermediates to be mobile on the surface.42,43 These elaborate models have important implications for perovskites, where light irradiation,45−47 applied electrical bias,48−50 or heat51 induce halide ion migration even within the perovskite lattice. Further complications arise when solution chemistry considerations (e.g., complexation, solution phase equilibria, multiple charge carrier redox processes) are taken into account.43,52−55 A detailed discussion of the different steps is given in the SI. Although these studies were exclusively conducted on single crystal surfaces, the profound effect of surface imperfections on the corrosion rate was realized early on.52,56,57
The stabilization effect of redox couples can also be included in kinetic models, as either competing reactions for surface charge carriers or reactants that regenerate the partially broken surface bonds. Generally, the stabilization efficiency of a given redox pair is light intensity dependent.44,58,59 As the light intensity increases, the branching ratio between photocorrosion and the redox reaction shifts in favor of the decomposition process. In comparison, the stabilization efficiency of redox couples in the case of layered 2D materials was independent from the light intensity.56 This behavior was explained by (i) the reversibility of the first charge carrier capture step and (ii) the fact that orbitals involved in driving the redox reaction, were not involved in bond formation.34 Similar enhanced stability was found for 2D perovskites compared to their 3D counterparts.60
Importantly, redox couples cannot be used (unless as mediators) in solar chemical or fuel production scenarios, as their presence will also suppress the desired reaction. In these situations, the kinetics (i.e., the branching ratio) will determine the degree of suppression. Therefore, the “holy grail” would be to drive an industrially relevant redox reaction that simultaneously is able to suppress photocorrosion. The thermodynamic criteria toward this reaction (i.e., redox potential) is ultimately limited by (i) the band positions, (ii) the corrosion potentials, and (iii) the bandgap of the perovskite.61 At this point, it is important to distinguish between photo(electro)catalytic or photo(electro)synthetic processes.2,62 Light-driven reactions that are thermodynamically “downhill” (ΔG < 0) can be termed photo(electro)catalytic, while thermodynamically “uphill” (ΔG < 0) reactions are considered photo(electro)synthetic processes. In the case of photo(electro)catalytic systems, no net energy is stored in the formation of chemical bonds, the role of light is only to accelerate the otherwise sluggish reaction.
Specifics of Perovskite Photocorrosion
Perovskites are nonconventional intrinsic semiconductors. Through the introduction of cation vacancies (lead and methylammonium) p-type behavior can be achieved, while from the presence of anion vacancies (iodide), n-type behavior can be achieved.63 These defect sites can also be formed at the charge extraction layer/perovskite interfaces during the operation of solar energy conversion devices, pointing toward the active ion conducting nature of these materials.64 The anion in the perovskite lattice affects the activation energy of ion migration, because of the difference in the Pb–X bond strength and the vacancy density (which are the active participants in ion migration).65 For example, halide ion migration is more pronounced in MAPbI3 compared to MAPbBr3. Interestingly, the migration of MA+ is also faster as a result of the expanded lattice of MAPbI3.65 While these mobile ions in the “soft” perovskite lattice are the main reasons behind the instability, they also allow the synthesis of perovskites with defect-free bulk structure using solution phase processes.9 Furthermore, these mobile ions are found to be the key reason for the self-healing properties of perovskites.66 The ambipolar charge carrier transport (with charge carrier diffusion length in the micrometer range) allows efficient electron or hole extraction from the bulk. Therefore, both oxidation and reduction reactions can be driven on perovskite surfaces, especially when appropriate charge extraction interfaces are used.67
Corrosion of Perovskite Photocatalysts
To enable efficient PC or photosynthetic reactions, photogenerated charge carriers must be efficiently transported to catalytic sites where they must live long enough (without recombining) to be consumed in the catalytic reactions. Consequently, the long charge carrier diffusion length5,6 and lifetime68 coupled with the suppressed surface recombination69 of perovskites sparked an interest in their possible PC application. Furthermore, by incorporation of different anions into the perovskite structure, the band edge position can be tailored, to activate (oxidize or reduce) different organic substrates. This allows fine-tuning of reaction mechanisms.70,71 The stability of perovskite-based PCs has been exclusively assessed through monitoring the product output and occasionally performing postrun measurements (X-ray diffraction (XRD), energy-dispersive X-ray microanalysis (EDX), X-ray photoelectron spectroscopy (XPS)).
CO2 Reduction
In PC CO2 reduction, mild polarity solvents such as ethyl-acetate72−78 or acetonitrile75−77 have been used as the media to ensure the stability of the perovskites. A further advantage is that CO2 is highly soluble in these solvents (240 mM in ethyl acetate72 and 270 mM in acetonitrile79). Interestingly, small quantities of water (<0.3% v/v) was also added as a proton source and hole scavenger, without seemingly compromising the stability. The other half reaction in such PC systems is oxygen evolution from the added water.72 This aspect is often overlooked, although both water and oxygen can compromise the stability of perovskites. Interestingly, the catalytic performance was relatively stable for hours.72−78
PC CO2 reduction was boosted by tailoring the shape of CsPbBr3 quantum dots. Through the introduction of thermodynamically less favored facets, enhanced CO and CH4 generation was detected. After PC experiments, the facet distribution was altered, yet the catalytic output remained stable.73 Fe(II) incorporation into CsPbBr3 was also performed to control the product distribution, but most of the Fe(II) leached out from the perovskite structure during the experiment.74 In the case of a MAPbI3/Fe-based metal–organic framework (MOF), the hybrid material was more stable than the MOF itself.
A heterojunction of g-C3N4 and CsPbBr3 nanocubes (NCs) was prepared to enhance the efficiency of charge separation. Poor recyclability was observed when the hybrid PCs were prepared via simple physical mixing.76 When the CsPbBr3 NCs were anchored on amino functionalized g-C3N4 through N–Br bonds, better adhesion and recyclability were achieved.77 Similar grafting strategies were employed in the case of graphene-oxide/CsPbBr3 NC72 and MXene/CsPbBr3.78 A common feature of these studies is the slight decomposition of the ethyl acetate solvent or the ligand shell, which might form CO or CH4 during the PC reaction.74,76,77 While considered “insignificant” in the studies, the resulting reactive reaction products can be either participants in the CO2R reaction (like the scavengers in water splitting80) or detected as reaction products (e.g., CO and CH4).76 The contribution of these unintended side reactions shall be quantified. Furthermore, there are no experiments on the formation of liquid phase products. Currently isotope labeling studies are only carried out in a small fraction of the studies.75−77 Such protocols for both reduction (C13O2)81 and oxidation (H2O18)82 reactions are well-established. Furthermore, the PC CO2R mechanism on perovskite NCs is still unknown, and simply observing steady product generation is not a sufficient indicator of stability.
PC Reactions in Organic Synthesis
A perovskite-friendly environment has to be ensured during PC organic syntheses as well. Different co-reactants and various intermediate products can all compromise the stability of the active material.71,83 Highly nonpolar solvents have to be used (e.g., dichloromethane (DCM), hexane, toluene) that are rigorously purified from water and stabilizing agents.70 Interestingly, upon prolonged illumination (>24 h), the decomposition of DCM can yield chloride ions, which also participate in halide ion exchange with the perovskite.71,84 Even easier halide ion exchange occurs using the halide salt of organic reactants.71 This can change the band edges of perovskite NCs during the PC reaction in situ, opening new reaction pathways.71 Acid binding on perovskite surfaces can passivate surface defects that participate as active sites in the organic reaction.85 The C–C bond formation between tertiary amines and aldehydes is dependent on the acidity of compounds in the reaction mixture.71 Furthermore, smaller sized perovskite NCs had higher initial reaction rate (due to the increased surface area) but their catalytic activity diminished quickly.71 These alterations can be monitored following the shift in the photoluminescence peak, the UV–vis absorbance onset, and the reflections on the XRD patterns.
Various perovskite-based heterojunctions (both type II and Z-scheme) were employed for the photooxidation of benzyl alcohol to benzaldehyde.83,86,87 In the type II case, the perovskite acts as a sensitizer that injects photoexcited electrons to the CB of TiO2 (Figure 3A).83,86 These electrons react with molecular oxygen and form reactive superoxide radicals. The holes, remaining on the VB of perovskite, oxidize benzyl alcohol to carbocations. These react in a subsequent step with the superoxide radical, forming initially benzyl aldehyde and ultimately benzoic acid. In these PC reactions, only bromide-based (or chloride) perovskites are viable as the iodide-based counterparts are sensitive to the formed superoxide radical (eq 3). The superoxide radical can deprotonate the organic cation in the perovskite lattice and oxidize the iodide in the lattice forming iodine (which can be released into the solution phase). Importantly, this reaction also forms the basis of the light and oxygen induced degradation of perovskite solar cells.88
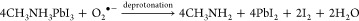

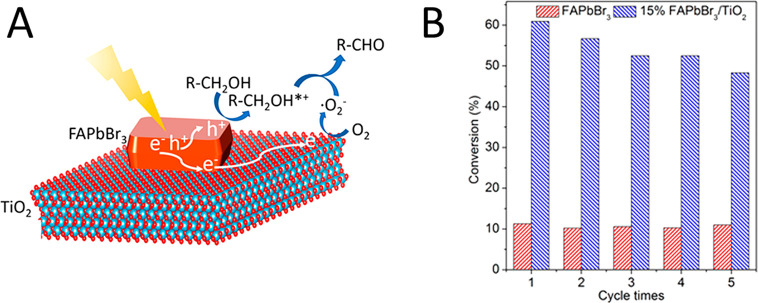
(A) Illustration of the mechanism of photocatalytic benzyl alcohol oxidation process over TiO2/FAPbBr3 heterojunction. (B) Recyclability of FAPbBr3 and 15% FAPbBr3/TiO2 in the photocatalytic oxidation of benzyl alcohol. Reproduced with permission from ref (83). Copyright 2018 American Chemical Society.
A Z-scheme heterojunction was formed in the case of Bi2WO6/FAPbBr3.87 Upon photoexcitation and subsequent vertical charge separation, the electrons in the perovskite CB drive CO2 reduction to CO, while the holes in the VB of Bi2WO6 facilitate direct benzyl alcohol oxidation, skipping the superoxide formation step. The product evolution rates did not match the expected 1:1 stoichiometry, and superoxide formation from O2 was also shown.87 Furthermore, the pristine FAPbBr3 showed signs of degradation, yielding CO, which calls for further studies. Perovskite PCs with varying amount of iodide vacancies were also evaluated in the PC degradation of organic compounds, and the beneficial role of iodide vacancies via the increased production of radical O2•– species was claimed.89
Surface termination can also play a role in the PC performance.90 When MAPbI3 was used for the PC conversion of 1,3-dihydroxyacetone to butyl lactate, MAI and PbI2 terminated surfaces were synthesized. The MAI terminated surface corroded rapidly to expose Pb(II) sites, which are photocatalytically active in the reaction. As expected, the catalytic activity initially increased but soon diminished as MAPbI3 was converted to PbI2.90 The importance of surface characteristics was further highlighted when poly(3,4-ethylenedioxythiophene) (PEDOT) was deposited on the surface of CsPbBrxI3–x NCs.91,92 When the oleic acid in the shell was replaced with methyl acetate, faster PEDOT deposition was achieved, due to the increased number of surface defects and the shorter ligand shell. The importance of the ligand shell in PC reactions was further demonstrated when the reduction of ferrocenium+ (Fc+) was only observed when the oleic acid/oleyl amine was exchanged to didodecyldimethylammonium bromide (in the case, the spontaneous reduction of Fc+ occurred).93
Hydrogen Evolution from Hydrogen Halide Solutions
When MAPbX3 interacts with water, monohydrate (eq 4) and dihydrate (eq 5) phases are formed.94 This is followed by the decomposition of the perovskite lattice, ultimately yielding insoluble PbX2.
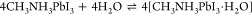
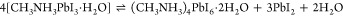

(A) Illustration of the dynamic equilibrium between solid MAPbI3 and a saturated HI solution. (B) Effect of pH and I– content on the chemical makeup of the precipitate formed during the equilibrium process. Reproduced from ref (95) with permission from Springer Nature.
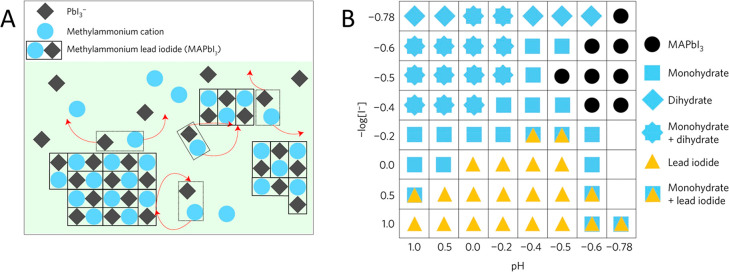
To enhance the performance (activity, selectivity, and stability) of perovskite photocatalysts in HX splitting reactions, different strategies were employed: (i) preparing mixed halide compositions,96,97 (ii) anchoring various HER catalysts (e.g., Pt,95,98,99 black-P,100 rGO,99 Ni3C,101 CoP,102 Mo2S103), or (iii) grafting additional hole extraction materials (e.g., PEDOT:PSS,104 carbonized polymer dots105) on the perovskite surface. In the case of TiO2/MAPbI3 hybrids, a distinct difference was found in both efficiency and stability between loading the Pt on the stable TiO2 or on the dynamically changing MAPbI3 surface.98 It was proposed that when the Pt is deposited on MAPbI3 and then combined with TiO2 in a subsequent step, the dissolution of the MAPbI3 surface removes the Pt catalyst. This was not the case when the Pt catalyst was predeposited on the TiO2 and then added to the MAPbI3 containing solution (Figure 5A,B). This difference was also observed in the H2 evolution rate (Figure 5C). Interestingly, no such behavior was observed in other cases, where Pt was directly deposited on MAPbI3.95,99−103 When one adapts this strategy to PEC applications,106 care must be exercised as the free-standing perovskite films can be slowly dissolved into the concentrated HI solution.98

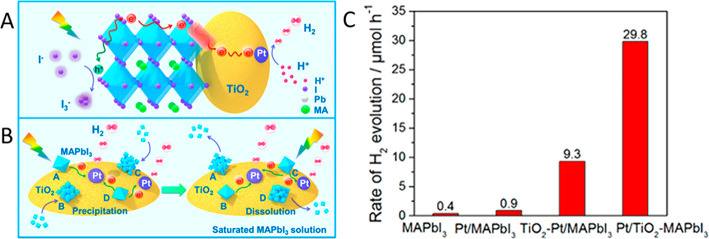
(A) Illustration of the flow of charge carriers in a MAPbI3 + Pt/TiO2 system during photocatalytic hydrogen evolution. (B) Proposed reaction scheme, where the TiO2/Pt surface acts as a temporary host for the deposition of MAPbI3 nanocrystals, where the charge transfer chain is established. (C) Rate of H2 evolution of pure MAPbI3, Pt decorated MAPbI3, TiO2–Pt/MAPbI3 (Pt on perovskite), and Pt/TiO2–MAPbI3 (Pt on TiO2) photocatalysts with illumination wavelengths λ > 420 nm. Reproduced with permission from ref (98). Copyright 2018 American Chemical Society.
Corrosion of Perovskite Photoelectrodes
The existence of a photocurrent response is not sufficient to assess the stability of a given photoelectrode and might even be misleading, as photocorrosion can be a major contributor to the photocurrent.107,108 Still, this is the widely used practice in the case of perovskite photoelectrodes. It is therefore vital to implement other characterization techniques (either ex situ or in situ) to assess their (in)stability. From the photoelectrode perspective, the initial stages of corrosion affect the surface, therefore surface-sensitive techniques (e.g., XPS) are of prime importance. As for the electrolyte solution, simple UV–vis spectroscopy can identify expelled halide species in the solution phase in micromolar concentrations.28 More sophisticated methods such as ion chromatography or inductively coupled plasma atomic emission spectroscopy (ICP-AES) can also detect degradation products in the electrolyte.109,110
PEC studies carried out on unprotected perovskite photoelectrodes are summarized in Table 1. To ensure stability, most were performed in nonaqueous media (such as DCM or ethyl acetate) in the presence of different redox couples. PEC studies using benzoquinone (BQ) were superior in terms of performance and stability, as the rapid electron transfer from the perovskite to BQ (rate of 1.0 × 1010 s–1 from CsPbBr3) can effectively suppress the cathodic photocorrosion.111 Interestingly, other redox couples that have also suitable redox potential to suppress the cathodic corrosion (see Figure 6) have not been investigated thoroughly.112 Similarly fast electron transfer rate from perovskites to different redox compounds was determined: 1.64 × 1010 s–1 to Fc+,93 0.3 × 1010 s–1 to tetracyano-ethylene,113 or 3.6 × 1011 s–1 to methylviologen (MV2+).114,115 In a similar fashion, the reduced forms of these species can accept holes from the VB of perovskites. If the binding of the redox active molecule to the surface of perovskites is strong enough and the lifetime of the charge-transfer state is long, it can participate in back-electron or hole transfer reactions.93 These studies always focus on the charge transfer from the perovskite to the redox active molecule. The fate of the other charge carrier that is left behind is often neglected, although it can also induce corrosion. Light screening effect of these redox couple containing electrolytes has to be also addressed, either by using dilute electrolytes or through the minimization of the optical path length of the PEC cell. So far, PEC cells with MAPbI3 and BQ/BQ• redox couple performed best (−5.0 mA cm–2 at −0.4 V vs Fc/Fc+ under 1 Sun).112 In comparison, lead-free tin-containing perovskites with varying halide composition (MASnBrxI3–x) showed inferior performance and stability in a similar setup.116

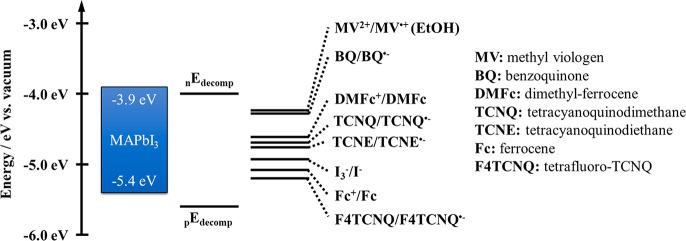
Band diagram of the prototypical perovskite MAPbI3, together with the experimentally determined corrosion potentials. Different redox couples that have suitable redox potential to suppress corrosion processes are also shown. Adapted with permission from ref (112). Copyright 2015 American Chemical Society.

| photoelectrode | reaction | electrolyte | performance | PEC stability | light source | comment | ref |
|---|---|---|---|---|---|---|---|
| MAPbI3 | BQ reduction | 0.1 M Bu4NPF6 DCM | –5.0 mA cm–2 (−0.4 V vs Fc/Fc+) | 50% at 22 h | 100 mW cm–2 AM1.5G | 30 μm layer | (112) |
| MAPbI3/PbI2 (2.5%) | BQ reduction | 0.1 M Bu4NPF6 DCM | –7.0 mA cm–2 (−0.4 V vs Fc/Fc+) | unknown | 100 mW cm–2 AM1.5G | 0–15% excess PbI2 | (123) |
| (MA)2CdCl4 | BQ reduction | 0.1 M Bu4NPF6 DCM | –0.35 mA cm–2 (−0.7 V vs Fc/Fc+) | 600 h | 100 mW cm–2 AM1.5G | EBG = 350 nm | (124) |
| MASnBrxI3–x | BQ reduction | 0.1 M Bu4NPF6 DCM | –1.0 mA cm–2 for MASnI3 (−0.7 V vs Fc/Fc+) | 50% at 40 min | 100 mW cm–2 AM1.5G | halide composition optimization | (116) |
| CNT/CsPbBrxI3–xNCs | BQ reduction | 0.1 M Bu4NPF6 DCM | –0.5 mA cm–2 (−0.4 V vs Fc/Fc+) | a | 150 mW cm–2 AM1.5G | Halide composition, carbon nanotube (CNT) and perovskite thickness optimization | (117) |
| MAPbI3 | none | 0.1 M Bu4NPF6 DCM | 0.50 μA cm–2 (unknown) | a | 100 mW cm–2 AM1.5G | positive current flow, initial rapid current decay | (102) |
| MAPbI3/CoP | none | 0.1 M Bu4NPF6 DCM | 2.00 μA cm–2 (unknown) | a | 100 mW cm–2 AM1.5G | positive current flow, initial rapid current decay | (102) |
| CsPbClxBr3–x NCs | none | 0.1 M Bu4NPF6 ethyl acetate | –4.4 μA cm–2 (unknown) | a | 200 mW cm–2 AM1.5G | (125) | |
| CsPbBr3 NCs | none | 0.1 M Bu4NPF6 ethyl acetate | 0.1 mA cm–2 (unknown) | a | 300 W Xe lamp (≥420 nm) | positive current flow | (126) |
| CsPbBr3NCs/MOF (UiO-66(NH2)) | none | 0.1 M Bu4NPF6 ethyl acetate | 0.4 mA cm–2 (unknown) | a | 300 W Xe lamp (≥420 nm) | positive current flow | (126) |
| CsPbBr3 nanocubes | none | 0.1 M Bu4NPF6 ethyl acetate | –0.18 mA cm–2(−0.4 V vs Ag/AgCl) | a | 150 mW cm–2 AM1.5G | change in PL and XRD reflection intensity | (73) |
| CsPbBr3 hexapods | none | 0.1 M Bu4NPF6 ethyl acetate | –0.10 mA cm–2 | a | 150 mW cm–2 AM1.5G | change in PL and XRD reflection intensity | (73) |
| CsPbBr3 nanocubes | none | 0.1 M Bu4NPF6 ethyl acetate | –0.05 mA cm–2 | a | 150 mW cm–2 AM1.5G | change in PL and XRD reflection intensity | (73) |
| CsPbBr3 NCs | none | 0.1 M Bu4NPF6 DCM | –30 μA cm–2 (−0.4 V vs Ag/AgCl) | a | 150 mW cm–2 (≥420 nm) | in situ chemical deposition of MO2 materials by the hydrolysis of precursors | (118) |
| CsPbBr3NCs/TiO2 | none | 0.1 M Bu4NPF6 DCM | –40 μA cm–2 | a | 150 mW cm–2 (≥420 nm) | in situ chemical deposition of MO2 materials by the hydrolysis of precursors | (118) |
| CsPbBr3–xClxNCs/SnO2 | none | 0.1 M Bu4NPF6 DCM | –60 μA cm–2 | a | 150 mW cm–2 (≥420 nm) | in situ chemical deposition of MO2 materials by the hydrolysis of precursors | (118) |
| CsPbBr3NCs/SiO2 | none | 0.1 M Bu4NPF6 DCM | –15 μA cm–2 | a | 150 mW cm–2 (≥420 nm) | in situ chemical deposition of MO2 materials by the hydrolysis of precursors | (118) |
| MAPbBr3 | CO2 reduction | 0.1 M Bu4NPF6 propylene carbonate | –3 μA cm–2 (−0.6 V vs Ag wire) | a | 100 mW cm–2 AM1.5G | unstable current response | (119) |
| GO/MAPbBr3 | CO2 reduction | 0.1 M Bu4NPF6 propylene carbonate | –5 μA cm–2 | a | 100 mW cm–2 AM1.5G | unstable current response | (119) |
| CsPbBr3 NCs | CO2 reduction | 0.1 M Bu4NPF6 ethyl acetate | –38.0 μA cm–2 (−0.4 V vs Ag/AgCl) | a | 150 mW cm–2 AM1.5G | EDX reveals Fe is leached out | (74) |
| Fe:CsPbBr3 NCs (25 at%) | CO2 reduction | 0.1 M Bu4NPF6 ethyl acetate | –120.0 μA cm–2 | a | 150 mW cm–2 AM1.5G | EDX reveals Fe is leached out | (74) |
| g-C3N4/CsPbBr3 NCs | CO2 reduction | 0.1 M Bu4NPF6 acetonitrile | –0.35 μA cm–2 (0 V vs Ag/AgCl) | a | 300 W Xe lamp (≥420 nm) | (77) | |
| CsPbBr3NCs | CO2 reduction | 0.1 M Bu4NPF6 ethyl acetate | –40 μA cm–2 (−0.4 V vs Ag/AgCl) | a | 150 mW cm–2 AM1.5G | (72) | |
| GO/CsPbBr3 NCs | CO2 reduction | 0.1 M Bu4NPF6 ethyl acetate | –50 μA cm–2 | a | 150 mW cm–2 AM1.5G | (72) | |
| CsPbBr3 NC | CO2 reduction | 0.05 M Bu4NPF6 ethyl acetate | –20 μA cm–2 (−0.2 V vs Ag/AgCl) | a | 150 mW cm–2 AM1.5G | (120) | |
| CsPbBr3 NC/a-TiO2 | CO2 reduction | 0.05 M Bu4NPF6 ethyl acetate | –200 μA cm–2 | a | 150 mW cm–2 AM1.5G | (120) | |
| CsPbBr3/Cs4PbBr6 | CO2 reduction | H2O without added electrolyte | –1.0 μA cm–2 (−0.4 V vs Ag/AgCl) | a | 100 mW cm–2 AM1.5G | perovskite suspension was measured | (127) |
| 2%Co:CsPbBr3/Cs4PbBr6 | CO2 reduction | H2O without added electrolyte | –3.0 μA cm–2 | a | 100 mW cm–2 AM1.5G | perovskite suspension was measured | (127) |
| c-TiO2/MAPbI3 | iodide oxidation | MAPbI3-saturated aqueous HI (57%) | 1.0 mA cm–2(0.14 V vs Ag/AgCl) | 8 h | 150 mW cm–2 AM1.5G | (106) | |
| c-TiO2/TiO2nanorod array/MAPbI3 | iodide oxidation | MAPbI3-saturated aqueous HI (57%) | 2.0 mA cm–2 | 8 h | 150 mW cm–2 AM1.5G | (106) | |
| MAPbI3 | H2 evolution | aqueous HI (57%) with H3PO2 | 0.75 μA (unknown) | a | 300 W Xe lamp (≥420 nm) | positive current flow | (101) |
| MAPbI3/Ni3C | H2 evolution | aqueous HI (57%) with H3PO2 | 1.50 μA (unknown) | a | 300 W Xe lamp (≥420 nm) | positive current flow | (101) |
| MAPbI3/black-P | H2 evolution | MAPbI3-saturated aqueous HI solution | 110 μA (unknown) | a | 300 mW Xe lamp (≥420 nm) | positive current flow | (100) |
| MAPbBrxI3–x | H2 evolution | mixed aqueous HBr/HI with H3PO2 | 1.75 μA cm–2 for MAPbBr0.45I2.55 (unknown) | a | 300 W Xe lamp (≥420 nm) | positive current flow | (97) |
| CsPbBr3 NCs | water reduction | 0.1 M Na2SO4, water pH = 6.8 | –3 μA cm–2 (−0.1 V vs NHE) | 6 h | 405 nm LED | initial current decay, with increasing dark current | (121) |
| CsPbBr3NCs/TiO2 | water reduction | 0.1 M Na2SO4, water pH = 6.8 | –5 μA cm–2 | 6 h | 405 nm LED | initial current decay, with increasing dark current | (121) |
| TiO2/CsPbBr3 | 2-mercapto-benzothiazole oxidation | 0.1 M Bu4NPF6 DCM | 0.15 mA cm–2 (−1.0 V vs NHE) | a | 100 mW cm–2 AM1.5G | n-type behavior, slight absorbance change after PEC | (128) |
a Not available.
Interestingly, PEC studies on perovskite photoelectrodes were only used to characterize the interaction among the constituents of the photoelectrode assemblies so far. Even during short PEC testing, some current decay was visible, which might become detrimental when translated to longer operations (Table 1). When charge-transporting layers were also employed, slightly increased current densities were observed, because of the enhanced charge carrier extraction.72,106,117−121 Importantly, the photocurrent densities are still orders of magnitude smaller, compared to perovskite solar cells (∼20 mA cm–2 with 1 Sun illumination (100 mW cm–2 AM1.5G)) for all but two entries in Table 1. This is especially striking for the CO2R and HER studies (1–100 μA cm–2). Sluggish reaction kinetics or mass transport limitations alone cannot explain this behavior, as perovskite photoelectrodes with deposited catalysts also show similarly low performance. This large difference is absent in the case of other photoelectrode materials that transitioned from solar cell applications (e.g., Si and GaAs). In many studies, the exact origin of the photocurrent is unknown, as no redox couples were added to the electrolyte, and reaction products were not analyzed. In these instances, several processes can be responsible for the photocurrent such as (i) slow corrosion of the perovskite layer, (ii) decomposition of the ligand shell, or (iii) decomposition of trace amount of water or impurities in the electrolyte. In cases where CO2 saturated electrolytes are employed, reference measurements should be carried out (under Ar-saturated conditions) as a minimum, but monitoring of the products (with isotopic labeling studies) is preferred.81 As CO2 often binds to the surface of the perovskite, the possibility of carbonate formation should also be addressed.122 As noted earlier, perovskite NCs are generally stabilized with nonconductive organic molecules; therefore charge transfer through this shell is often hindered and can contribute to photocurrent losses.114
Perovskite-saturated hydrogen-halide electrolytes were also studied in the PEC scenario.97,100,101,106 As the electrolyte is complex and contains different species, it is difficult to assign the photocurrent to any given process. Furthermore, the photoelectrode surface is constantly dissolving and redepositing in this media. It was also shown that perovskite layers instantly dissolved when immersed in this media.98
In mixed halide perovskites the bandgap monotonously decreases in the series of Cl– > Br– > I–. For MAPbBrxI3–x, this translates to a bandgap variation between 1.6 eV (x = 0) and 2.3 eV (x = 3). Continuous light irradiation results in phase segregation, where iodide- and bromide-rich domains form (eq 6).129 This process is not confined to the iodide/bromide system but was also encountered for the bromide/chloride composition.130 Interestingly, this process is reversible, as the original mixed phase can be restored when segregated devices are stored in the dark and no solid/liquid interface is present.28,29,45
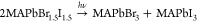
Electrochemical Methods to Diagnose Corrosion
Electrochemistry allows selective electron or hole injection to the electrode material, which can help to uncover the elementary steps of the photocorrosion processes.28,36,137 So far, the stability of unprotected perovskite electrodes was assessed through spectroelectrochemical experiments without external illumination.28,36,137 The sensitivity of perovskite electrodes toward both electrons36,137 and holes28,36 was demonstrated. When electrons were injected to MABr-doped FAPbI3 electrodes, selective dissolution was observed at the grain boundaries, followed by seemingly random intragrain pitting (Figure 7). The chemical composition of the grain boundaries were identified as a FAPbI3·DMSO complex that can be easily corroded. Planes terminated in Pb2+ were identified as corrosion sites, and the formation of β-PbO through the oxidation of metallic Pb intermediates was proposed. This corrosion pathway was significantly suppressed by adding BQ as an electron scavenger.137

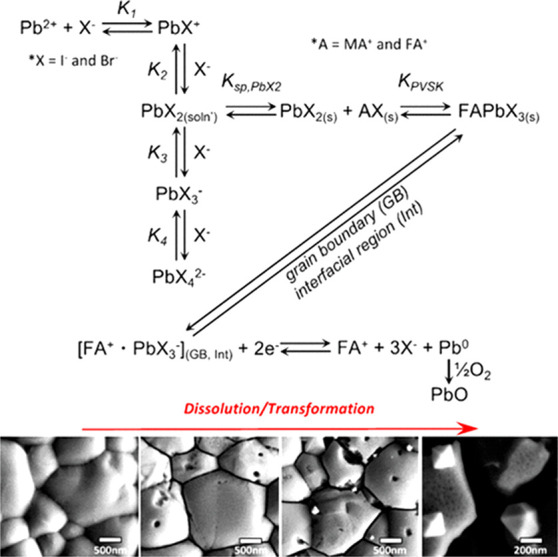
Summary of the reaction mechanism of formation and electrochemical corrosion of FAPbI3 perovskite electrodes. SEM images showing the initial removal of the material located at the grain boundary, followed by pitting and subsequent destruction of the grains. Reproduced with permission from ref (137). Copyright 2017 American Chemical Society.
Electrochemical hole injection to CsPbBr3 and MAPbI3 can oxidize the halide anions in the perovskite lattice, resulting in its decomposition.36 The situation becomes more complex in the case of mixed halide perovskites. Controlled hole injection to MAPbBr1.5I1.5 reveled two distinct processes.28 The initial step of hole-trapping is not accompanied by any spectral changes (region 1 in Figure 8A). When sufficient electrochemical potential is reached to achieve hole injection into the VB of MAPbBr1.5I1.5 (region 2 in Figure 8A), however, the absorbance decreased and the bandgap changed. Hole injection gradually expelled iodide from the perovskite lattice into the solution phase, ultimately yielding pure MAPbBr3 (Figure 8B), as described by eq 7.28,29 The process possesses the hallmarks of electrochemical corrosion (Figure 8C–E). Similarly to electron injection, the corrosion also propagates from the grain boundaries.
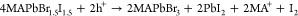

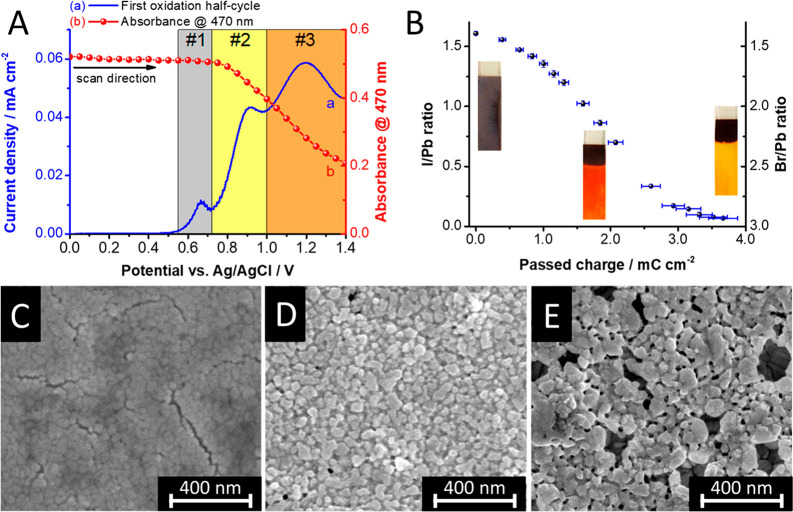
(A) Linear sweep voltammogram of FTO/MAPbBr1.5I1.5 film together with the absorbance change at 470 nm. Regions 1 and 2 show hole-trapping and injection to the VB, respectively. (B) Alteration of the perovskite composition as a function of passed charge during potentiostatic treatment (E = 0.9 V vs Ag/AgCl). (C–E) Top-down SEM images recorded at different stages of the hole induced corrosion. Reproduced with permission from ref (28). Copyright 2019 American Chemical Society.
While detailed mechanistic studies on the photocorrosion of perovskite photoelectrodes are still lacking, different tools are available to overcome this shortcoming (see a summary in Table 2, and detailed description in the SI). Briefly, the branching ratio between corrosion and a given redox reaction can be determined by using a rotating ring disk electrode method to properly select the appropriate redox active components.38,42,58,59,138−146 The response of a photoelectrode to periodic alteration of the incident light (studied by intensity modulated photocurrent spectroscopy, IMPS) can reveal the underlying kinetics and competition between surface recombination and the photocorrosion reaction.147,148 Operando electrochemical techniques offer the possibility to monitor the accompanying chemical changes and evolution of corrosion intermediates during PEC reaction (either on the surface of the electrode or in the solution phase).109,110,149−156 Furthermore, accelerated stability tests can be carried out by using ultrafast transient spectroscopic techniques, where the (sub-)femtosecond-pulsed irradiation can greatly accelerate the corrosion process.157 Even subtle changes in the quality of the interfaces (trap state formation, presence of corrosion intermediates) might have an immediate impact on the charge carrier dynamics of the photoelectrode.158,159 Careful analysis can also reveal the role of dynamically forming and recovering trap states in corrosion processes.160

| technique | obtainable information | refs |
|---|---|---|
| photoelectrochemistry with illuminated rotating ring disk electrode | stabilization efficiency, reorganization energy of redox couple, active site identification of corrosion reactions | (38, 42, 58, 59, 138−146) |
| electrochemical photocapacitance spectroscopy | energy and distribution of surface states, and identification of states responsible for corrosion, efficiency of defect passivation, separation of bulk and surface states | (161−164) |
| PEC impedance spectroscopy | position of band edges, position of interband states participating in corrosion | (38, 52, 146, 165−169) |
| band edge shift measurements with corrosion current (“Tafel-plot” like representation) | stabilization efficiency, corrosion mechanism validation | (38, 44, 53, 54) |
| photocorrosion quantum efficiency measurements (monochromatic) | percentage of photocurrent attributed to corrosion (stabilization efficiency) | (53, 146, 147) |
| intensity modulated photocurrent/photovoltage spectroscopy | determination of the rate constants of distinct steps in photocorrosion, charge carrier capture cross section and activation energy of steps, mechanism evaluation | (147, 148) |
| PEC quartz crystal nanogravimetry | mass changes during PEC operation (overlayer formation, corrosion) | (156, 170) |
| in situ IR spectroelectrochemistry | identification of adsorbed species on the electrode surface, corrosion intermediate identification | (155, 171) |
| in situ Raman spectroelectrochemistry | photocorrosion product/intermediate detection | (153, 154, 156) |
| in situ UV–vis spectroelectrochemistry | material loss related to corrosion with the simultaneous evolution of dissolved species in the electrolyte; surface roughening of the electrode | (28, 36, 137, 172) |
| in situ photoluminescence spectroelectrochemistry | monitoring the formation of corrosion states, kinetics of surface corrosion state emptying and refilling | (173−175) |
| in situ PEC ICP-MS | dissolution rate during PEC operation, stoichiometry change of complex materials | (109, 110) |
| in situ PEC X-ray photoelectron spectroscopy | identification of chemical alteration of the surface/electrolyte during photocorrosion, time-evolution of corrosion products, band-alignment measurements under operating conditions | (149−152) |
| in situ UV–vis ellipsometry | change in surface layer thickness, morphology, and composition during photocorrosion | (171) |
| in situ scanning electrochemical microscopy | kinetics of photocorrosion processes | (176) |
Design Strategies to Enhance Stability
The high degree of interchangeability of all components within the ABX3 perovskite lattice and the vast number of different components used in related devices (e.g., charge transfer materials, surface/defect passivation agents, hydrophobic capping layers) offer different strategies to inhibit photocorrosion. Many of these are yet to be implemented in PC and PEC systems. As the first step, the relative energy positions of the components and the redox potential of the target redox reaction as well as the corrosion process have to be compared (Figure 9). To suppress corrosion, redox reactions should be introduced that are more favorable than the corrosion levels (Figure 6). As an example, through the exchange of bromide to iodide in the lattice, apart from the decrease in the optical bandgap, both band edges shift (as halogen orbitals contribute to both).177 This decreases the pool of redox processes that can be used to stabilize iodide-containing perovskites.

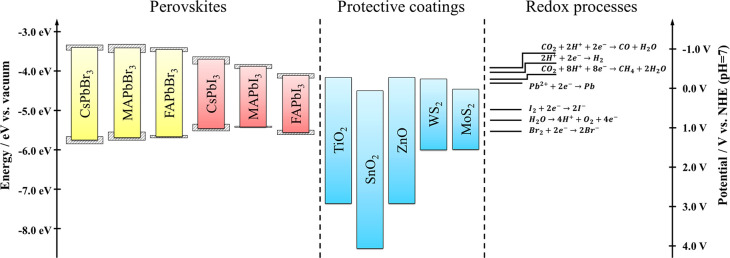
Band edge positions of different perovskites together with the band edge positions of some protective or catalytic coatings. The gray area around the band edges denotes the dispersion in reported values. Redox potentials of commonly encountered redox processes are also shown.
The use of protective coatings is a widely employed strategy in both PC and PEC energy conversion. Possible materials combinations for driving reduction reactions are shown in Figure 9. In PC and PEC applications, however, apart from efficient charge extraction, fast transfer kinetics toward the desired substrate is also required. To overcome kinetic limitations, catalysts are often deposited on the protecting layer, which also influences the product distribution of the reactions. As shown previously, the current focus is on HER, CO2R, or oxygen evolution reaction (OER) on perovskites. Thermodynamically, these reactions are feasible, as many perovskites have proper CB and VB positions for these reactions, except for FAPbI3. Notably, the reported redox potentials are generally determined in aqueous media, and deviations can occur in organic solvents. In unprotected cases, interference from the reduction of lead is expected in the HER and CO2R. In a similar manner, iodide oxidation can be problematic during OER, while bromide oxidation is less favored thermodynamically.
Compositional Engineering
The chemical instability of perovskites is mainly ascribed to the volatile organic cations (MA+, FA+) in the structure.178,179 The incorporation of inorganic elements (Cs+, Rb+) into the structure can enhance the stability180,181 and also suppresses phase segregation in mixed halide perovskites.182,183 In terms of PC and PEC applications, only the mixed halide variants were studied.89,96,97,116,117,125 With increasing bromide content in the MAPbI3 lattice, the resistance to moisture was improved.184 The susceptibility to reactive oxygen, however, remains the same.185 The simultaneous incorporation of mixed cations, where improved stability would be expected, however, is yet to be explored. The addition of small quantities of chloride during the synthesis can suppress phase segregation.31,186,187 It influences the perovskite film quality (morphology,188 lattice strain,189 grain size,190 and crystallinity191) and halide defect density.192 With decrease of the available sites that participate in phase segregation, the stability is increased in an indirect manner. Recently, triple halide perovskites were shown to be resilient toward phase segregation and operate under 100-sun intensity with less than 4% degradation for 1000 h.186
Crystal Facet Engineering
The various facets of perovskite crystals, which inherently have different atomic terminations, behave differently under PC or PEC operation. Properties such as (i) electronic structure, (ii) built-in electric fields, (iii) specific adsorption, (iv) reaction kinetics, (v) reaction product distribution, and (vi) stability are all affected by the exposed facets.32,39,193 Furthermore, when multiple facets are simultaneously present, the formed heterojunction must also be considered. This can affect the charge carrier dynamics by either facilitating charge separation or enhancing charge carrier trapping.193 The specific accumulation of charge carriers on low stability facets can accelerate corrosion. Facets with low surface energy tend to form under mild conditions, while excess energy or external stabilization (through the utilization of capping agents) is necessary to form facets with high energy. Consequently, tailored exposure of selected facets can be achieved,32,73,193 exploiting the difference in adsorption of capping agents on the various facets. Finally, when ionic species are involved in the specific adsorption, the formed electric double layer will also affect the separation of charge carriers and ultimately the kinetics of corrosion.38 Studies on single crystal perovskites have been mainly focused on PV cells and photodetectors,194,195 which can be later extended to PC and PEC studies.
2D Perovskites
A unique combination of materials and crystal facet engineering is the preparation of 2D perovskite derivatives.196 In these 2D materials, the inorganic network is disconnected along one axis, which in turn restricts charge transport to a specific plane. The perovskite lattice can be cut at crystallographic planes of ⟨100⟩, ⟨110⟩, and ⟨111⟩, resulting in the over-representation of that particular orientation (Figure 10). The ⟨100⟩ cut perovskite lattice is the most popular, as the optoelectronic properties can be varied through control over the inorganic slab thickness.197 Two further subclasses (Ruddlesden–Popper and Dion–Jacobson perovskites) exist for the ⟨100⟩ deconstructed perovskite, based on the stacking of the inorganic layers. The large organic cations, separating the inorganic layers of the perovskite lattice, can bestow increased resistance toward humidity.60,198,199 These organic ligands can be tailored toward specific PEC applications, providing catalytic sites for the desired reactions. Four different photoelectrode architectures can be envisioned: (i) self-standing 2D, (ii) mixed 2D/3D, (iii) 3D with 2D capping layer, and (iv) 2D passivated 3D perovskites.196 These combinations have already attracted significant attention in the PV community, as they show superior stability but slightly inferior efficiency compared to their 3D counterparts.60,200,201

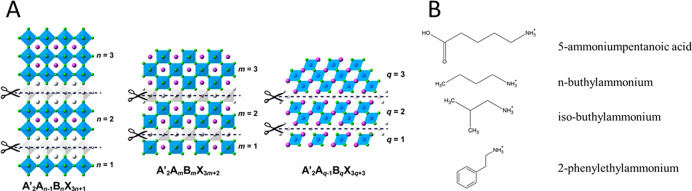
(A) Illustration of the different types of 2D perovskites cut through ⟨100⟩, ⟨110⟩, and ⟨111⟩ orientation. Reproduced from ref (196) with permission from Wiley-VCH. (B) Depiction of common cations used to form 2D perovskites.
Surface Passivation
The ionic nature of perovskites inherently produce defects with positive (undercoordinated Pb(II) sites) or negative (undercoordinated halide ion) charge. Therefore, surface passivation is a convenient strategy to reduce the density of potentially corrosion susceptible defect states on the surface.202 Different electron donor (Lewis-base) or acceptor (Lewis-acid) molecules can be employed to neutralize these defects. Halide containing compounds were studied as surface passivating agents, such as metal halides,203−205 quaternary ammonium halides,206,207 and phenylethylammonium iodide.208,209 As an additional benefit, the efficiency of surface-passivated devices also increases, as recombination through these passivated energy levels is suppressed (Figure 11). Finally, a further physical barrier is formed against the penetration of moisture into the perovskite structure when hydrophobic ligands are employed.210

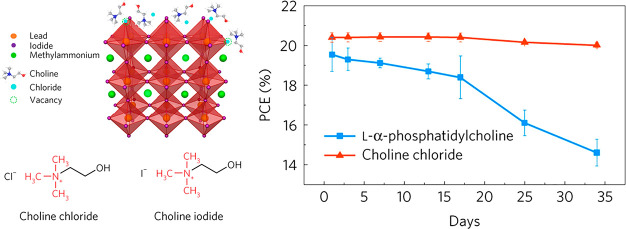
Depiction of surface passivation with choline chloride a quaternary ammonium halide and its beneficial effect on solar cell performance and stability. Reproduced from ref (206) with permission from Springer Nature.
Corrosion processes propagate from defect sites as the chemical makeup of the intergranular layer is often susceptible to chemical attack or corrosion.137,211 Surface passivating agents can be effectively used in the corrosion inhibition of PC and PEC systems (if their electrical conductivity is ensured). Furthermore, bifunctional molecules that possess inherent catalytic property can be also employed, therefore merging two functionalities.
Physical Coatings
Perovskite-based photoelectrodes dominantly use physically protected architectures, because with this approach the light absorber perovskite surface can be isolated from the electrolyte (buried junction concept).212 Protection layers include metals (e.g., Ni,213−215 Ti,216 Field’s metal217−225), carbon-based materials,226,227 and atomic layer deposited TiO2.228 These protected photoelectrode designs employ a full (or partial) perovskite solar cell buried beneath the protective coatings. Based on the structure (n-i-p or p-i-n), both reduction and oxidation reactions can be driven on these photoelectrodes. The target reaction will dictate which charge transport layer and catalyst need to be used. Device failures are mainly caused by the penetration of electrolyte through the protective layer, due to structural imperfections. A mitigation strategy can be the incorporation of additional hydrophobic layer(s) into the structure, that can prolong the stability during PEC operation.215,226 Further protection layers (such as polyethylenimine) were used to prevent the iodide migration to the metal contacts.221,223−225
Field’s metal is a nontoxic eutectic alloy of bismuth (32.5%), indium (51%), and tin (16.5%), which has received considerable attention as a protective and catalytic coating. The low melting point of the alloy allows the fabrication of thick protective layers on top of the sensitive perovskite electrodes. Its use in PEC HER,217−221,223 OER,214,218 and CO2R222,224,225 reactions was demonstrated. Field’s metal inherently possesses catalytic activity; for example, the alloy composition had a pronounced effect on the product distribution in CO2R.222 Furthermore, different catalytically active materials such as Pt,217 cobalt(II)-based molecular catalysts,224 hydrogenase enzyme,223 or Cu94In4225 can also be loaded on the surface of the photoelectrode assembly. Field’s metals, however, suffer from low stability in acidic media and high cost. As a cheaper alternative, a hybrid layer of mesoporous graphite/hydrophobic graphite sheets was employed to perform OER with CsPbBr3 perovskite photoanodes.227 In this design, the mesoporous layer was responsible for charge collection, while the hydrophobic graphite sheet was used as a protective layer. An operational stability of 7–30 h (in a pH range of 2–13 in aqueous electrolyte) was demonstrated.
Atomic layer deposition (ALD) has been widely used to deposit different components of perovskite solar cells.229,230 Care must be exercised when using ALD on perovskite surfaces for the deposition of (i) passivation layers, (ii) charge transfer overlayers, or (iii) encapsulation layers. The sensitivity of the perovskite surfaces toward temperature and reactants (e.g., H2O, O2, ozone, metal precursor), and the volatile nature of different components of perovskites (MA+ and the I–) have to be considered when designing the ALD protocol.229 While ALD is considered as the state-of-the-art in depositing uniform and thin protective coatings, so far only an ALD deposited TiO2 layer was used in the case of perovskite photoelectrodes.228 This photocathode assembly with Pt HER catalyst (Figure 12A) was stable for 2 h in highly acidic media (pH = 0.32) under 0.5 Sun illumination (∼70% activity retention) (Figure 12B). The semitransparency will enable the integration of this design into tandem PEC cells for overall solar-driven water splitting.

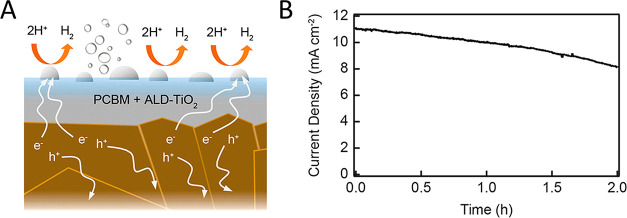
(A) Schematic representation of the photocathode architecture and the involved processes during photoassisted HER. (B) Stability of the photocurrent density for an ALD TiO2 protected perovskite photocathode with a nominally 15 nm thick Pt catalyst (E = 0 V vs RHE, 0.5 Sun in 0.5 M H2SO4). Reproduced with permission from ref (228). Copyright 2019 American Chemical Society.
Summary and Future Directions
PC and PEC scenarios are recent additions to the potential research arenas where the optoelectronic properties of perovskites can be harnessed. Elaborating on the experience gathered from pioneering studies on photocorrosion processes and from stabilizing perovskite layers in PV devices will greatly accelerate the progress of this field. As the first step, careful characterization protocols are necessary for both PC and PEC studies (with quantitative product detection), to understand reasons and processes behind the instability. Furthermore, combined in situ electrochemical measurements will furnish mechanistic insights, with the possibility of revealing the elementary steps of corrosion. For nanoscale studies, in situ electrochemical atomic force microscopy231−234 and PEC transmission electron microscopy will be useful to visualize morphological changes that occur on the surface of photocatalysts and photoelectrodes. Pinpointing structural weaknesses in these systems (e.g., sensitive facets, morphological features) will guide better design of stable solar energy conversion devices. In a similar fashion, coupled ultrafast techniques can shed light on how corrosion affects the charge carrier dynamics of these complex systems.158,235 Ultrafast spectroscopic studies under electrochemical control have just been employed for perovskite electrodes recently, and further breakthroughs are expected once the pulse duration is decreased. With setups allowing few femtoseconds (or even sub-femtosecond) resolution,236 the initial stages of charge carrier generation can be followed, providing new insights for subsequent rational materials design. While single crystal surfaces can be used to identify elementary steps in photocorrosion processes (including identification of stable and active facets), crystal facet engineering will help to translate this knowledge to nanostructured materials of practical importance.
Once the elementary steps of the photocorrosion process are uncovered, different strategies can be employed to stabilize perovskites. Compositional engineering, various surface protection approaches and the preparation of 2D perovskites are all viable options. As a particularly promising direction, bifunctional coatings can be developed, where the catalytic and protective functions are merged. Clearly, there is room for optimization, as protective coatings other than TiO2, catalysts, and reactions (CO2R, OER, HER) are yet to be explored (see Figure 9 for potential candidates). In this regard, emerging ALD methods will provide thin and homogeneous coatings of a rapidly broadening materials portfolio. Finally, the gathered insights on both the photocorrosion process itself and the efficiency of the mitigation strategies will provide feedback from a fresh perspective to the PV community.
Notably, corrosion processes can also be turned to our advantage. Selective removal of photoirradiated portions of samples can micropattern photoelectrodes. Furthermore, in the case of certain photoelectrodes (e.g., Cu2O), the delicate balance between photocorrosion and the desired redox reaction is responsible for creating and maintaining the catalytically active surface (self-healing).237 These aspects are yet to be explored in perovskite-based systems. Finally, while discussions in this Perspective focused on perovskite/electrolyte interfaces, there is a growing field of PC and PEC studies on solid/gas interfaces. Concepts borrowed from “dark” electrochemistry (such as gas-diffusion electrodes) are fueling the growth of this area, and some initial studies on perovskites are already available.238,239
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c10348.
Discussion on photocorrosion kinetics and summary of different techniques for studying corrosion (PDF)
Notes
The authors declare no competing financial interest.
Acknowledgments
The authors thank Dr. Bíborka Janáky-Bohner and Ádám Balog (both from the University of Szeged) for their constructive comments on an earlier version of the manuscript. Support from the EU’s Horizon 2020 research and innovation program (Grant Agreement Nos. 716539 (ERC) and 862453) is acknowledged. The ELI-ALPS project (GINOP-2.3.6-15-2015-00001) is supported by the European Union and co-financed by the European Regional Development Fund. This project was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (G.F.S.).
 Photocorrosion
at Irradiated Perovskite/Electrolyte
Interfaces
Photocorrosion
at Irradiated Perovskite/Electrolyte
Interfaces