
- Altmetric
Catalytic kinetic resolution of amines represents a longstanding challenge in chemical synthesis. Here, we described a kinetic resolution of secondary amines through oxygenation to produce enantiopure hydroxylamines involving N–O bond formation. The economic and practical titanium-catalyzed asymmetric oxygenation with environmentally benign hydrogen peroxide as oxidant is applicable to a range of racemic indolines with multiple stereocenters and diverse substituent patterns in high efficiency with efficient chemoselectivity and enantio-discrimination. Late-stage asymmetric oxygenation of bioactive molecules that are otherwise difficult to synthesize was also explored.
Catalytic kinetic resolution of amines is a longstanding challenge in chemical synthesis. Here, the authors report on titanium‐catalysed asymmetric oxygenation with hydrogen peroxide for kinetic resolution of secondary amines through oxygenation to produce enantiopure hydroxylamines involving N–O bond formation.
Introduction
Enantiopure cyclic secondary amines are key constituents of natural products, pharmaceuticals, and agricultural chemicals1. Catalytic kinetic resolution (KR) of racemic amines represents a practical and robust approach to access optically pure targets, especially in cases where the racemates are readily available and the enantiopure materials are not2–8, though the theoretical yield of the expected optically pure target can never exceed a limit of 50%9–11. The current nonenzymatic KR of secondary amines predominantly relies on asymmetric N-acylation strategy involving N–C bond formation, which typically requires the use of stoichiometric pre-prepared acylating agents involving lengthy reagent synthesis (Fig. 1a)12–18. Development of a catalytic KR of secondary amines based on other elementary reaction bearing an economy-oriented mind-set would be highly desirable.

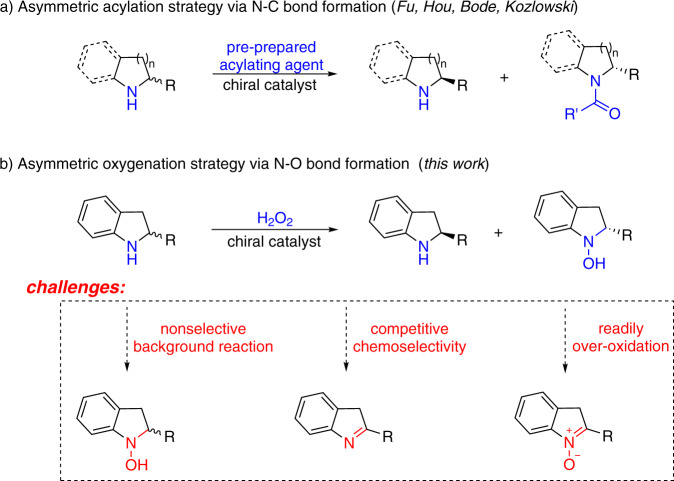
Overview of KR of cyclic secondary amines.
a Asymmetric acylation strategy via N–C bond formation. b Asymmetric oxygenation strategy via N–O bond formation.
Oxygen atom transfer (OAT) reaction is ubiquitous in biological systems, organic synthesis, and industrial processes19–23. Current asymmetric OAT studies predominantly focused on oxygenation of alkenes and sulfides involving C–O and S–O bond formation24–34. We envisioned that asymmetric OAT to secondary amines to produce enantiopure hydroxylamines involving N–O bond formation would be an ideal template for KR design based on economical and environmental factors (Fig. 1b). However, asymmetric OAT to amines has remained a formidable challenge35,36. To the best of our knowledge, asymmetric oxygenation of secondary amines to produce hydroxylamines through either enzymatic or non-enzymatic catalysis has never been established to date, which might be ascribed to three key challenges. First, the inherent high reactivity of amines results in easy nonselective oxygenation without the intervention by a catalyst. Second, competitive dehydrogenation of secondary amines to imines usually accompanies the expected OAT process37–39. Third, chirality in hydroxylamine products could be facilely destroyed through further oxidation to nitrones40. On the other hand, aqueous hydrogen peroxide is a desirable oxidant from the viewpoints of atom efficiency (48%), easy-to-handle, and ecological benignity32,41. Given the significance of optically pure indolines in modern pharmacology, we herein report a titanium-catalyzed KR of indolines using H2O2 as the oxidant through N–O bond formation. Late-stage asymmetric oxygenation of bioactive molecules that are otherwise difficult to synthesize was also explored.
Results
Reaction condition optimization
Initially, asymmetric oxygenation of racemic indoline 1a was selected as a reference reaction using aqueous H2O2 as the oxo-transfer agent to search for a suitable chiral catalyst (Table 1). Chiral monomeric (salen)titanium(IV) C1 exhibited no oxidation catalysis reactivity (entry 1). We then explored dimeric metallosalen complex as catalyst. Delightedly, di-μ-oxo titanium(salen) C2 effected the expected asymmetric oxygenation, though poor chiral recognition and notable over-oxidation were observed (entry 2). The substituent patterns on 1,2-ethanediamine proved to be crucial to catalytic reactivity and asymmetric induction. Replacing the 1,2-cyclohexanediamine moiety in C2 with 1,2-diphenylethylenediamine one (C3) significantly reduced the oxidation catalytic reactivity (entry 3). Di-μ-oxo titanium(salalen) C4 was prepared by reducing one of the process (entry 4). Displacing the phenyl group on salicylaldehydes with other two imine bonds of C2, was beneficial for suppressing undesired over-oxidation substituents afforded inferior results, which prompted us to introduce another chiral element at C3 site of the basal salalen ligand to enhance the enantio-differentiating ability of the catalyst. The “hybrid” titanium(salalen) C5 bearing a (Ra)-binaphthyl unit on the imine side was not an effective catalyst (entry 5). Promising chiral recognition was observed when di-μ-oxo titanium(salalen) catalyst C6 bearing two (Ra)-binaphthyl units on both amine and imine sides were used (entry 6). The absolute configuration of (R)-1a was assigned to be R by comparing the optical rotation and HPLC analysis with reported data. See the Supplementary Information for details. Reversing the absolute configuration of 1,2-cyclohexanediamine in catalyst C7 provided a mismatch recognition (entry 7). Extensive optimization of the solvent identified CHCl3 to be optimal (entries 8–10). The level of chiral discrimination was further enhanced by lowering the loading of titanium(salalen) C6 from 2.5 mol % to 1.0 mol %, though a slightly prolonged time period was required (entry 11).

 | |||||
|---|---|---|---|---|---|
| Entry | Cat. | t (h) | Yield (%)b 1a/2a/3 | ee (%)c (R)-1a/(S)-2a | sd |
| 1 | C1 | 24 | > 95/< 5/< 5 | n.d. | n.d. |
| 2 | C2 | 12 | 44/23/27 | 35/30 | 2.5 |
| 3 | C3 | 24 | > 95/< 5/< 5 | n.a. | n.a. |
| 4 | C4 | 1 | 48/32/13 | 43/45 | 3.9 |
| 5 | C5 | 24 | 58/16/20 | 35/54 | 4.7 |
| 6 | C6 | 8 | 49/35/10 | 74/76 | 16 |
| 7 | C7 | 12 | 78/9/8 | 7/30 | 2 |
| 8e | C6 | 12 | 67/17/9 | 37/90 | 28 |
| 9f | C6 | 12 | 51/36/9 | 65/75 | 14 |
| 10g | C6 | 4 | 47/43/5 | 94/89 | 60 |
| 11g,h | C6 | 6 | 47/45/3 | 92/92 | 79 |
Asymmetric oxygenation of indolines bearing one stereocenter
The scope of di-μ-oxo titanium(salalen)-catalyzed asymmetric oxygenation of racemic indolines was explored (Fig. 2)40. Substrates 1a-1k bearing a wide range of electronically varied aryl and heteroaryl groups at C2 position with different substituent patterns proceeded with excellent chemoselectivity and chiral recognition (Fig. 2a). Indolines 1l-1s bearing diverse C2-alkyl substituents were suitable components with excellent selectivity factors (Fig. 2b). Diverse functional groups including phenyl motif (1o and 1p), silyl ether (1q), carboxylic acid ester (1r), and terminal alkyne (1s) were tolerated as additional functional handle. Spirocyclic 1r and 1s containing the variant of the geminal disubstitution at C3-position were also tolerated (Fig. 2c). Simple indoline 1t without C3-substituent was competent substrate, though the oxidized hydroxylamine 2t was unstable and underwent decomposition during purification probably due to the existence of reactive benzylic C3–H bonds.

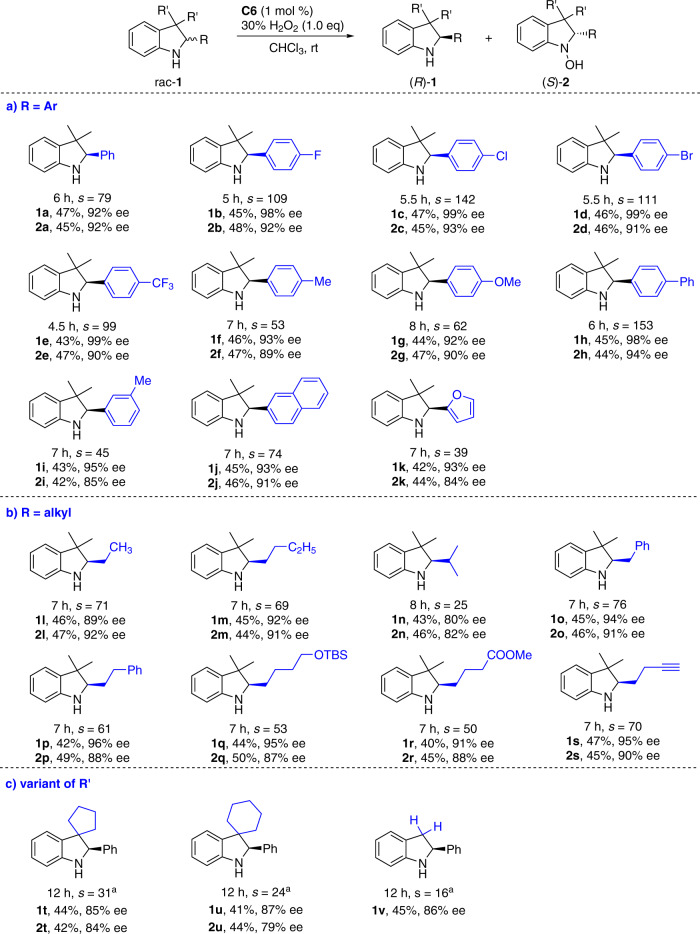
Asymmetric oxygenation of racemic C2-substituted indolines.
Conditions: rac-1 (0.1 mmol), 30% aqueous H2O2 (0.1 mmol), and C6 (1 mol %) in CHCl3 (1.0 mL) at rt for indicated time period. aReaction with 2.0 equiv of aqueous H2O2 at 0 °C.
The substituent effects on the indoline arene were then investigated (Fig. 3). A range of electron-withdrawing and -donating substituents at either C4, C5, or C6 position of substrates 4a–4j were tolerated with high level of chiral discrimination (Fig. 3). Bromo (4a–4c), chloro (4d), and fluoro (4e) substituents were compatible with the oxidation system for further diversification.

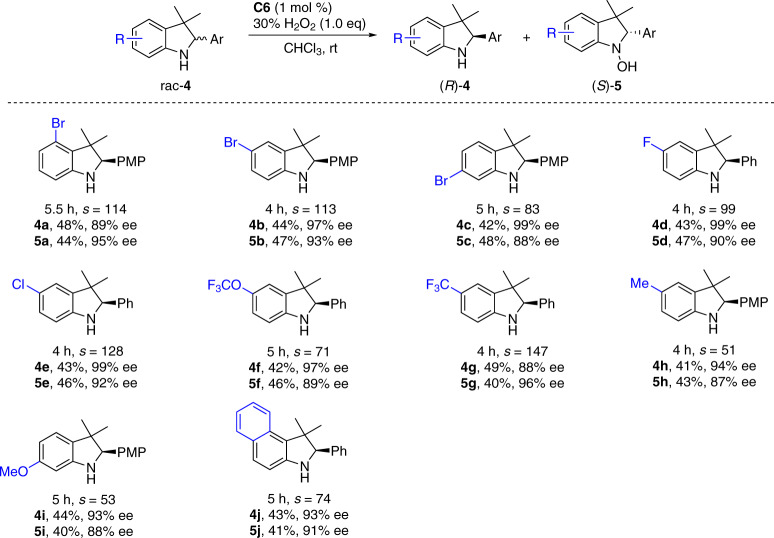
Substituent effects on the indoline arene.
Conditions: rac-4 (0.1 mmol), 30% aqueous H2O2 (0.1 mmol), and C6 (1 mol %) in CHCl3 (1.0 mL) at rt for indicated time period.
Scope of indolines bearing two stereocenters
The success in asymmetric oxygenation of racemic indolines bearing one stereocenter prompted us to further investigate the tolerance of substrates bearing two stereocenters (Fig. 4)42–47. Enantio-differentiating oxygenation of trans-2,3-trisubstituted indoline rac-6a bearing C3 quaternary chiral center proceeded smoothly, providing (2R, 3S)-6a in 46% yield with 88% ee together with hydroxylamine (2S, 3R)-7a in 45% yield with 93% ee (s = 81). Cis-2,3-trisubstituted indoline rac-6b was also compatible with the asymmetric oxygenation conditions (s = 46). Common functional groups at the C3 quaternary center, like aryl (6c-6f) and cyano (6f and 6g), were well tolerated with good selectivity factors of 49–87. Cyclohexane-fused indolines, key structural motifs in a number of Aspidosperma alkaloids, were competent substrates, as demonstrated by effective enantio-differentiating oxygenation of rac-6h and 6i48–50. Trans-2,3-disubstituted indoline rac-6j participated in asymmetric oxygenation, and (2S, 3S)-6j was recovered in 42% yield with 84% ee. Due to the existence of reactive benzylic C3–H bond, the oxidized hydroxylamine 7j was not stable under the oxidation conditions, and was further oxidized to several unexpected compounds (see the Supplementary Information for details)51.

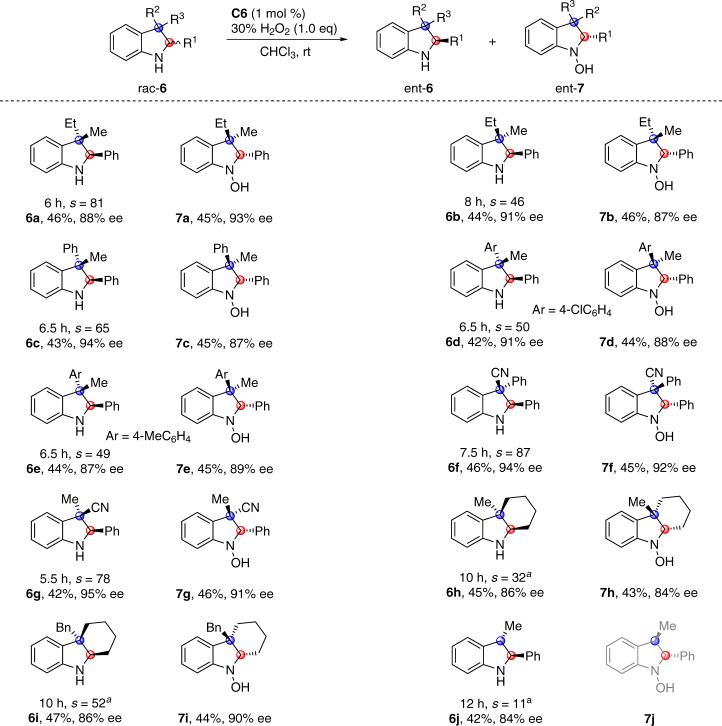
Scope of indolines bearing two stereocenters.
Conditions: rac-6 (0.1 mmol), 30% aqueous H2O2 (0.1 mmol), and C6 (1 mol %) in CHCl3 (1.0 mL) at rt for indicated time period. aReaction with 2.0 equiv of aqueous H2O2 at 0 °C.
Synthetic applications
The synthetic utilities of the method were next examined (Fig. 5). The optically pure recovered indolines and oxidized hydroxylamines can undergo interconversion with the ee highly conserved. Hydroxylamine (S)-2a was reduced to (S)-1a in the presence of Zn and AcOH (Fig. 5a). The absolute configuration of (S)-2a was assigned to be S by HPLC analysis of the reduction product (S)-1a. See the Supplementary Information for details. Indoline (R)-1a was selectively oxidized to hydroxylamine (R)-1a in 92% yield when the combination of racemic titanium(salalen) C4 and aqueous H2O2 was employed (Fig. 5b). Severe over-oxidation to nitrone was observed when common oxidants such as 3-chloroperoxybenzoic acid (mCPBA), NaWO4/H2O2, and methyltrioxorhenium (MTO)/H2O2, were used. The reaction in a gram-scale proceeded without obvious loss of enantioselectivity (Fig. 5c). The late-stage enantio-differentiating oxygenation advanced intermediates of bioactive molecules that would be otherwise difficult to access was further explored. Trans-2,3-trisubstituted indoline 8 was identified as a potent and selective inhibitor of the histone lysine demethylases KDM2A/7A (Fig. 5d)52. Under the standard conditions, rac-9 participated in the asymmetric oxygenation reaction, furnishing (2R, 3R)-9 in 45% yield with 96% ee together with (2S, 3S)-10 in 47% yield with 91% ee (s = 85) (Fig. 5d). C2-Cyclopropane-substituted indoline 11 was discovered as a general pharmacophore for gonadotropin-releasing hormone (GnRH) antagonists (Fig. 5e)53. Asymmetric oxidation of rac-12 proved to be relatively sluggish, and when 2.0 equiv of aqueous H2O2 was used, (R)-12 was recovered in 49% yield with 83% ee (s = 23) together with over-oxidized nitrone 13 in 45% yield (Fig. 5e).

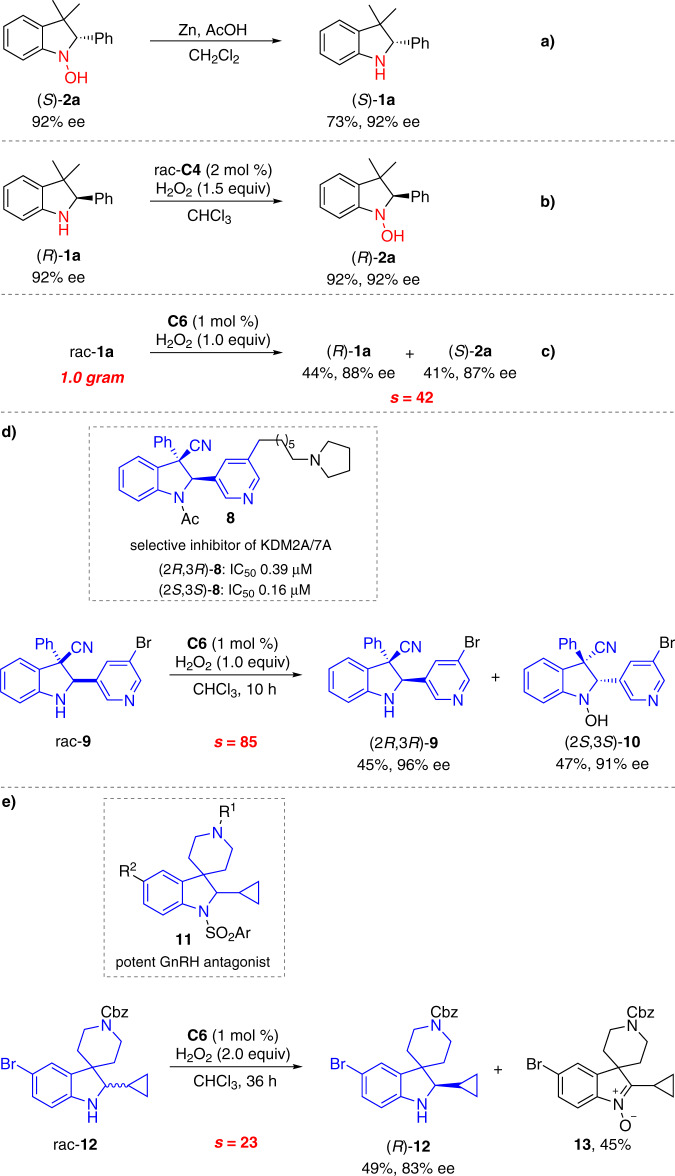
Synthetic applications.
a Reduction of hydroxylamine (S)-2a. b Oxidation of indoline (R)-1a. c Gram-scale experiment. d Late-stage enantio-differentiating oxygenation intermediates of KDM2A/7A. e Late-stage enantio-differentiating oxygenation intermediates of GnRH antagonists.
Discussion
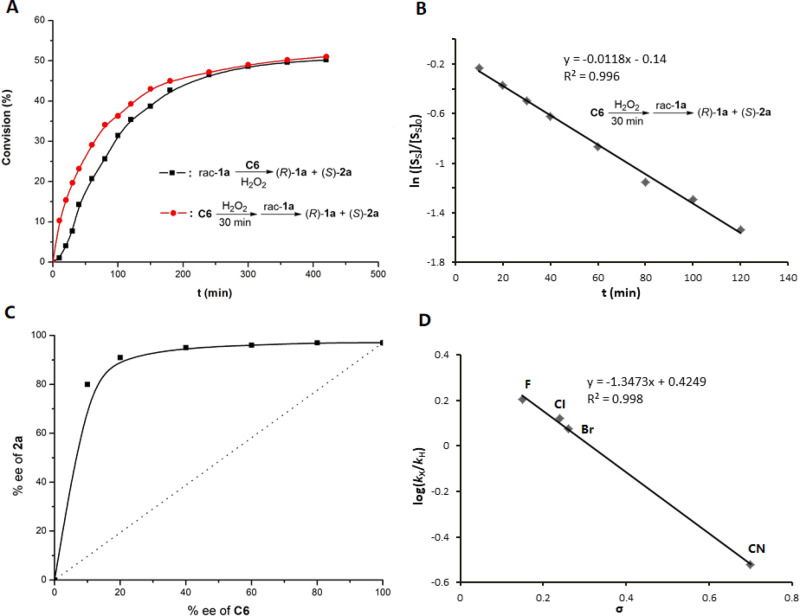
To get a preliminary understanding of the catalysis role of titanium(salalen) C6 in asymmetric oxygenation of indolines, conversion (%) of rac-1a was plotted against time (min) for reaction under standard conditions and that with pre-mixed catalyst and H2O2, respectively (Fig. 6A). For the standard reaction, conversion followed a sigmoidal curve, and an induction period of about 30 min was observed. Such induction period was not observed in the reaction using pre-mixed C6 and H2O2, suggesting that the generation of the real active species for oxygenation reaction is a slow process. Moreover, the latter reaction exhibited an apparent steady-state regime, resulting in linear ln([SS]/[SS]0) vs. time dependence (Fig. 6B).

Mechanistic studies.
A Kinetic plots for the oxygenation of rac-1a with C6/H2O2. B ln([SS]/[SS]0) versus time dependences for the oxygenation of (S)-1a. [SS]0 = [(S)-1a]0 = 0.05 M, [SS] = [(S)-1a] ≈ [0.5 – C][rac-1a]0, [rac-1a]0 = 0.1 M, conversion = C = (ee1a)/(ee1a + ee2a). C Plot of the enantiomeric excess of 2a versus the enantiomeric excess of C6 at 50% conversion. The dotted line symbolizes the linear correlation. D Hammett plot of log(kX/kH) vs σ for the competitive oxidation of C5-substituted indolines by C6/H2O2.
Control experiments were performed to further understand the identity of the generated species by mixing C6 with H2O2. The relationship between the excesses of 2a and the catalyst C6 was investigated, and a positive nonlinear relationship was observed, suggesting the possible intervention of a dimeric complex (Fig. 6C)54,55. ESI mass-spectrometry analysis of the mixture of di-μ-oxo titanium(salalen) C6 and 30% aqueous H2O2 after 30 min showed the peak of 1797.6, which is equal to [MC6 + O + H]+, implying the formation of a μ-oxo-μ-peroxo species S (Fig. 7a)56. However, a lower intensity of this peak was observed for mixing C6 and H2O2 after 15 min, implying that the formation of S might be a slow process. No reaction was observed when mixing one equiv of S with rac-1a (Fig. 7b). However, the addition of aqueous hydrogen peroxide rendered S catalytically active with comparable results to C6 (Fig. 7c). These results clearly indicate that S is neither the real active species nor a dead-end species but an active intermediate. Crossover experiments involving two different catalysts C4 and C6 were next performed. Mass spectra analysis of equivalent C4 and C6 in the presence of 30% aqueous H2O2 showed the peak of 1445.5, which is equal to [MC4/2 + MC6/2 + O + H]+, suggesting the generation of a crossover dimerization peroxo complex (Fig. 7d). No such peak was detected during the mass spectra analysis of the mixture of C4 and C6 in the absence of H2O2 (Fig. 7e). These observations indicated that a disassembly and reassembly process might occur after the formation of μ-oxo-μ-peroxo species S. We envisioned that the real active species involved in the catalytic cycle might be the disassembled monomeric peroxo Ti(salalen) complex57.

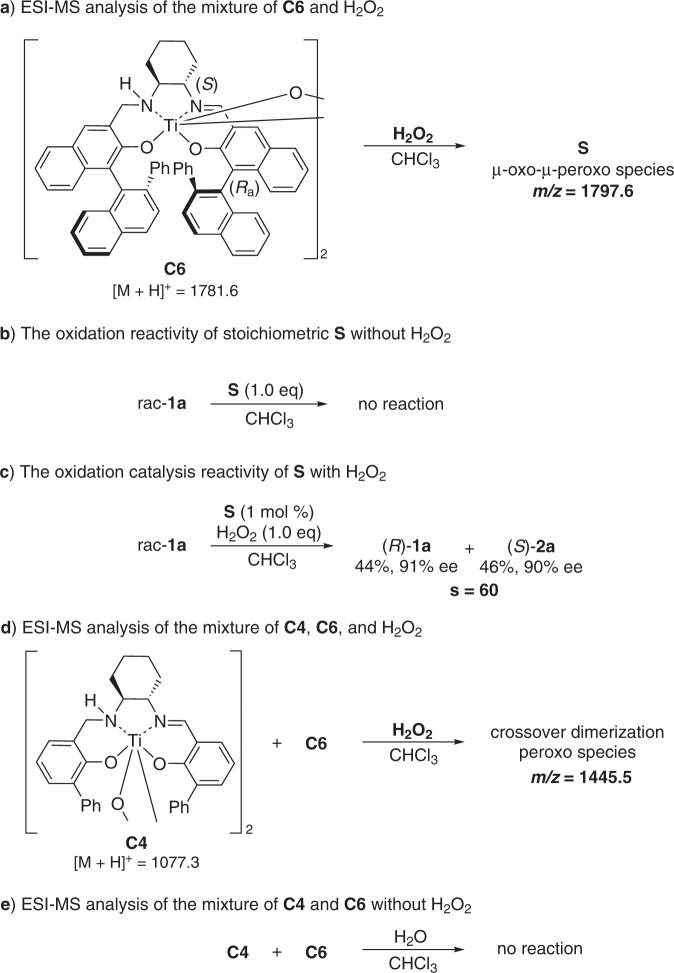
Control experiments of titanium(salalen) catalyst with aqueous H2O2.
a ESI-MS analysis of the mixture of C6 and H2O2. b The oxidation reactivity of stoichiometric S without H2O2. c The oxidation catalysis reactivity of S with H2O2. d ESI-MS analysis of the mixture of C4, C6, and H2O2. e ESI-MS analysis of the mixture of C4 and C6 without H2O2.
Asymmetric oxygenation of racemic indolines with various kinds of C5-substituents (X) on indoline arene were performed and the reaction rates were dependent on the electronic effects of the substituents (see the Supplementary Information for details)58,59. The Hammett plot (log(kX/kH) versus σ) for the competitive oxidation of 1a and respective variants was exhibited in Fig. 6D. The observed plot displayed linear correlation with a ρ value of −1.347 (R2 = 0.99). Good linearity suggests that the oxidation proceeds through a single mechanism. The negative value of ρ indicates a positive charge build-up on nitrogen in the transition state60. The use of Hammett parameter σ+ gave a relatively poorer correlation (R2 = 0.97, see the Supplementary Information for details). These data are consistent with a concerted mechanism, in which an electrophilic active oxygen species might be directly attacked by a nucleophilic nitrogen61.
Based on the above studies and literature survey, a plausible mechanistic pathway for asymmetric oxygenation of racemic indolines was suggested (Fig. 8). Treating di-μ-oxo titanium(salalen) C6 with hydrogen peroxide gave μ-oxo-μ-peroxo species S, which is the incubation period for the oxygenation process. In the presence of hydrogen peroxide, relatively stable S underwent a disassembly process providing monomeric peroxo Ti(salalen) complex 14, which was proposed to be the real active species involved in the catalytic cycle62,63. The asymmetric nucleophilic attack of the nitrogen of indoline rac-1a onto electrophilic oxygen of chiral complex 14 through 15 generated complex 16. 16 reacted with hydrogen peroxide providing hydroxylamine 2a together with 14 for the catalytic cycle. According to the absolute configuration of recovered indolines, we envisioned that amine (S)-1a was oxidized more preferentially than (R)-1a, and therefore unreacted (R)-1a was isolated with high enantioselectivity.

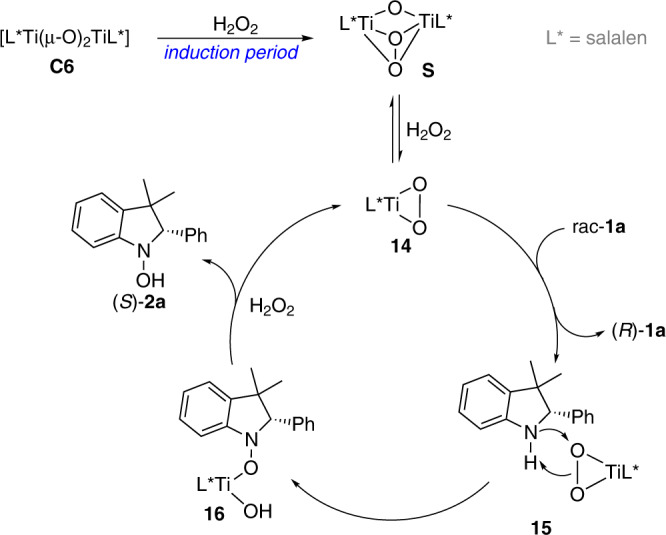
Proposed mechanistic pathway.
The possible reaction pathway based on our studies and the previous literatures.
The stereochemical induction model was suggested in Fig. 9. The structure of monomeric peroxo Ti(salalen) complex 14 is proposed based on the crystal structure of di-μ-oxo titanium(salalen) C664,63. The quadrant diagrams elaborate the origins of the chiral recognition. The steric repulsion between two (R)-binaphthyl unites and indoline 1a prevents the nucleophilic attack of the nitrogen from the third and fourth quadrants. In addition, locating the less sterically demanding phenyl moiety of indoline skeleton in the first quadrant is preferred to avoid the steric repulsion between the (R)-binaphthyl unite and the α-substituent of 1a. The transition state TS2 for (R)-1a has obvious steric repulsion between the α-phenyl substituent of indoline with the cyclohexane moiety of salalen ligand. Such steric repulsion is absent in the transition state TS1 for (S)-1a. Therefore, (S)-1a was oxidized more preferentially than (R)-1a, and unreacted (R)-1a was recovered with high enantioselectivity.

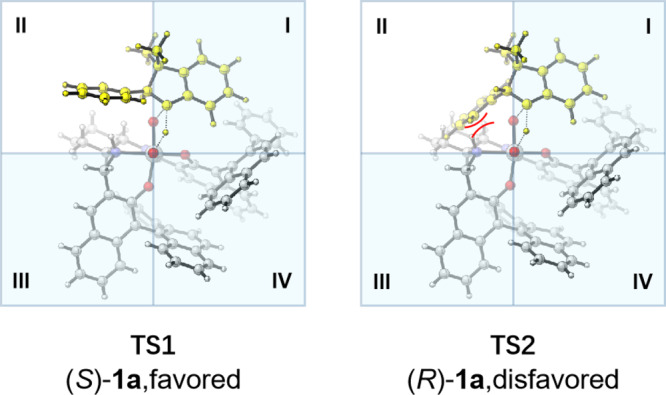
Proposed transition state models.
The transition state TS1 for (S)-1a is favored. The transition state TS2 for (R)-1a is disfavored.
In summary, an oxidative KR of secondary amines based on N–O bond formation is reported. The practical titanium(salalen) catalyzed asymmetric oxygenation with environmentally benign hydrogen peroxide as oxidant is applicable to a range of indolines with multiple stereocenters and diverse substituent patterns in high efficiency with efficient chemoselectivity and enantio-discrimination. Late-stage asymmetric oxygenation of bioactive molecules that are otherwise difficult to synthesize was further explored. The KR of secondary amines based on N–O bond formation described herein represents an advance in the field of asymmetric oxidation.
Methods
General procedure
To a solution of racemic indoline (0.1 mmol, 1.0 eq) in CHCl3 (1.0 mL) was added 30% aqueous hydrogen peroxide (0.1 mmol, 10 μL, 1.0 eq) and C6 (0.001 mmol, 1.8 mg, 1 mmol %) at room temperature. The reaction was vigorously stirred for 4–12 h. Then the mixture was diluted with CH2Cl2 (20 mL), washed with water (10 mL), dried over MgSO4, filtered, and concentrated. The residue was purified by silica gel chromatography (EtOAc/petroleum ether) to give the desired product.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22658-3.
Acknowledgements
Financial supports were provided by the National Science Foundation of China (21971148, 21722204), Shenzhen Special Funds (JCYJ20190807093805572), and Youth Interdiscipline Innovative Research Group of Shandong University (2020QNQT009).
Author contributions
G.W. conducted the asymmetric oxygenation experiments and mechanistic studies; R.L. and C.H. prepared the substrates; L.L. designed the experiments and wrote the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
 Kinetic resolution of indolines by asymmetric hydroxylamine formation
Kinetic resolution of indolines by asymmetric hydroxylamine formation
