
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Corynebacteriales are Actinobacteria that possess an atypical didermic cell envelope. One of the principal features of this cell envelope is the presence of a large complex made up of peptidoglycan, arabinogalactan and mycolic acids. This covalent complex constitutes the backbone of the cell wall and supports an outer membrane, called mycomembrane in reference to the mycolic acids that are its major component. The biosynthesis of the cell envelope of Corynebacteriales has been extensively studied, in particular because it is crucial for the survival of important pathogens such as Mycobacterium tuberculosis and is therefore a key target for anti-tuberculosis drugs. In this study, we explore the biogenesis of the cell envelope of Corynebacterium glutamicum, a non-pathogenic Corynebacteriales, which can tolerate dramatic modifications of its cell envelope as important as the loss of its mycomembrane. For this purpose, we used a genetic approach based on genome-wide transposon mutagenesis. We developed a highly effective immunological test based on the use of anti-cell wall antibodies that allowed us to rapidly identify bacteria exhibiting an altered cell envelope. A very large number (10,073) of insertional mutants were screened by means of this test, and 80 were finally selected, representing 55 different loci. Bioinformatics analyses of these loci showed that approximately 60% corresponded to genes already characterized, 63% of which are known to be directly involved in cell wall processes, and more specifically in the biosynthesis of the mycoloyl-arabinogalactan-peptidoglycan complex. We identified 22 new loci potentially involved in cell envelope biogenesis, 76% of which encode putative cell envelope proteins. A mutant of particular interest was further characterized and revealed a new player in mycolic acid metabolism. Because a large proportion of the genes identified by our study is conserved in Corynebacteriales, the library described here provides a new resource of genes whose characterization could lead to a better understanding of the biosynthesis of the envelope components of these bacteria.
Introduction
The Corynebacteriales order is a group of Gram-positive bacteria widely distributed in nature that includes corynebacteria, mycobacteria, nocardia, rhodococci and other related microorganisms [1]. Some of these bacteria are human pathogens, known to cause severe infectious diseases (Mycobacterium tuberculosis or Mycobacterium leprae) or opportunistic pathologies (Mycobacterium abscessus, Corynebacterium jeikeium or some species of Nocardia). All these bacteria have in common a cell envelope of unusual composition and architecture [2, 3]. Their cell wall core is made up of a peptidoglycan (PG) covalently bound to arabinogalactan (AG) chains, which in turn are linked to mycolic acids (forming the mycoloyl-arabinogalactan-peptidoglycan or mAGP complex). Mycolic acids (MA) are α-branched, β-hydroxylated fatty acids, exclusively synthesized by Corynebacteriales, whose length can reach up to 100 carbon atoms in mycobacteria [4]. The mycolic acid-containing part of the mAGP complex associates with other mycolates containing compounds, essentially trehalose mono or di-mycolates (TMM and TDM respectively), to form the backbone of an outer bilayer named the mycomembrane [5, 6]. This outer membrane, that contains porin-like proteins, is thought to be the functional equivalent of the outer membrane of gram-negative bacteria, although it is more impermeable to most compounds and especially antibiotics [7].
Biosynthesis of compounds specific to the cell wall of Corynebacteriales, i.e. MA and AG, has been the subject of numerous studies over several decades primarily because the production of these compounds is essential for mycobacterial survival. Hence, AG and MA biosynthesis is the target of several known antituberculous drugs, e.g. ethambutol, isoniazid and ethionamide, as well as several candidate drugs in pre-clinical or clinical development [8]. In comparison, Corynebacterium glutamicum, a non-pathogenic bacterium widely used in industrial glutamate production, is much more robust against major disruptions of its cell envelope. For example, C. glutamicum can grow in the complete absence of MA [9] or with an AG devoid of the arabinose domain [10]. This peculiarity has made this species an indispensable model for the study of the biosynthesis of the Corynebacteriales cell envelope. Notwithstanding differences in the fine structure of AM and AG within the Corynebacteriales, the major steps of their biosynthesis seem to be conserved among the different genera as evidenced by the presence, in their genome, of genes encoding the enzymes involved in these pathways [11]. Although the cytoplasmic part of these biosynthetic pathways is well understood, a number of unknown factors remain to be discovered regarding the distribution, transit and assembling of these compounds within the cell envelope. Random mutagenesis, using transposons, is a popular approach for identifying such factors. In Corynebacterium, only two studies, based on the screening of a transposon-insertion library for mutants with an altered envelope phenotype, have been published [12, 13]. In the first, to identify genes involved in MA synthesis, Wang et al. [12] analyzed approximately 400 insertional mutants of Corynebacterium matruchotii using their corynomycolic acid content as a screen. They found one mutant of particular interest with a transposon insertion in a gene encoding a membrane protein conserved in the Corynebacteriales (Cg1766 in C. glutamicum). However, a subsequent characterization of this protein showed that it is actually an α(1,6) mannopyranosyl-transferase (termed MptB) involved in the synthesis of cell envelope lipoglycans [14]. In a very recent study, Lim et al. [13] generated the first high-density library of transposon insertion mutants of C. glutamicum and screened their library for an hypersensitivity to the AG synthesis inhibitor ethambutol. Among the 49 loci identified by their screen (named ste for sensitive-to-ethambutol), they found genes encoding proteins already known to be involved in envelope biogenesis but also identified a new locus implicated in cytokinesis.
Because the function of the mycomembrane is to serve as a selective permeability barrier, any defect in the synthesis or assembly of any of the outer membrane components will affect its structure and, consequently, will alter its permeability. Such a relationship between mycomembrane permeability and alteration in MA synthesis has already been shown in C. glutamicum using a MytA-deficient mutant [15]. Indeed, disruption of mytA, one of the six genes encoding mycoloyltransferases present in the C. glutamicum genome [16], produces a significant decrease in cell wall bound corynomycolate and TDM contents, together with an increase in TMM. In this context, Puech et al. [15] showed that the diffusion rate of two hydrophilic molecules was significantly greater in the mutant compared to the parental strain, strongly suggesting an increase in cell wall permeability. We took advantage of this observation to develop a screen that allowed us to readily identify mutants with an altered cell envelope permeability. However, rather than using the passive diffusion of a small molecule through the cell wall to the cytoplasm, as is frequently done in this kind of screen, we searched for the possibility that increased mycomembrane permeabilization could lead to excretion of easily monitored cell wall compounds. For this purpose, we used antibodies directed against cell wall to screen a transposon mutant library of C. glutamicum and identified new genes involved in cell envelope biogenesis, one of which is very likely involved in the biosynthesis of mycolic acids.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains used in this study are shown in Table 1. Corynebacterium strains were grown in brain heart infusion (BHI) liquid medium with shaking (250 rpm) or in BHI-agar at 30˚C. Escherichia coli DH5α was grown at 37˚C in Luria-Bertani (LB) medium. When necessary, appropriate antibiotics were supplemented as follows: chloramphenicol (Cm) 15 μg/ml; kanamycin (Km) 25 μg/ml; ampicillin (Amp) 100 μg/ml. Electro-transformable C. glutamicum cells were obtained as described in Bonamy et al. [17], with cells collected in early exponential phase (OD600 = 1.5) and in the presence of Tween 80 (0.1% v/v final concentration) for strain 2262. Electro-transformable cells were resuspended in 1/500 of the initial culture volume and 100 μl of the cells were pulsed in the presence of 20 to 100 ng of DNA for replicative plasmids, or 500 ng to 3 μg for integrative plasmids (MicroPulserTM electroporator Biorad in 2 mm cuvettes (Eurogentec) at 25 μF, 2.5 kV and 200 Ω). The cell suspension was immediately diluted with 1 ml of BHI medium and incubated for 1h (replicative plasmids) or 2 hours (integrative plasmids) at 30°C before plating.

| Strain or plasmid | Relevant genotype and/or phenotype | Source or Reference |
|---|---|---|
| E coli strains | ||
| DH5α | F- φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK-,mk+) phoA supE44 λ- thi-1 gyrA96 relA1 | Invitrogen |
| TOP10 | F- mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacΧ74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(StrR) endA1 nupG λ- | Invitrogen |
| C. glutamicum strains | ||
| 2262 | An industrial strain of C. glutamicum | [18] |
| RES167 | Restrictionless derivative of ATCC13032 | [19] |
| CGL2005 | Restrictionless derivative of ATCC21086, Rifampicin resistant | [20] |
| CGL2022 | mytA disrupted, KmR, derivative of CGL2005 | [15] |
| CGL2029 | ΔmytA-ΔmytB::Km double mutant, KmR, derivative of CGL2005 strain | [21] |
| Δcg-pks | Δcg-pks::Km, KmR, derivative of RES167 | [9] |
| ΔaccD3 | ΔaccD3::Km, KmR, derivative of RES167 (originally the gene was named accD4) | [22] |
| Δcg1246 | Δcg1246 derivative of RES167 | This work |
| Δcg1247 | Δcg1247 derivative of RES167 | This work |
| Plasmids | ||
| pCR®2.1-TOPO® | E. coli cloning vector with f1 and pUC origins, lacZα, KmR, AmpR | Invitrogen |
| pCGL0040 | pBluescript II SK(+) containing the cat gene of Tn9 and the Tn5531 transposon | [23] |
| pK18MobSac | Kmr sacB RP4 oriT ColE1 ori | [24] |
| pK18MobSac-Δcg1246 | pK18MobSac containing the upstream and downstream regions of the cg1246 ORF, construct for cg1246 deletion | This work |
| pCGL482 | Shuttle vector E coli/C. glutamicum, CmR | [25] |
| pCGL2420 | Derivative of pCGL482 containing the cg1246 gene under its own promoter (304 bp upstream the ATG of cg1247), CmR | This work |
DNA manipulations
Plasmid DNA was extracted from E. coli using a Wizard® Plus SV Minipreps DNA Purification System (Promega). C. glutamicum chromosomal DNA was extracted as described by Ausubel et al. [26]. Oligonucleotide primers were synthesized by Eurogentec. PCR experiments were performed with a 2720 thermocycler (Applied Biosystems) using GoTaq® (Promega) or PhusionTM High Fidelity (Thermo scientific) DNA polymerases. All DNA purifications were performed using a Roche High Pure PCR product purification kit or a QIAquick Gel Extraction Kit (Qiagen). Standard procedures for DNA digestion and ligation were used in conditions recommended by the enzyme manufacturer (Promega or Fermentas). All DNA sequencing was carried out by Beckman Coulter or Eurofins Genomics.
Preparation of cell envelop components and antiserum production
AG from strain ATCC13032 was purified according to [27] and used to produce antiserum in rabbits (CovaLab).
Cell envelop components used for testing the anti-cell wall (CW) antiserum were prepared as follow. The core cell wall arabinogalactan-peptidoglycan complex from Cg-Pks- strain (PG-AG) was purified according to [28], freeze-dried and suspended in Milli-Q water before use. To obtain AG, the PG-AG suspension was centrifuged (17,000 g, 10 minutes) and the pellet was treated with H2SO4 0.05 M (6 ml for 30 mg of PG-AG) for 4 days at 37°C. After centrifugation (3,000 g, 30 minutes), the supernatant (containing AG) was recovered, neutralized with NaOH, freeze-dried and suspended in Milli-Q water before use. The pellet obtained after the H2SO4 treatment and containing the PG, was recovered, washed 3 times with Milli-Q water, freeze-dried, and suspended in Milli-Q water before use. However, analysis of the glycosyl composition of this PG preparation indicated incomplete acid hydrolysis with approximately 30% of the AG remaining associated to PG (named PG-AGh in S2 Fig).
LM/LAM were extracted from delipidated cells. Briefly, dried cells were suspended in 50% ethanol, disrupted by sonication and incubated 5h at 65°C. After centrifugation (4,000 g, 15 minutes), supernatant was recovered, evaporated and freeze-dried. The lyophilized material was re-hydrated in phosphate buffered saline (PBS) 0.1M and extracted with an equal volume of saturated phenol for 2 h at 80°C. The aqueous layer (containing lipoglycans) was recovered and dialyzed against Milli-Q water before being freeze-dried. The LM/LAM preparation was suspended in Milli-Q water before use.
MytA was purified from a culture supernatant of a ΔMytA strain expressing a MytAhis, as decribed in [28].
Mutant generation and immunological screening
Plasmid pCGL0040 (a non-replicative delivery vector containing Tn5531 [23]) was used to transform fresh electrocompetent Corynebacterium 2262 cells [17] and Km resistant transformants were selected on BHI-agar plates supplemented with Km. Subsequently, 10,073 mutants were picked from the original plates and transferred both on BHI-Km-agar plates (12 x 12 cm x 14 mm) entirely covered with a nitrocellulose sheet (Protran® BA-85, Schleicher and Schuell) and to 96-well microtiter plates containing BHI-Km. To grow bacteria, microtiter plates were incubated at 30°C with shaking overnight before sterile glycerol was added (26.5% v/v final concentration). Plates were stored at—80°C until further use. BHI-Km-agar plates covered with nitrocellulose membranes were also placed at 30°C. Generally, the nitrocellulose sheet was recovered after 16 hours of culture. Nevertheless, when slow-growing colonies were detected, the corresponding mutants were transferred on a new nitrocellulose/BHI plate and allowed to grow for a longer period (24 to 30 h). In order to obtain a replicate of the agar plate, immediately after removal of the nitrocellulose membrane on which the colonies have grown, a new membrane was placed on the plate by gently pressing it to allow a total contact with the agar. After 1 hour of contact at room temperature, the membrane was recovered. All nitrocellulose membranes were treated in the same way. They were washed 3 times for 5 min with phosphate-buffered saline containing 0.05% (v/v) Tween 20 (PBST), in particular, to completely remove bacteria from the surface of the culture membranes. Membranes were then incubated overnight in blocking buffer (PBST, 5% skim milk powder at 4°C), followed by 3 washes in PBST and a 2 h incubation with primary antibodies against CW (rabbit serum diluted 1:2,000 in PBST). Membranes were then washed 3 times for 5 min in PBST and incubated 1 h with alkaline phosphatase-conjugated secondary antibody (Anti-Rabbit IgG (Fc), AP Conjugate antibody, Promega, 1:7,000 in PBST). After 2 subsequent 5 min washes in PBST, membranes were incubated in a 0.165 mg/ml BCIP (5-bromo-4-chloro-3-indolyl phosphate, Promega)/ 0.33 mg/ml NBT (p-nitroblue tetrazolium chloride, Promega) containing solution (100 mM Tris pH 9.5, 100 mM NaCl, 5 mM MgCl2). The reaction development (the appearance of a halo around the colony mark on a culture membrane or of a signal on the corresponding replicate membrane, see above) was followed by comparison to the signal produced by control colonies present on the same culture membrane (the WT strain C. glutamicum 2262 and mutants Cg-Pks- and MytA- derived from RES167 and CGL2005, respectively, or from C. glutamicum 2262 i.e. mutants 1928 and 308). The reaction was stopped by rinsing membranes in deionized water.
At the end of this first screening round, the positive mutants (350) were selected and subjected to the same analysis a second time.
Identification of disrupted genes containing transposon insertions
Identification of the flanking regions adjacent to the transposon insertions was carried out by inverse PCR [29] or arbitrary-primed PCR [30]. Primers used for these PCR reactions are provided in S1 Table. For inverse PCR, we used the protocol described in Green and Sambrook [29]. Briefly, chromosomal DNAs extracted from the different mutants were digested with either SalI or EcoRI restriction endonucleases and then self-ligated with T4 ligase. PCR were performed using primer pairs Isb01/CdsX or Isb04/CdsVIII with the circular DNAs from EcoRI or SalI digestions, respectively. Depending on the quantity and purity obtained, PCR products were either purified on columns or purified from agarose gels and, in most cases, sub-cloned into plasmid pCR®2.1-TOPO® using the TOPO TA cloning kit (Invitrogen) prior to sequencing. PCR products were sequenced using primers Isb04 or Isb01 or Rev and F-20. For arbitrary-primed PCR, the first PCR round was performed with approximately 100 ng of genomic DNA as template, using the Phusion High Fidelity DNA polymerase. We used a primer specific for the transposon (Isb01) and a first arbitrary primer that we designed after a search for pentameric sequences present at least 6,000 times in the genome of ATCC13032 strain but absent from the transposon (ARB4020). PCR was performed as follows, with primers at a final concentration of 0.5 μM: 5 min 98°C, 6 cycles (10 s 98°C, 30 s 30°C, 1 min 30 s 72°C), 30 cycles (30 s 98°C, 30 s 45°C, 2 min 72°C) and finally 72°C for 4 min. The PCR products obtained from this first PCR were purified, and one tenth served as template for a second-round reaction. We used a second arbitrary primer (ARBq) that paired with the 5’ end of ARB4020 and a primer that pairs with the transposon downstream of the Isb01 sequence (Isb012). Second-round PCR was performed as follows, with primers at a final concentration of 0.2 μM: 1 min 98°C, 30 cycles (30 s 98°C, 30 s 55°C, 2 min 72°C) and finally 72°C for 4 min. If only one major band was visible on an agarose gel after this second-round PCR, the product was purified and sequenced. However, when several bands of comparable intensity were present on an agarose gel, the different products were purified from the gel and submitted to a third round of PCR in conditions identical to that of the second round. PCR products were sequenced using primer Isb013.
Bioinformatic analyses
Sequences interrupted by the transposon were aligned using BLASTn online software at the NCBI (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov) using Corynebacterium genomes as the search set. Best matches were systematically obtained with sequences from SCgG1 and SCgG2 genome strains. However as neither of these two genomes is annotated, transposon insertion locations were established with respect to the sequence of the ATCC13032 genome (NCBI Reference Sequence: NC_006958.1).
Conserved domains, or functional units within proteins, were searched using the Conserved Domain Database (CDD, https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) at the NCBI [31] and the Integrated Microbial Genomes and Microbiomes web resources (IMG/M: https://img.jgi.doe.gov/m/) [32]. Unknown proteins were classified into general categories using EggNOG (Evolutionary genealogy of genes: Non-supervised Orthologous Groups) [33] (http://eggnog5.embl.de).
Analysis of the C. glutamicum transcriptome published by Pfeifer-Sancar et al. [34] was used to predict genes inactivated in operons.
Construction of plasmids and bacterial strains
The different plasmids used in this study are described in Table 1. In order to delete cg1246, we used the strategy described by Schafer et al. [24]. In brief, two DNA fragments overlapping the gene at its 5’ and 3’ extremities were amplified by PCR from C. glutamicum total DNA using appropriate primers (1246-del1/1246-del2 and 1246-del3/1246-del4, see S1 Table) and cloned in the non-replicative vector pK18mobSac. The resulting plasmids (pK18mobsacΔ1246) was sequenced and transferred into C. glutamicum RES167 by electroporation. Transformants in which the construct was integrated into the chromosome by single crossing-over were selected on BHI plates containing Km. The second crossover event was selected by plating KmR clones on BHI plates containing 10% sucrose. Km-sensitive and sucrose-resistant colonies were screened by PCR for the correct deletion of the gene using appropriate primers. After verification of PCR products by sequencing, one strain carrying the cg1246 deletion (Δ1246 strain) was selected for further studies.
A complementation vector encoding Cg1246 (pCGL2420) was constructed using pCGL482 as the cloning vector [25]. Two DNA fragments were amplified by PCR from C. glutamicum RES167 chromosomal DNA: the coding sequence of cg1246 (using the primer pair 1246-RcaI/1246-XhoI) and the promoter region of the operon cg1247-cg1246 (304 bp upstream the ATG of cg1247, using the primer pair p1246-RcaI/p1246BglII) (see S1 Table). The amplicons were ligated together, digested with RcaI and XhoI and inserted into the BamHI/XhoI digested pCGL482 to obtain plasmid pCGL2420.
Biochemical analyses of selected mutants
Protein analyses
Cells grown overnight (equivalent of 1 ml bacterial suspension at an OD650 = 10) were centrifuged at 16,000 g for 5 min. Then, 800 μl of the supernatant, which contained the secreted proteins, were added to 200 μl of 50% TCA and the mixture incubated for 1 h at 4°C. The precipitated proteins were collected by centrifugation, the pellet was washed with acetone and solubilized in 50 μl of Laemmli denaturing buffer. The bacterial pellet was incubated in 50 μl of 50 mM Tris-HCl pH 6.8, 2% (w/v) SDS at 100°C for 3 min and centrifuged at 16,000 g, for 5 min at 4°C. The supernatant, which contained the cell wall proteins, was recovered. Proteins were separated by SDS-PAGE and gels were stained with Coomassie brillant blue R-250.
Lipid analysis
Lipids were extracted from wet cells for 16 h with CH3OH/CHCl3 (2:1 v/v) at room temperature. The organic phase was evaporated to dryness and lipids were solubilized in CHCl3 (typically 100 μl for lipids extracted from 20 ml of exponentially growing cells or 10 ml of cells in stationary phase). Lipids were analysed by Thin Layer Chromatography (TLC) on silica gel-coated plates (G-60, 0.25 mm thickness, Macherey-Nagel) developed with CHCl3/CH3OH/H2O (65:25:4, v/v/v). Detection of all classes of lipids was performed by immersion of the TLC plates in 10% H2SO4 in ethanol, followed by heating at 110°C; glycolipids were revealed by spraying plates with 0.2% anthrone (w/v) in concentrated H2SO4, followed by heating at 110°C.
The various classes of extractable lipids were also analysed by TLC after radiolabelling. Briefly, 1 μCi of [1-14C]-palmitate (2.22 GBq mmol-1, Perkin Elmer) was added to 10 ml culture medium of exponentially or stationary phase-grown bacteria and further incubated for 1.5 h at 30°C. After centrifugation, the cell pellets were extracted twice with CHCl3/CH3OH (1:2, v/v, then 2:1, v/v) for 2x24 h. The organic solutions were separated from the delipidated cells by filtration, then pooled and dried. The crude lipid extracts were resuspended in CHCl3 at 20 mg/mL and 15 μL were spotted onto a Silica Gel 60 TLC plate run in CHCl3/CH3OH/H2O (65:25:4, v/v/v). Labelled lipids were visualized with a Typhoon phosphorImager (Amersham Biosciences). The relative percentage of radioactivity incorporated in TMM and in TDM was determined using Image Quant software (GE Healthcare).
Results and discussion
Development of an original screen to detect mutants with envelope disruption
To identify new genes involved in Corynebacterium cell envelope biogenesis, we developed an effective and rapid test using polyclonal antibodies directed against CW. For this purpose, we took advantage of an observation that we made with well-characterized mutants partly or totally devoid of mycolic acids. Indeed, as shown in Fig 1, these mutants released, to the external medium, molecules that reacted with anti-CW antiserum. This excretion was clearly visible as a halo around a colony when bacteria were spotted and grown on a nitrocellulose membrane on a BHI plate, after immunoblotting with anti-CW antiserum. No signal was visible when the corresponding pre-immune serum was used instead of the anti-CW antiserum (S1A Fig). Moreover, the size of the excretion halo is commensurate with the importance of the alteration, as can be seen from a comparison of the wild type (WT) strain, the MytA- mutant [16], the double MytA-/MytB- mutant [21] and the Cg-Pks- [9] or AccD3- mutants [22] which produce 100%, 60%, 40% or no mycolic acids, respectively. The same pattern was also obtained with 2 other mutants defective in AG biosynthesis: AftB- [35] and DprE2- [36] (S1B Fig).

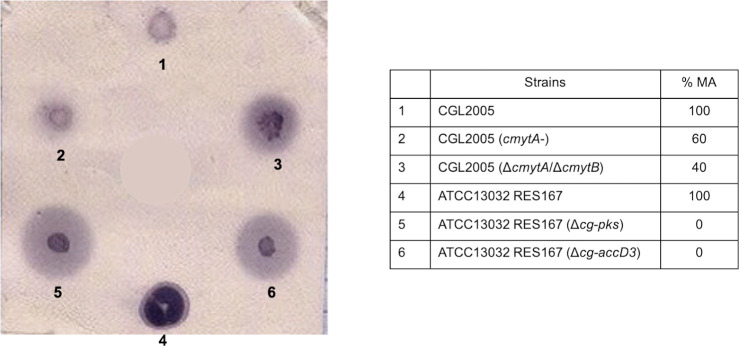
Immunological test for the detection of mutants with an altered cell envelope permeability.
Different C. glutamicum strains were spotted and cultivated on a nitrocellulose membrane placed on a BHI plate as described in Materials and Methods. The membrane was first treated with primary antibodies against CW and then by a classical western blot procedure (phosphatase alkaline coupled secondary antibodies and NBT/BCIP revelation). Clone outlines are colored in dark purple and are visible for all strains. Mutants are surrounded by a very distinct halo (light purple around the colony), the size of which is proportional to the importance of the cell envelope perturbation. The strains used in this test (numbered 1 to 6 in the blot) are given in the table. The % MA indicates the proportion of MA in the mutants as compared to the corresponding parental strain.
In order to identify which components were actually detected by the anti-CW antiserum, we performed an immunoblot with individual envelope components. These include: AG, PG-AG, cell-wall associated lipoglycans, “free” lipids (lipids extracted with organic solvents), MytA (which is one of the most abundant proteins found in the cell wall of C. glutamicum), and also some monosaccharides of interest (arabinose, galactose, mannose and glucose). In order to mimic the conditions used to screen our mutants, all solutions containing envelope components were dropped on a nitrocellulose membrane placed on a BHI agar plate and incubated for 10 minutes at room temperature. Possible diffusion of the molecules across the membrane to the agar was also examined by making a replicate of the plate, as described in materials and methods. As can be seen in the S2 Fig, none of the monosaccharides, nor free lipids, are recognized by the anti-CW antiserum. A very low response is obtained for MytA. Purified AG and PG-AG preparations produce very weak signals. Surprisingly, PG-AG partially hydrolyzed (in which 70% of the AG was removed) is much more recognized by the anti-CW antiserum. This could be due to a better access of antibodies to their epitopes, as a result of the alteration of the polymer conformation. Finally, the highest signal was obtained with the lipoglycan solution, containing a mixture of lipoarabinomannan (LAM) and lipomannan (LM). Interestingly, lipoglycans are the only molecules that rapidly diffuse on and, through the membrane, giving a signal on the “imprint” membrane, reminiscent of what is observed for excreted compounds in the cell envelope mutants (see also S3 Fig). Overall, this experiment suggests that more than one cell envelope component is recognized by the anti-CW antiserum, but that lipoglycans are among the most likely excreted molecules detected by antibodies in our screen. Although we were unable to precisely determine the nature of the excreted compounds reacting with the anti-CW antibodies, we believe that this information was not essential for mutant screening and that this assay could thus be a very powerful means to identify new genes involved in envelope biogenesis.
Construction of a transposon library in C. glutamicum and large-scale immuno-screening of mutants with an altered envelope
A transposon mutant library of C. glutamicum was generated using an IS1207-based transposon, Tn5531, cloned into a non-replicative delivery vector [23]. This system was shown to be effective for random mutagenesis in C. glutamicum 2262, a strain that does not contain the IS1207 sequence in contrast to the reference strain ATCC13032 ([23] and unpublished results). We generated 10,073 insertion mutants in this strain that we analysed by means of our immunological test.
The scheme of the library screening is depicted in Fig 2. Because differences in growth kinetics of mutants and in plate humidity could generate variability in the diffusion rate of the antigenic molecules from the nitrocellulose to the agar, we tested each mutant for both the presence of a halo around the colony and/or the presence of a positive signal on the plate footprint (S3 Fig). To ensure reliable immunological responses, two rounds of screening were performed. In this way, we were able to select 133 mutants that unambiguously excrete into the medium a compound recognized by anti-CW antiserum. In order to refine these data, we searched for characteristics that are commonly observed in cell wall mutants and that could be present in these pre-selected mutants. These mainly concerned (i) growth and phenotype differences and (ii) differences in cell wall and secreted protein profiles as compared to the parental strain. For the first parameter, we analysed colony phenotypes on agar plates, the rate of growth on solid and liquid media, the tendency to aggregate in liquid culture and the presence of cell shape or division defects visible by optical microscopy. The second parameter was based on observations previously made that an alteration of cell-wall architecture makes the strain more sensitive to SDS treatment, leading to the extraction of a greater number of cell envelope proteins [21]. Cell envelope alteration also often produces a leakage of cell wall proteins into the culture medium [16]. We then sorted the mutants by assigning them a score as follows: we rated 1 or 2 the immunological signal produced by a mutant according to the size of its diffusion halo (see S3 Fig). We assigned 1 point to each mutant exhibiting at least one of the phenotypic or growth differences listed above and 1 point to each mutant with significant differences in cell wall/secreted protein profiles (see S4 Fig for examples). After summing these three scores, only mutants with a total score ≥ 2 were retained, reducing their number from 133 to 80 (i.e. 0.8% of all the 10,073 mutants analysed by our immunological screening). Details of the scores attributed to each of the mutants finally selected are provided in S2 Table.

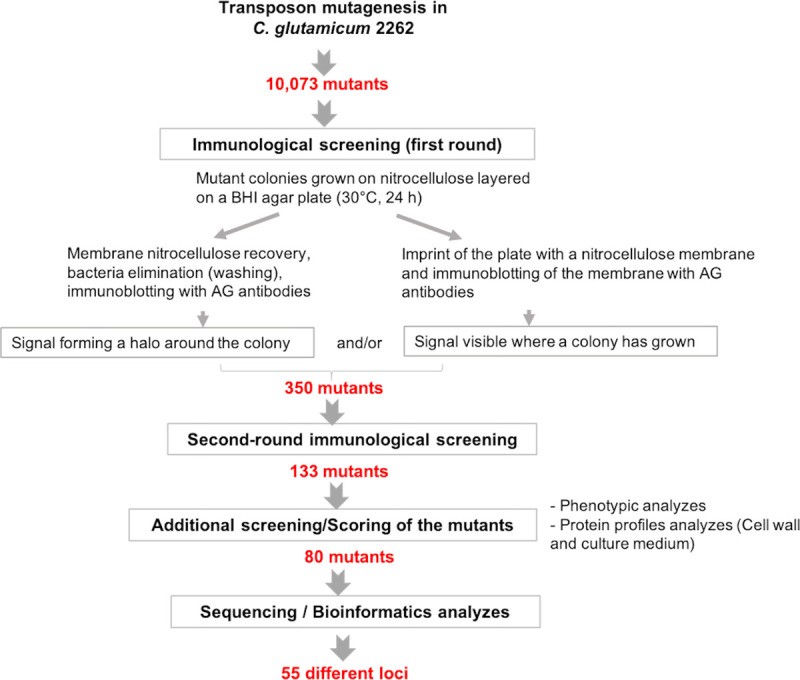
Flow chart of the screening procedure used in this study.
See Materials and Methods for details.
Analyses of Tn insertions
The precise insertion site of the transposon in the genome of each of these 80 mutants was then determined by PCR analyses (inverse or arbitrary PCR) and DNA sequencing. Because the sequence of the C. glutamicum 2262 strain is not available, a nucleotide BLAST analysis was performed for each of the amplicons against all C. glutamicum genomes available in the National Center for Biotechnology Information (NCBI) database. In all cases, similar DNA sequences were found with the highest identity scores systematically obtained for strains SCgG1 (NC_021351.1) and SCgG2 (NC_021352.1), two industrial glutamate hyper-producing strains. As shown in Fig 3A, the selected mutations were distributed all along the chromosome, but with a number of loci hit several times and a substantial number of insertions in a region known to be involved in envelope biosynthesis (Fig 3A and 3B, see below). Of the 80 sequences interrupted by the transposon, 79 could also be unambiguously mapped on the ATCC13032 strain chromosome sequence. The only sequence that could not be mapped was identified at the upstream region of the mytC gene orthologue in SCgG1 and SCgG2. Because the ATCC13032 strain is the most documented of the C. glutamicum strains, we chose to refer to it, and in particular to the NCBI Reference Sequence: NC_006958.1 [37]. In this context, and in the absence of genome annotation for C. glutamicum 2262, we annotated genes in this strain by the locus tag identifier and the gene symbol of the orthologous locus found in ATCC13032 preceded by ort- for the locus tag.

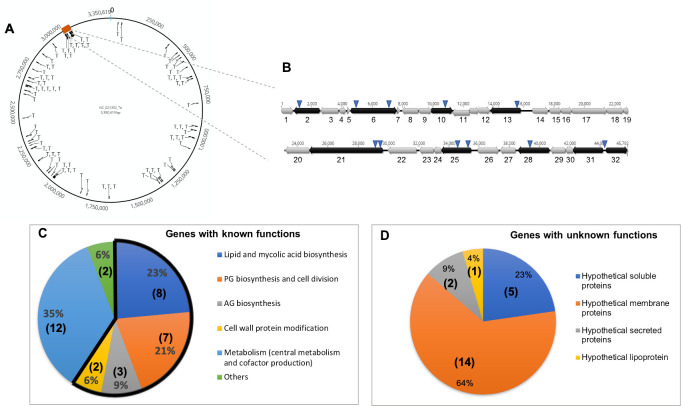
Overview of the mutant analyses.
(A) Location of transposon insertions (indicated by T) corresponding to the 80 selected mutants, mapping to the genome of C. glutamicum SCgG2. The numbers outside the circle represent the base pairs (from 0 to 3,350,619). The red rectangle corresponds to the large cluster of genes known to be involved in cell envelope biogenesis, which is detailed in (B). (B) Schematic representation of the cell wall biosynthetic gene cluster of C. glutamicum ATCC13032. This large cluster includes many genes involved in mycolic acid and AG biosynthesis. The cluster is given from cg3156 to cg3192 (about 45.8 kb) but its limits are not precisely known. The distances on the chromosome are indicated. Gene orientation is indicated by arrows; genes that were insertionally-inactivated are in black; the locations of transposon insertions are indicated by inverted triangles. For better readability, the genes are numbered from 1 to 32. Details of their annotations (locus tag, protein name and function) are provided below. 1: cg3156 (HsecP), 2: cg3157 (HsecP), 3: cg3158 (NagA2: putative β-N-acetylglucosaminidase), 4: cg3159 (UspA), 5: cg3160 (HsecP), 6: cg3161 (AftD, arabinosyltransferase, AG biosynthesis), 7: cg3162 (HP), 8: cg3163 (TmaT, TMCM mycolylacetyltransferase), 9: cg3164 (HMP), 10: cg3165 (HMP), 11: cg3166 (HP, putative glycosyltransferase), 12: cg3167 (HP), 13: cg3168 (MtrP, methyltransferase), 14: cg3169 (PhosphoenolPyruvate Carboxykinase), 15: cg3170 (Tellurite resistance protein or related permease), 16: cg3172 (tRNA (guanine-N(7)-)-methyltransferase), 17: cg3173 (HP), 18: cg3174 (CmpL1 mycolic acid transporter), 19: cg3175 (HMP), 20: cg3176 (HP), 20: cg3177 (AccD3, subunit of Acyl-CoA carboxylase complex, mycolic acid biosynthesis), 21: cg3178 (Cg-Pks, mycolic acid condensase), 22: cg3179 (Cg-FadD2, fatty acyl-AMP ligase, mycolic acid biosynthesis), 23: cg3180 (Elrf, envelope lipid composition regulator), 24: cg3181 (HSecP), 25: cg3182 (MytA, mycoloyltransferase), 26: cg3185 (Pcons, HP), 27: cg3186 (MytB, mycoloyltransferase), 28: cg3187 (AftB, AG biosynthesis), 29: cg3189 (UbiA, decaprenylphosphoryl-D-arabinose (DPA) biosynthesis), 30: cg3190 (5'-phosphoribosyl-monophospho-decaprenol phosphatase, DPA biosynthesis), 31: cg3191 (Glft2, galactosyltransferase, AG biosynthesis), 32: cg3192 (HMP). (C) Pie chart representing the distribution, by functional categories, of the proteins of known function identified by our screening procedure. The part of the circle outlined in black represents the categories that are directly related to the biogenesis of the cell envelope. Numbers in parenthesis represent the number of genes in the corresponding category. (D) Pie chart representing the distribution, by putative localizations, of the proteins of unknown or poorly characterized functions identified by our screen. Numbers in parenthesis represent the number of genes in the corresponding category.
In 70% of the mutants, the transposon insertion was found to occur inside an open reading frame (ORF). However, in the remaining 30%, transposon insertion site was found in a non-coding region at the 5’-end of an ORF (between 10 base pairs (bp) and up to 200 bp upstream of the start codon, depending on the mutants), that we assumed to be the promoter region (noted pr-), the interruption of which may, more or less dramatically, affect downstream ORF expression.
Of these 80 mutants, only 55 corresponded to different disrupted loci. Indeed, in 15 cases the same ORF and/or promoter region was found to be disrupted by the insertion in 2 (7 cases), 3 (7 cases) or 5 times (1 case). Nevertheless, in 3 cases, several insertions were in the exact same position (3 in pr-fasI, 2 in pr-lgt and 2 in steA, see S2 Table). We cannot exclude that identical insertions came from cross-contaminations although this seems unlikely because the different strains did not originate from the same plates and that, at least for the fasI and steA mutants, the transposon (and consequently the aphIII gene) was not inserted in the same direction in all the mutants. The difference in the orientation of the aphIII gene at the 5’ end of the fasI gene probably lead to different polar effects that could explain the variations observed in the scores obtained for the three mutants with the same transposon insertion point.
It has been shown that only one-third of the approximately 3000 protein-coding genes of the ATCC13032 strain are transcribed monocistronically while the remaining two-thirds are part of operons [34]. If we assume that the transcriptional pattern of C. glutamicum 2262 is similar to that of the ATCC13032 strain, then, 35 of the interrupted loci lie within operons, which represent 64% of the total number of impacted loci, as would be expected if the transposon was randomly inserted into the genome.
All information concerning the mutants obtained in our library (score, transposon insertion sites, locus tags, prediction of a gene in an operon, characteristic of gene products) are given in S2 Table.
Immuno-screening with anti-CW antibodies is effective to identify genes involved in cell wall biogenesis of Corynebacteriales
Bioinformatic analyses of DNA sequences interrupted by the transposon showed that 34 loci (51 different mutants) correspond to ORFs or promoter regions of previously characterized genes (Fig 3C). These loci are shown in Table 2. Among them, 20 are directly involved in cell wall processes. Two genes encode proteins that synthetize essential molecules for cell envelope building: (i) fatty acids, the precursors of phospholipids and mycolic acids (5 hits) and (ii) decaprenyl-pyrophosphate, the lipid carrier of many precursors of cell wall compounds (insertion in the promoter region of uppS1). Thirteen interrupted loci were found to be directly involved in mAGP complex biosynthesis: cg-pks, pr-pptT, cmrA, pr-dtsR2, mytA, mtrP (mycolic acid biosynthesis), aftB, aftD, pr-glfT2 (AG biosynthesis), ponA, ftsI, alr, ltsA (PG biosynthesis). Three transposon insertions affected genes involved in cell division (fhaA, ftsK and mraW which, with ftsI, belongs to the dcw cluster). Two loci encoding enzymes that modify envelope proteins post-translationally were also interrupted: lgt, and pr-mytC, responsible for the transfer of a diacylglyceride or a mycolate onto proteins, respectively. Twelve of the 14 other genes are mainly related to metabolic functions and energy production, most of which may have an indirect influence on the biosynthesis of envelope compounds. This is most probably the case for genes encoding enzymes of central metabolism (lpd, mqo, deoC and zwf) or encoding proteins involved in the assembly of the cytochrome bc1–aa3 supercomplex (ctiP and pr-ctaD and surf1), the inactivation or under-expression of which will certainly modify respiratory chain activity and consequently carbon fluxes. Two genes (otsA and otsB), belonging to one of the three different trehalose synthesis pathways present in C. glutamicum, were also interrupted by the transposon. Because trehalose is the main acceptor of mycolates in the cell envelope [38], it is not very surprising to obtain such mutants by our screening. We found 3 independent insertions in pdxR, a gene encoding a positive transcriptional regulator of the pyridoxal 5’-phosphate synthase genes. Many enzymes use pyridoxal 5’-phosphate (PLP) as a cofactor, primarily thus involved in the biosynthesis of amino acids and their derivatives. For example, meso-diaminopimelate (m-DAP), an essential amino-acid in PG, is synthetized by two pathways, one of which (the succinyl pathway) uses DapC (the succinyl-diaminopimelate transaminase), a PLP-dependent enzyme, and it has been shown that mutations in this pathway led to loss of cell wall integrity [39]. We also found an insertion in bioM, which is part of the bioYMN operon encoding the biotin transport system. C. glutamicum is a biotin auxotrophic bacterium, and must import the cofactor from its environment by the ATP-dependent BioYMN transport system [40]. Biotin is essential for acyl-CoA carboxylases involved in fatty acid and mycolate biosynthesis and it has been shown that biotin limitation can lead to a small decrease in mycolic acid content but also to an important change in their chain length [41].

| Gene/locus | Nb | Protein and function | Ref |
|---|---|---|---|
| Lipid and isoprenoid biosynthesis | |||
| pr-fasI and fasI (ort-cg2743) | 5 | FasI-A: Fatty Acid Synthase | [42] |
| pr-uppS1 (ort-cg1130) | 1 | UppS1: catalyzes the synthesis of the lipid carrier decaprenyl pyrophosphate | [43] |
| Mycolic acid biosynthesis | |||
| pr-dtsR2 and dtsR2 (ort-cg0811) | 3 | DtsR2/AccD2: β subunit of the acetyl-CoA carboxylase involved in MA biosynthesis | [44] |
| pptT (ort-cg2171) | 1 | PptT: 4’-phosphopantetheinyl transferase that activates the mycolic acid condensing enzyme Cg-Pks | [45] |
| mtrP (ort-cg3168) | 1 | MtrP: a methyltransferase required for optimal transport of TMM, precise function unknown. | [46] |
| pr-cg-pks and cg-pks (ort-cg3178) | 2 | Cg-Pks: fatty acid condensase that synthetizes MA | [9] |
| cmrA (ort-cg2717) | 1 | CmrA: MA reductase. | [47] |
| mytA/cop1 (ort-cg3182) | 2 | MytA: mycoloyltransferase A, catalyzes the transfer of a MA onto TMM (to give TDM) and AG | [15, 16] |
| Arabinogalactan biosynthesis | |||
| aftD (ort-cg3161) | 2 | AftD: arabinosyltransferase D involved in the biosynthesis of the arabinan domain of AG | [48] |
| aftB (ort-cg3187) | 1 | AftB: arabinosyltransferase B involved in the biosynthesis of the arabinan domain of AG | [49] |
| pr-glfT2 (ort-cg3191)* | 1 | GlfT2: galactosyltransferase that transfers Galf to the arabinan domain of AG | [50] |
| Peptidoglycan biosynthesis and cell division processes | |||
| fhaA (ort-cg0064) | 1 | FhaA: cell-division associated protein. fhaA is the first gene of an operon involved in cell division. | [51] |
| ponA (ort-cg0336) | 1 | PonA/Pbp1a: Penicillin-Binding Protein 1A | [52] |
| alr (ort-cg0681) | 1 | Alanine racemase: converts L-alanine to D-alanine used in PG biosynthesis | [53] |
| ltsA (ort-cg2410) | 1 | LtsA: glutamine amidotransferase that catalyzes the amidation of cell wall PG diaminopimelic acid (DAP) residues | [54] |
| pr-ftsK (ort-cg2158) | 2 | FtsK: putative cell division protein involved in chromosome partitioning | [51] |
| pr-ftsI (ort-cg2375) | 1 | FtsI: Penicillin-Binding Protein. ftsI is the first gene of an operon which is part of the dcw cluster | [55] |
| mraW (ort-cg2377) | 1 | MraW: putative S-adenosylmethionine-dependent 16SRNA methyltransferase. The mraw gene is part of the dcw cluster (orthologous to MraW of E. coli) | [56] |
| Post-translational modifications of envelope proteins | |||
| pr-mytC (ort-cg0413) | 1 | MytC: mycoloyltransferase that catalyzes protein mycoloylation | [57] |
| pr-lgt and lgt (ort-cg2292) | 3 | Lgt: prolipoprotein diacylglyceryl transferase | [58] |
| Metabolism and energy production | |||
| lpd (ort-cg0441) | 1 | Lpd: dihydrolipoamide dehydrogenase, a component of the pyruvate dehydrogenase complex and the oxoglutarate dehydrogenase complex (TCA cycle) | [59] |
| deoC (ort-cg0458) | 1 | DeoC: deoxyribose-phosphate aldolase, produces D-glyceraldehyde 3-phosphate and acetaldehyde which enters central metabolism through the glycolytic pathway and the TCA cycle, respectively | [60] |
| proC (ort-cg0490) | 1 | ProC: pyrroline-5-carboxylate reductase catalyzes the formation of L-proline from pyrroline-5-carboxylate | [61] |
| Zwf (ort-cg1778) | 1 | Zwf: glucose-6-phosphate 1-dehydrogenase (pentose phosphate pathway) | [60] |
| mqo (ort-cg2192) | 1 | Mqo: malate:quinone oxidoreductase (TCA cycle) | [60] |
| surf1 (ort-cg2460) | 1 | Surf1: involves in the assembly of cytochrome aa3 oxidase probably in relation to the heme a insertion into the CtaD apo-protein (respiration) | [62] |
| ctiP (ort-cg2699) | 1 | CtiP: involves in the biogenesis of cytochrome aa3 oxidase by transporting and transferring copper to the CuB center of CtaD (respiration) | [63] |
| pr-ctaD (ort-cg2780) | 2 | CtaD: cytochrome aa3 oxidase subunit I (respiration) | [64] |
| otsA (ort-cg2907) | 1 | OtsA: trehalose 6-phosphate synthase (trehalose biosynthesis) | [38] |
| otsB (ort-cg2909) | 1 | OtsB: trehalose 6-phosphate phosphatase (trehalose biosynthesis) | [38] |
| Cofactor biosynthesis and transport | |||
| pdxR (ort-cg0897) | 3 | PdxR: transcriptional regulator of genes involved in pyridoxal 5'-phosphate (PLP) synthesis | [65] |
| bioM (ort-cg2148) | 1 | BioM: biotin transport protein (ATPase) | [40] |
| Others | |||
| pr-ohr (ort-cg0038) | 1 | Ohr: Organic Hydroperoxide Resistance protein | [66] |
| pr-nusG or nusG (ort-cg0562) | 3 | NusG: orthologue of Rv0639, a NusG paralogue which interacts with ribosomal protein S10, and thereby participates in transcription–translation coupling. | [67] |
Nb: Number of mutants interrupted in the same locus
*: The transposon was inserted in a non-coding region at an equivalent distance from the ATG of the glfT2 and cg3192 genes
These results unambiguously showed that our immunological screen is a powerful tool for the identification of proteins involved in cell wall compound biosynthesis and, more widely, in cell wall biogenesis (Fig 3C).
Identification of 22 putative new players in cell wall biogenesis of C. glutamicum
Twenty-two loci (corresponding to 30 different mutants) of unknown, or poorly characterized functions have been identified by our screening method. As shown in Table 3, approximately 60% of the uncharacterized proteins fall into the category “function unknown” according to the EggNOG functional classification [33]. Although we have very limited (or no) indications of their function, as expected from the panel of known genes identified in Table 2, at least one half of these unknown proteins could be involved in cell wall biogenesis. To support this hypothesis, an analysis of the translated sequences showed that, while 5 correspond to hypothetical cytosolic proteins, 13 correspond to hypothetical membrane proteins and 3 to putative secreted proteins (Table 3 and Fig 3D). Thus, 73% of the unknown proteins found in this study are predicted to localize in the bacterial cell envelope (inner membrane or cell wall), a result that reinforces the idea that most of the proteins targeted by our screen are associated with cell envelope functions. It should be noted that for 2 mutants (5267 and 3464 see S2 Table), we do not know if only one gene was affected by the transposon and, if so, which one. In mutant 5267, the transposon insertion was localized both at the very beginning of the coding sequence of ort-cg1137 but also presumably in the ort-cg1136 promoter. Both genes encode unknown proteins. In mutant 3464, the transposon was inserted in an intergenic region at an equal distance from each of the ATG codons of ort-cg3191 (glfT2) and ort-cg3192 (encoding an unknown function). Because of its role in AG biosynthesis, it is tempting to favor an impact on glfT2 (Table 2), but an impact on ort-cg3192 cannot be excluded.

| Locus | Nb | EggNOGa | Localizationb | Conserved domain/predicted function |
|---|---|---|---|---|
| ort-cg0530 | 1 | S | Mb | DUF4229 (PF14012) domain-containing protein. |
| ort-cg0575 | 2 | S | Mb | DUF3068 (PF11271) domain-containing protein. |
| ort-cg0853 | 1 | S | Cyt | DUF3499 (PF12005) domain-containing protein. First gene in an operon involved in the synthesis of Man6P the precursor of the sugar donor GDP-Man. |
| pr-ort-cg0947 | 1 | S | Cyt | DUF3071 (PF11268) domain-containing protein. |
| ort-cg11371 | 1 | K | Cyt | Putative transcriptional regulator of LysR type (cd05466 conserved domain). |
| ort-cg1246 | 3 | S | Cyt | DUF402 (PF04167) domain-containing protein. Also classified in COG2306: Predicted RNA-binding protein, associated with RNAse of E/G family. |
| ort-cg1254 | 1 | S | Mb | Classified in COG2246 and PF04138: putative flippase GtrA (transmembrane translocase of bactoprenol-linked glucose). Proteins of the GtrA family are predicted to be involved in the biosynthesis of cell surface polysaccharides. |
| ort-cg1270 | 1 | E | Cyt | Classified in COG4122 and PF13578: Predicted O-methyltransferase. |
| ort-cg1603 and pr-ort-cg1603 (steA) | 3 | S | Mb | Classified as membrane-anchored protein (COG4825), with a thiamine pyrophosphokinase C terminal domain—TPPK_C (PF12555). Function unknown, but recently identified by Lim et al. [13] as part of a complex involved in cytokinesis and named SteA. |
| pr-ort-cg1735 | 1 | D/M | Env | Putative cell wall-associated hydrolase with a NlpC C-terminal domain (COG0971 and PF00877: NLPC_P60). The 134 last amino-acids are homologous to the C-terminal domain of RipA (Rv1477) a PG endopeptidase that cleaves the bond between D-glu and meso-DAP |
| ort-cg2157 (terC) | 2 | P | Mb | Classified in COG0861 and PF03741: integral membrane protein of the TerC family, possibly involved in tellurium resistance |
| ort-cg2207 (rspE) | 1 | M | Mb | Predicted Zn-dependent protease homologous to the Rip1 metalloprotease which is a determinant of M. tuberculosis cell envelope composition and virulence (COG0750: Membrane-associated protease RseP) |
| ort-cg2397 | 1 | S | Mb | Classified in acyltransferase family (Acyl_transf_3, PF01757) transferring acyl groups other than amino-acyl groups. Also classified in COG4763: uncharacterized membrane protein YcfT. |
| ort-cg2424 | 1 | S | Mb | DUF4191 (PF13829) domain-containing protein. |
| pr-ort-cg2657 | 1 | S | Mb | No conserved domain |
| ort-cg2811 | 3 | V | Mb | Classified as putative permease component of an ABC-type transport system, involved in lipoprotein release, (COG4591: LolE domain). 2 MacB_PCD domains (MacB-like periplasmic core domain, PF12704) and 2 FtsX domains (FtsX-like permease family, PF02687) |
| ort-cg2861 | 1 | S | Mb | Classified in COG1272 and PF03006: predicted membrane channel-forming protein, Hemolysin III related. |
| pr-ort-cg2971 | 1 | E/G/P | Mb | Lincomycin resistance protein LmrB. Predicted arabinose efflux permease, MFS family (COG2814) and major facilitator superfamily (MFS_1, PF07690). Identified by Kim et al. [68] as potentially involved in lincomycin efflux. |
| ort-cg3052 | 1 | S | env/mb | Putative secreted protein with prokaryotic membrane lipoprotein lipid attachment site profile. No conserved domains. |
| ort-cg3157 | 1 | V | Env | Putative secreted protein. Classified in COG2720: vancomycin resistance protein YoaR (function unknown), contains putative peptidoglycan-binding domain (PG_binding_4, PF12229) |
| ort-cg3165 | 1 | S | Mb | No conserved domain |
| ort-cg31922 | 1 | S | Mb | DUF5129 (PF17173) domain-containing protein. |
Nb: Number of mutants interrupted in the same locus
a Letters refer to the EggNOG (Evolutionary genealogy of genes: Non-supervised Orthologous Groups) classification of functions [33]. D: cell cycle control, cell division, chromosome partitioning, E: amino acid transport and metabolism, G: carbohydrate transport and metabolism, K: transcription M: cell wall/membrane/envelope biogenesis, O: post-translational modification, protein turnover, and chaperones, P: inorganic ion transport and metabolism, Q: secondary metabolite biosynthesis, transport, and catabolism, S: function unknown, V: defense mechanisms.
b Predicted localization from the primary sequence of the putative protein: cytoplasm (cyt), membrane (mb) and envelope (env).
1 The transposon integration into this locus could also affect the transcription of cg1136, as the insertion is also presumably in the promoter of cg1136, a gene encoding a protein of unknown function and without any conserved domain.
2 The transposon was inserted in a non-coding region at an equivalent distance from the ATG of the glfT2 and the cg3192 genes
Hypothetical membrane proteins
Among the 13 hypothetical membrane proteins uncovered here, 2 are most likely related to envelope dynamics. The first one is ort-Cg1603 (whose locus was inserted 3 times by the transposon) a membrane-anchored protein with a putative cytoplasmic domain and a poorly conserved thiamine diphosphokinase domain (PF12555) at its C-terminal. In ATCC13032, cg1603 is predicted to be transcribed with cg1604, which encodes a protein orthologous to the mycobacterial outer membrane protein MctB, involved in copper efflux [69]. Surprisingly, cg1603 and cg1604 were very recently identified as conferring ethambutol hypersensitivity and cell separation defects when inactivated [13]. The authors proposed that Cg1603 (named SteA) and Cg1604 (named SteB) both localized in the inner membrane at the division site where they form a complex. This complex was hypothesized to connect other division proteins and in particular a putative periplasmic PG endopeptidase (Cg1735) that we also identified in this study. The second protein is ort-Cg2207, a putative membrane-embedded Zn-dependent protease of the RseP (Regulator of Sigma E protease) family that may play a role in cell biogenesis regulation. Indeed, the orthologue in M. tuberculosis is the Rip1 protein which controls 4 extracytoplasmic function (ECF) sigma (σ) factor pathways (K,L,M and D) [70, 71] and influences the lipid composition of the mycobacterial envelope, including MAs, by controlling the transcription of many specific genes [72]. It is tempting to hypothesize that Cg2207 could act on the σD pathway that controls the integrity of the cell envelope in C. glutamicum [73, 74]. However, it is important to note that (i) unlike SigD (the sigma factor), the anti-sigma factor RsdA is not conserved between C. glutamicum and M. tuberculosis, (ii) the σD regulons are different between the two genera [73–75] and (iii) there is no experimental evidence for a proteolytic function of Cg2207 in vivo.
Four loci hit by the transposon encode potential membrane transporters (ort-Cg1254, ort-Cg2811, ort-Cg2157 and ort-Cg2971). Cg1254 is annotated as a putative flippase of the GtrA family (COG2246), a group of proteins predicted to be involved in the biosynthesis or translocation of precursors of cell surface polysaccharides. Cg2811 (whose gene was inserted 3 times) is annotated as a predicted ABC-type permease transport system member, with conserved domains that belong to protein families involved in lipoprotein or lipid transport across the envelope (COG4591). In ATCC13032, cg2811 is predicted to be transcribed with cg2812 that encodes the putative cognate ATPase component of the system. Cg2157 (whose gene was inserted 2 times) is annotated as TerC (for Tellurite resistance protein, COG0861) and classified in the general category “inorganic ion transport and metabolism”. It is interesting to note that cg2157 is located just downstream of the ftsK gene (cg2158) encoding a DNA translocase essential for cell division, that was also a target of the transposon (see Table 2). It appears that, neither the nature of the substrates transported by these three proteins, nor their role in the biosynthesis of the cell envelope can be deduced from these annotations. The fourth putative transporter (ort-Cg2971) is annotated as LmrB in ATCC13032 strain and was previously described to be involved in the proton-dependent efflux of the antibiotic lincomycin [68]. It is not currently known if Cg2971 transports other substrates in connection with envelope biosynthesis. Two other hypothetical membrane proteins identified here have sequences that matched with conserved domains or families (ort-Cg2397 and ort-Cg2861). Cg2397 is an uncharacterized membrane protein possessing a predicted acyl transferase domain transferring acyl groups other than amino-acyl groups (PF01757). Interestingly in ATCC13032, cg2397 is located in the vicinity of genes encoding proteins involved in envelope biogenesis: MptA (cg2385), MptD (cg2390) MptC (cg2393) and PimB’ (cg2400) (lipoglycan biosynthesis), MytF (cg2394) (mycolate biosynthesis), PlsC (cg2398) (phospholipid biosynthesis) and Cg2401 a putative secreted PG lytic protein. Cg2861 is a predicted membrane channel-forming protein belonging to the hemolysin III family (COG1272). Finally, 5 putative membrane proteins have been identified whose sequences matched with domains of unknown function (DUF) indexed in the PFAM database (ort-Cg0530, ort-Cg0575 (2 mutants) and ort-Cg2424) or has no conserved domain (ort-Cg2657 and ort-Cg3165). Although lacking any information on their putative function, 2 of these genes were found to be located in clusters involved in the biosynthesis of cell envelope compounds implying that they are relevant in this context. Indeed, cg0530 is surrounded by genes encoding proteins responsible for the biosynthesis of respiratory chain components (quinone, cytochrome c, heme) and cg3165 is within a large cluster dedicated to cell envelope biosynthesis. It is interesting to note that, eight genes from this locus were inserted by the transposon (ort-cg3157, aftD (ort-cg3161), mtrP (ort-cg3165), ort-cg3168, cg-pks (ort-cg3178), mytA (ort-cg3182), aftB (ort-cg3187) and potentially glfT2 (ort-cg3191) and/or ort-cg3192 (Fig 3B).
Hypothetical secreted proteins
Two proteins identified in this work possess a putative sec-type signal sequence (ort-Cg1735 and ort-Cg3157) and are likely related to PG metabolism. Indeed, Cg1735 possesses a C-terminal NlpC/P60 domain generally associated with a cell wall peptidase activity [76]. Although the protein was named RipC by Lim et al. [13], it is not orthologous to the protein of the same name in M. tuberculosis. Nevertheless, in accordance with a putative function in PG hydrolysis, and despite the absence of enzymatic data, two studies have shown that inactivation of the protein lead to important defects in cell separation, a result that links the protein to the division process in C. glutamicum [13, 77]. Cg3157 possesses both a PG-binding domain (PF12229) and a VanW domain (COG2720, associated to vancomycin resistance, a PG modification), which suggests a role of Cg3157 in PG metabolism.
One protein possesses a predicted lipoprotein lipid attachment site (ort-Cg3052) but has no discernable conserved domain.
Hypothetical soluble non-secreted proteins
Five proteins are predicted to be non-membranous and non-secreted (ort-Cg0853, ort-Cg0947, ort-Cg1137, ort-Cg1246 and ort-Cg1270). One of them (Cg1246) could be linked to cell envelope biosynthesis. Indeed, although the putative protein has unknown function, the corresponding gene (inserted by the transposon 3 times) is part of the σD regulon that regulates mycomembrane biosynthesis and PG structure [73, 74]. It is also possible that Cg0853 is indirectly involved in the construction of the cell envelope. Indeed, in ATCC13032, its gene is surrounded by genes involved in GDP-mannose biosynthesis (manA, encoding a mannose-6-phosphate isomerase and pmmA, encoding a phosphomannomutase which form an operon with cg0853 and rmlA2 encoding a mannose-1-phosphate guanylyl transferase). As this nucleoside-diphosphate-sugar is an important provider of mannose for lipopolysaccharide and protein mannosylation in the cell envelope, disruption of its synthesis could have negative effects on cell wall integrity. There are no current indications linking the three remaining proteins (ort-Cg1270, ort-Cg1137 and ort-Cg0947) to any process related to the cell envelope: Cg1270 is annotated as a putative O-methyltransferase (COG4122), Cg1137 as a putative regulator of the LysR family and Cg0947 a protein with a conserved domain of unknown function.
Approximately half of the non-characterized proteins are conserved among the Corynebacteriales
Since Corynebacteriales share unique properties in relation to their cell wall, we determined whether the proteins of unknown function identified in this work are conserved within this order. We chose 5 species, representative of the main genera that compose this order (M. tuberculosis, Rhodococcus erythropolis, Nocardia farcinica, Gordonia bronchialis, Tsukamurella paurometabola) and M. leprae because of its highly degenerate genome and searched for orthologues among these different species using BLASTp. As shown in S3 Table, 7 proteins identified by our screen (Cg0853, Cg0947, Cg1270, Cg1603, Cg2207, Cg2424 and Cg3165) have orthologues in all 6 species, with Cg1603 and Cg2207 being the best conserved between them (score > 200). Four proteins possess orthologues in all species except M. leprae (Cg1246, Cg2157, Cg2861 and Cg2971) with Cg2157 being the best conserved (score > 200). Thus, about half of the proteins found in this study is conserved among Corynebacteriales and in particular in M. tuberculosis. Of the 12 M. tuberculosis orthologous proteins, 5 are essential according to Sasseti et al. (Rv0883c, Rv1697, Rv2219, Rv0226c, Rv0224c) [78]. Five proteins appear to be specific to the Corynebacterium genera (Cg0575, Cg1254, Cg2397, Cg3052 and Cg3192) with Cg3052 restricted to C. glutamicum.
Cg1246 is very likely involved in mycolic acid metabolism
To identify transposon insertion in genes that are potentially involved in mycolate biosynthesis, we performed TLC analysis of organic solvent-extractable lipids from the 30 mutants interrupted in loci encoding proteins of unknown function and searched for those with a severe decrease, or a complete absence of TMM. Three mutants (4954, 6935 and 7968, S2 Table) clearly showed a significant decrease in TMM (Fig 4A). The 3 mutants were all interrupted in a single gene, ort-cg1246. We thus attempted to delete cg1246 in the C. glutamicum RES167 strain. For that purpose, a non-replicative pK18mobsac-derivative plasmid was constructed (pK18mobsacΔ1246) carrying sequences adjacent to the gene [24]. Kanamycin-sensitive and sucrose-resistant clones resulting from two recombination events between the plasmid and chromosome were easily obtained. Colonies in which the second recombination event led to proper deletion of the desired DNA fragment were identified using PCR with primers designed up- and downstream of this fragment. One mutant, corresponding to the expected deletion, was chosen for further characterization (Δ1246 strain). As expected from the results obtained with the ort-cg1246 interrupted mutants, the TLC profile of the Δ1246 extractable lipids showed a significant decrease in the TMM pool as compared to the WT strain, which was restored by complementation with a plasmid bearing a copy of the wild type cg1246 gene (Fig 4B). Quantification of trehalose lipids, performed after radiolabelling with 1-14C palmitate, and TLC analysis of the extractable lipids, confirmed the importance of the TMM deficiency in the Δ1246 strain (Fig 4C), which could be estimated as four-fold when compared to the WT strain in exponentially growing cells (Fig 4D). In contrast, the TDM pool remained comparable between these two strains under the same growth conditions. Thus, the Δ1246 mutant displayed a TMM/TDM ratio lower than that of the WT cells in exponential growth (Fig 4E). From these data, we conclude that Cg1246 has an important impact on the pool of TMM. How Cg1246 is connected to the mycolate pool could not be deduced from these preliminary data nor from the protein sequence itself. However, two lines of evidence argue in favor of a direct involvement of Cg1246 in mycolate biosynthesis. First, as highlighted above, the gene was found to be upregulated by σD, the ECF sigma factor which was shown to regulate MA biosynthesis in C. glutamicum [73, 74]. Second Cg1246 is well conserved in most of Corynebacteriales members and is mostly associated with bacteria of this order, a specificity that is in accordance with a metabolic function (mycolate biosynthesis) that is unique to bacteria of this taxon.

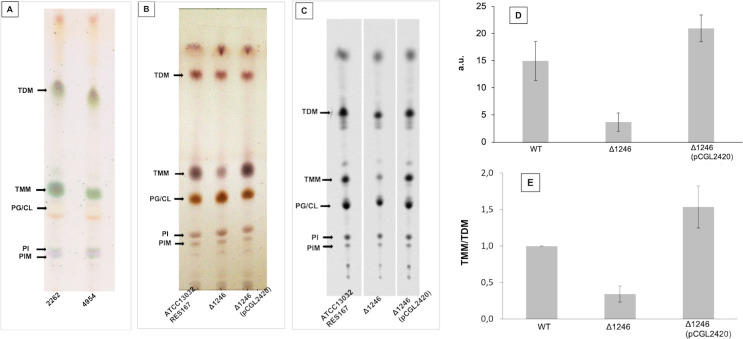
Lipid analyses of Cg1246 mutants.
(A) and (B): TLC analysis of crude lipid extracts isolated from exponentially growing cg1246-inactivated mutants. Lipids were extracted as described in Materials and Methods and comparable amounts were loaded on TLC plates. (A): parental strain C. glutamicum 2262 and its isogenic mutant 4954. Glycolipid spots were visualized by spraying 0.2% anthrone in H2SO4, followed by charring. (B): parental strain ATCC13032 and its isogenic mutant strains Δ1246 and Δ1246(pCGL2420). Lipids were visualized after immersion of the plate in 10% H2SO4 in ethanol, followed by charring. Arrows indicate the position of trehalose mono- and di-mycolate (TMM and TDM), respectively. (C) (D) and (E): lipid quantification of RES167 strain (WT) and its derivative Δ1246 and Δ1246(pCGL2420). Exponentially growing cells were labeled with [1-14C] palmitate for 1.5 h and then extracted with organic solvents as described in Materials and Methods and, the radiolabeled extractable lipids were analyzed by TLC-phosphorImaging (C). (D): TMM quantities in arbitrary units (a.u.). (E): TMM/TDM. The relative radioactivity incorporated into TMM and TDM was determined for each strain, the ratio was calculated and normalized to 1 for the WT strain (RES167). The values in (D) and (C) are the means ± SD of at least three independent experiments. PG/CL: Phosphatidylglycerol/Cardiolipine; PIM: Phosphatidylinositol mannoside; PI: Phosphatidylinositol.
Conclusions
Compared to the large knowledge base acquired concerning cell envelope biogenesis in Gram-negative bacteria, little is known about the biosynthesis and assembly of the didermic cell envelope of Corynebacteriales. However, since the direct visualization of a mycolate containing outer membrane [5, 6], an ever-increasing number of studies have been published on the biosynthesis and assembly of the cell envelope of these bacteria. An important part of the current knowledge on this subject came from the use of C. glutamicum because, unlike mycobacteria, most of the known genes involved in MA and AG biosynthesis are not essential in this bacterium [3]. It is thus natural that C. glutamicum constitutes a model of choice to search for new genes involved in envelope biosynthesis processes. In this context, we used a classical transposon mutagenesis strategy, combined with an original immunological screening. We retained 80 mutants out of approximately 10,000 screened colonies, which corresponded to 55 independent loci. The effectiveness of our screening method was attested to by the identification of 34 interrupted loci encoding already known functions, more than half of which is involved in cell envelope biogenesis, i.e. biosynthesis of cell envelope components as well as cell envelope dynamics and assembly including cell division. It is therefore legitimate to assume that a significant part of the 22 loci of unknown or poorly characterized function that we identified in this work are also involved in cell envelope biogenesis. Consistent with this hypothesis is the fact that some of the genes identified in this study were also found by Lim et al. [13] in their large-scale transposon mutagenesis study associated with sensitivity to ethambutol (ste genes). Indeed, among the 49 ste genes, 12 were also found here (indicated in S2 Table), five of which are uncharacterized genes (cg0575, cg0853, cg1254, cg2811, cg3165) and two encode poorly characterized proteins linked to cell division (cg1603 and cg1735). Five additional ste mutations were found in predicted operons that were also inactivated by our transposon and detected by our screen (also indicated in S2 Table).
Here we identified cg1246, a gene encoding a protein of unknown function well conserved in Corynebacteriales and relatively specific to this order. We characterized the corresponding mutant and showed the involvement of Cg1246 in MA metabolism. The functional characterization of this protein is currently underway.
Acknowledgements
We are very grateful to Pr N. Bayan and Dr M. Daffé for helpful discussions and to C. Dietrich for his kind help in the purification of cell wall components used in S2 Fig. We want to acknowledge Pr M. Dubow for critical reading of the manuscript and corrections. We thank M. Millot, L. Graffagnino and C. Couteau for all their technical help.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
 Genome-wide identification of novel genes involved in Corynebacteriales cell envelope biogenesis using Corynebacterium glutamicum as a model
Genome-wide identification of novel genes involved in Corynebacteriales cell envelope biogenesis using Corynebacterium glutamicum as a model
