
Severe acute respiratory coronavirus 2 (SARS-CoV-2) infection, which has caused the current coronavirus disease 2019 (COVID-19) pandemic, is the most significant health issue facing humankind in the past century. Since the initial reports of COVID-19 from China ≈1 year ago, the virus has spread to virtually all countries with >69 million people infected and 1.6 million deaths as of December 10, 2020.1 Experience with COVID-19 indicates that, in addition to age, comorbidities such as cardiovascular disease (mainly ischemic heart disease, atrial fibrillation, and stroke), hypertension, diabetes, and chronic respiratory disease elevate the risk of mortality among SARS-CoV-2–infected individuals.2,3 Hypertension not only predisposes to more severe SARS-CoV-2 disease, but widespread antihypertensive treatment with blockers of the renin-angiotensin system (RAS) was initially questioned due to the possible facilitation of SARS-CoV-2 infection. However, multiple reports, including 2 large retrospective cohort studies, among others, have provided evidence that renin-angiotensin-aldosterone system inhibitors may be safely continued in hypertensive individuals without increasing the risk for severe COVID-19.4,5
See related article, pp [Related article:] 833–842
At the present time, a large number of cohort studies and meta-analyses have generally confirmed the lack of association of RAS inhibitor use with severity of COVID-19, including a recently published systematic review and meta-analysis of 7 high-quality studies in 164 064 patients.6 Because the majority of studies have been conducted in hospitalized patients testing positive for SARS-CoV-2 and many patients had comorbidities associated with increased risk of COVID-19, however, these reports are subject to potential indication and collider bias and confounding.
In the current issue, Semenzato et al7 report a retrospective observational study involving essentially the entire adult population of France (67 million), of which 2 million adults with uncomplicated hypertension were subdivided into 3 mutually exclusive cohorts: those taking ACE (angiotensin-converting enzyme) inhibitors or angiotensin receptor blockers (ARBs) or calcium channel blockers. Subjects with known COVID-19 comorbidities including diabetes, known cardiovascular disease, chronic renal failure, or chronic respiratory disease during the previous 5 years were excluded. Whereas the vast majority of studies evaluating the association of antihypertensive drug classes with severity of COVID-19 have been in hospitalized patients, the present study is unique in that it was based on drug class comparisons among ambulatory adults on long-term stable antihypertensive drug therapy. The subjects were followed for 16 weeks; time to hospitalization (primary end point) and time to intubation or death (secondary end point) were compared among the three cohorts. ACE inhibitors and ARBs were associated with a 26% and 16% lower risk of COVID-19 hospitalization, respectively, compared with calcium channel blockers (hazard ratio [HR], 0.74 [95% CI, 0.65–0.83] for ACE inhibitors and 0.84 [95% CI, 0.76–0.93] for ARBs) and a 34% (HR, 0.66 [95% CI, 0.51–0.84]) and 21% (HR, 0.79 [95% CI, 0.64–0.98]) lower risk of intubation or death, respectively, than for calcium channel blockers. The hospitalization (HR, 0.87 [95% CI, 0.79–0.96]) and intubation (HR, 0.83 [95% CI, 0.67–1.03]) risks were slightly lower for ACE inhibitor compared with ARB users.
This well-conducted study has several strengths, including size, a comprehensive, uniform data collection system, a baseline population of uncomplicated, treated hypertensive patients, independence from SARS-CoV-2 test results, comparison of end points among users of 3 mutually exclusive drug classes, and exclusion of comorbidities, which would be likely to influence the results. However, there are a few potential limitations. Antihypertensive drug adherence could not be assessed or verified beyond the record of drug dispensation (minimum 3, average 6 medication deliveries the previous year) to the subjects during the study period. Although 60% of the patients took only monotherapy, the remaining 40% took additional antihypertensive agents, including diuretics and β-blockers, which might have influenced the results. Altogether, the study strengths far outweigh its limitations, and the results, if verified, suggest that RAS inhibitors could be protective of severe COVID-19.
While the present study does not provide any information on the pharmacological basis of the observed protection from severe COVID-19, it is intriguing to speculate on potential mechanisms. SARS-CoV-2 uses the membrane-bound ACE-2 receptor as its portal of entry into affected cells.8–11 ACE-2 is a key enzyme of the RAS that facilitates conversion of the octapeptide Ang II (angiotensin II) to the heptapeptide Ang (1–7) (angiotensin (1–7)). Ang II, via actions at the AT1R (Ang II type 1 receptor), induces a host of potentially detrimental actions including vasoconstriction, antinatriuresis, inflammation, fibrosis, oxidative stress, apoptosis, and cellular proliferation. On the contrary, Ang (1–7) acts via the mas receptor to oppose the Ang II-AT1R pathway by inducing vasodilation and anti-inflammatory, antifibrotic, antiapoptotic, and antiproliferative effects. Thus, the ACE-2/Ang (1–7)/mas receptor pathway serves as a major anchor for the beneficial endogenous counterregulatory components of the RAS.
Activation of the RAS due to binding of SARS-CoV-2 to the extracellular domain of ACE-2 engenders loss of ACE-2 directly by cellular internalization and indirectly by proteolytic processing and shedding of its ectodomain (Figure). Loss of ACE-2 is a major driving force for the systemic manifestations of COVID-19, and ACE inhibitors and ARBs upregulate the ACE-2 pathway.8–10 Injection of SARS-CoV spike protein into mice induces acute lung failure that is attenuated by inhibitors of the RAS.11 In general, but with some tissue variability, ACE inhibitors and ARBs increase ACE-2 mRNA and protein expression in tissues, Ang (1–7) concentrations in plasma, and ACE-2 catalytic activity in diverse experimental animal models.10 The exact mechanisms underlying the augmentation of ACE-2 biosynthesis by RAS inhibitors require further clarification. However, if SARS-CoV-2 downregulates membrane-bound ACE-2 by promoting extracellular domain shedding and cellular internalization, without reducing intracellular viral propagation, this sequence would be predicted to downregulate the ACE-2/Ang (1–7)/mas receptor pathway leading to increased disease severity. ACE inhibitors and ARBs then would be expected to compensate for virus-induced Ang II/AT1R activation through a combination of Ang II/AT1R inhibition and upregulation of the protective ACE-2/Ang (1–7)/mas receptor pathway.

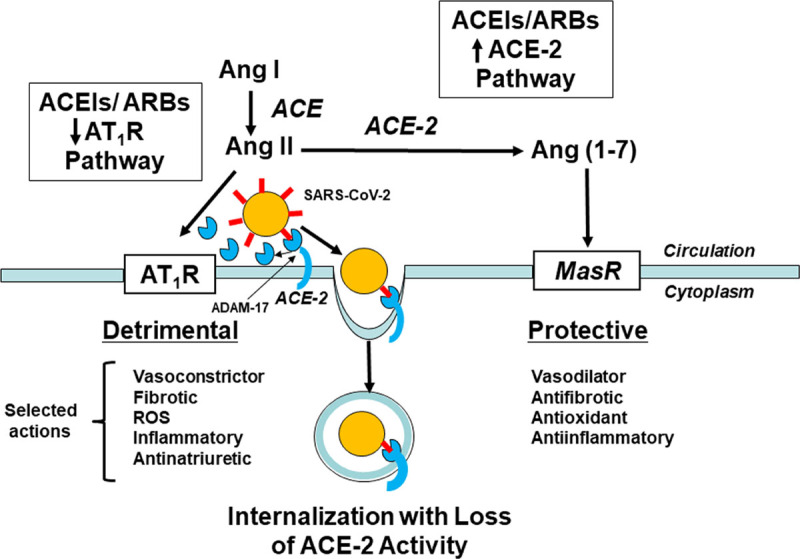
Schematic diagram of the role of ACE (angiotensin-converting enzyme)-2 in the pathogenesis of coronavirus disease 2019 (COVID-19). The severe acute respiratory coronavirus 2 (SARS-Cov-2) viral spike protein (red symbols) binds to the extracellular domain of ACE-2, and the virus and ACE-2 are internalized enabling viral entry into cells and reducing ACE-2 activity. Extracellular ACE-2 also is shed into the circulation during the process of SARS-CoV-2 infection by an ADAM-17 (A disintegrin and metalloproteinase-17)–dependent process. The net effect is increased action of Ang II (angiotensin II) at AT1Rs (Ang II type 1 receptors) promoting detrimental inflammatory responses and reduced Ang (1–7) (angiotensin (1–7)) formation and consequent activity at the Mas receptor—a beneficial counterregulatory receptor of the renin-angiotensin system. ACE inhibitors and angiotensin receptor blockers (ARBs) upregulate the protective ACE-2 pathway and downregulate the detrimental Ang II-AT1R pathway, theoretically protecting the infected host from COVID-19. ACE indicates angiotensin converting enzyme; and ROS, reactive oxygen species.
Because of the relatively large reductions of COVID-19 hospitalization, intubation, and death using ACE inhibitors and ARBs reported by Semenzato et al7 and in light of the worldwide public health disaster presented by COVID-19, the results of their study bear urgent confirmation.
R.M. Carey is the Principal Investigator and Project Director of a National Institutes of Health Research Grant (R01-HL-128189) and Program Project Grant (P01-HL-074940), respectively.
None.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.