
Edited by Natasha V. Raikhel, Center for Plant Cell Biology, Riverside, CA, and approved November 17, 2020 (received for review November 11, 2020)
Author contributions: L.Z., F.M., Y.-L.R., and M.L. designed research; L.Z., B.L., H.L., Z.W., and X.W. performed research; L.Z., L.W., B.M., Y.Z., Y.-L.R., and M.L. analyzed data; L.Z., Y.-L.R., and M.L. wrote the paper; and F.M., Y.-L.R., and M.L. supervised research.
Sugar transport across membranes is essential for maintaining cellular sugar homeostasis and metabolic balance in plant cells. However, it remains unclear how this process is regulated among different classes of sugar transporters. Here, we identified an apple tonoplast H+/glucose symporter, MdERDL6-1, that exports glucose to cytosols to up-regulate the expression of H+/sugar antiporter genes TST1 and TST2 to import sugars from cytosol to vacuole for accumulation to high concentrations in apples and tomatoes. The findings provide insights into the regulatory mechanism underlying sugar exchange between cytosol and vacuole.
Sugar transport across tonoplasts is essential for maintaining cellular sugar homeostasis and metabolic balance in plant cells. It remains unclear, however, how this process is regulated among different classes of sugar transporters. Here, we identified a tonoplast H+/glucose symporter, MdERDL6-1, from apples, which was highly expressed in fruits and exhibited expression patterns similar to those of the tonoplast H+/sugar antiporters MdTST1 and MdTST2. Overexpression of MdERDL6-1 unexpectedly increased not only glucose (Glc) concentration but also that of fructose (Fru) and sucrose (Suc) in transgenic apple and tomato leaves and fruits. RNA sequencing (RNA-seq) and expression analyses showed an up-regulation of TST1 and TST2 in the transgenic apple and tomato lines overexpressing MdERDL6-1. Further studies established that the increased sugar concentration in the transgenic lines correlated with up-regulation of TST1 and TST2 expression. Suppression or knockout of SlTST1 and SlTST2 in the MdERDL6-1–overexpressed tomato background reduced or abolished the positive effect of MdERDL6-1 on sugar accumulation, respectively. The findings demonstrate a regulation of TST1 and TST2 by MdERDL6-1, in which Glc exported by MdERDL6-1 from vacuole up-regulates TST1 and TST2 to import sugars from cytosol to vacuole for accumulation to high concentrations. The results provide insight into the regulatory mechanism of sugar accumulation in vacuoles mediated by the coordinated action of two classes of tonoplast sugar transporters.
Soluble sugars play important roles not only as nutrients but also as signal molecules in plant growth and development (1). In fleshy fruits, sucrose (Suc) and monosaccharides (glucose [Glc] and fructose [Fru]) are also central to fruit quality as they are the main nutrients and sweetening compounds. The distribution of sugars in plant cells is dependent on several transport steps across cell membranes in addition to long-distance translocation through the phloem (2).
In most plants, Suc is transported from photosynthetically active leaves (source), through the phloem, to nonphotosynthetic sink organs, such as fruits, roots, and stems. Suc is then either directly transported into sink cells or taken up in the form of hexoses (Glc and Fru) after cleavage by an extracellular invertase (3, 4). Within the sink cells, soluble sugars are first metabolized to meet the requirements for energy and carbon skeleton production. Excessive sugars are generally converted into starch for storage in the plastid or imported into vacuoles for transient or long-term storage mediated by vacuolar sugar transporters (2, 5). The vacuole occupies up to 90% of the cell volume, where most sugars are compartmentalized, typically in the parenchyma cells of many sugar-accumulating organs such as sugar cane stems, sugar beet roots, and fresh fruits of tomato, apple, and orange (56–7). While considerable effort has been made to understand sugar transport and accumulation in plants, our knowledge of the mechanism by which cells accumulate high concentrations of sugars in the vacuole remains rather limited.
Sugar input into vacuoles is largely determined by different classes of sugar transporters located on the tonoplast (6). The tonoplast monosaccharide transporter (TMT), now renamed as tonoplast sugar transporter (TST), and the vacuolar glucose transporter (vGT) families encode proteins to mediate sugar influx into vacuoles as H+/sugar antiporters (89–10). In Arabidopsis, AtvGT1 is a tonoplast transporter specific for Glc with a role in seed germination and flowering (10), while AtTMT1/2 exhibits a broad substrate specificity and could transport Glc, Fru, and Suc into the vacuole (8, 11). More recently, SWEETs (sugars will eventually be exported transporters) have been identified as energy independent uniporters from Arabidopsis, rice, and other species, mediating concentration-dependent sugar influx or efflux across membranes (12). In the SWEET family of Arabidopsis, AtSWEET16 and AtSWEET17 were localized on the tonoplasts of root and leaf cells, where the former transported Suc, Glc, and Fru (13), while the latter appeared specific to Fru (14).
Among the transporters that had been identified, only one member from the TST subfamily, namely, the TST2 subfamily, has been shown to play important roles in sugar accumulation (9, 15, 16). TSTs from different plants displayed different substrate specificities. For example, the watermelon ClTST2 protein exhibited similar transport activity for Suc, Fru, and Glc (16), while the BvTST2.1 protein from sugar beets has a specific affinity for Suc and is responsible for Suc accumulation in the taproots (9).
Apart from the H+/sugar antiporter, TSTs, two types of H+/sugar symporters, the Suc exporter SUC4 (17) and the Glc exporter early response to dehydration like 6 (ERDL6), have also been identified to function on the tonoplast (18, 19). The operation of H+-coupled importers (e.g., TSTs, as an antiporter) and exporters (e.g., SUC4 and ERDL6, as symporters) on the tonoplast regulates sugar homeostasis between vacuole and cytoplasm (6). In Arabidopsis, AtSUC4 is weakly expressed in Suc-accumulating leaf mesophyll cells, and high SUC4 activity is unfavorable for sugar accumulation (17). It was reported that AtERDL6 acts as a Glc exporter in Arabidopsis to transfer Glc from the vacuole to the cytosol, as indicated by the finding that the corresponding erdl6 mutant accumulated more Glc in the vacuole (18, 19).
It is noteworthy that orthologous genes of AtERDL6 exhibited higher expression levels in a wide range of sugar-accumulating fleshy fruits, such as those from pineapples (20), tomatoes (21), oranges (22), and apples (23, 24). The expression level of these ERDL6 orthologs genes generally matches with the fruit sugar content. In apples, the mRNA level and protein abundance of multiple MdERDL6 family members were strongly and positively correlated with Suc and Fru concentrations in the fruits, in parallel to high expression of MdTST1/2 (23, 24). The high expression of MdERDL6 genes in the sugary apple fruits is intriguing given that ERDL6 is an exporter of Glc from vacuoles and its overexpression in Arabidopsis impaired cold tolerance and seed germination (19). It remains unknown if ERDL6s contribute to sugar accumulation in fruits and, in particular, how ERDL6s and TSTs may coregulate sugar levels in the vacuoles.
To explore the potential roles and cross talks of tonoplast H+/sugar symporters and antiporters in sugar accumulation, we first characterized MdERDL6-1 expression patterns and the transport activities of the encoded protein. This was followed by overexpression of MdERDL6-1 in apple and tomato (Solanum lycopersicum cv. Micro-Tom). The data obtained indicated that MdERDL6-1 functions as a H+/Glc symporter on the tonoplast to export Glc from the vacuole into the cytosol. Overexpression of MdERDL6-1 in apples and tomatoes substantially increased Glc, Fru, and Suc concentrations in leaves and fruits. This promoting effect was owing to the increased expression of TST1 and TST2 in the transgenic lines because the positive effect of MdERDL6-1 overexpression on sugar level was abolished or reduced once TST1 and TST2 were knocked out or suppressed, respectively. Overall, these results, together with other findings documented in this paper, identified a tonoplast H+/Glc symporter from apple that exports Glc from vacuoles and established that the increased Glc efflux mediated by MdERDL6-1 into the cytosol up-regulated TST1 and TST2 expression to promote sugar import into the vacuoles. The findings provide insights into the regulatory mechanism on sugar transport between cytosol and vacuole across tonoplast and offer potential opportunities to improve fruit sugar levels, hence fruit quality and yield.
To identify major energy-dependent sugar transporters operating on tonoplast in apple, the AtERDL6, AtTST, or AtvGT protein sequences were used as queries to search Malus × domestica genome GDDH13 v1.1 (25). The exercise identified 11 MdERDL6s, six MdTSTs, and three MdvGTs genes from the updated Malus × domestica genome. Phylogenetic analysis revealed that the 11 candidate MdERDL6s could be divided into three groups. Among them, MdERDL6-1 and MdERDL6-2 were clustered with AtERDL6, AtERDL6-5, and BvIMP (an AtERDL6 ortholog from sugar beet) (SI Appendix, Fig. S1), all of which function as H+/Glc symporters in Arabidopsis (18, 19). The identified MdTST1, 2, and 3 were all orthologous to AtTMT2 (SI Appendix, Fig. S1).
Transcriptional patterns of MdERDL6s, MdTSTs, and MdvGTs were analyzed in developing fruit and other tissues, including shoot tips and mature leaves based on our RNA sequencing (RNA-seq) data (Fig. 1A and Dataset S1A). Six of the 11 MdERDL6 orthologs (from MdERDL6-1 to MdERDL6-6), along with MdTST1 and MdTST2, exhibited an increased expression as fruit develops, which correlated with fruit Fru and Suc levels (Fig. 1A). Among the 11 MdERDL6 genes, MdERDL6-1 exhibited a higher expression level in developing fruit based on the reads per kilobase per million mapped reads (RPKM) values (Dataset S1A). qRT-PCR analyses also confirmed that MdERDL6-1 was highly expressed in fruits, with a trend similar to those of MdTST1 and MdTST2 (Fig. 1B), implying a potential link between the MdERDL6-1 and MdTST1/2. Thus, MdERDL6-1 was selected as a candidate gene for further studies on its role in sugar accumulation and to test whether there is a functional coupling with MdTST1 and 2.

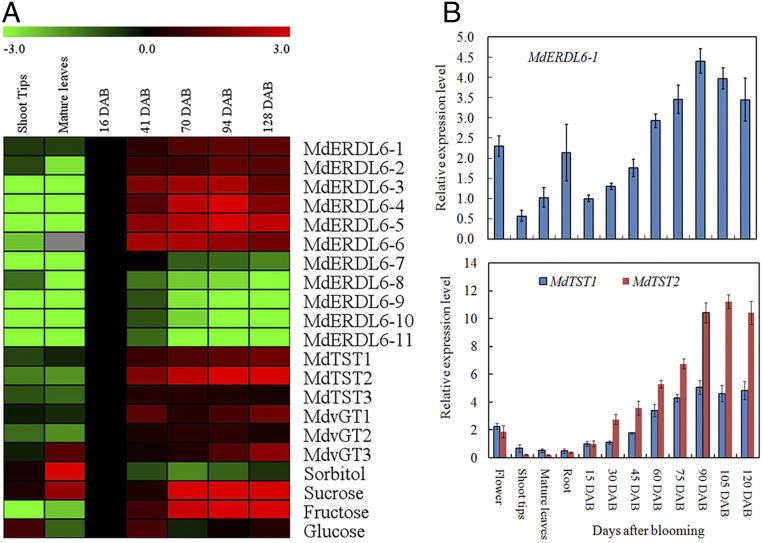
The expression profiles of vacuolar sugar transporter genes, including MdERDL6s, MdTSTs, and MdvGTs, in apples. (A) A heat map of gene expression levels based on RNA-seq and sugar concentrations in different tissues including developing fruit. RPKM values measured with RNA-seq are shown in Dataset S1A. Fold difference is designated as a log2 value, with the data in young fruit at 16 DAB set as 1. (B) Relative transcript levels of MdERDL6-1, MdTST1, and MdTST2 in different tissues, including developing fruits based on qRT-PCR. For each sample, transcript levels were normalized with those of MdActin. Relative expression levels for each gene were obtained via the ddCT method, setting its expression in young fruit at 15 DAB as 1. The bars represent the mean value ± SD (n ≥ 3).
To determine the subcellular localization of MdERDL6-1, we transiently expressed a green fluorescent protein (GFP)-fused MdERDL6-1 protein in protoplasts, isolated from Arabidopsis, tobacco leaves, and apple calli. The Arabidopsis protoplast displayed red auto-fluorescence from chlorophyll outside the MdERDL6-1-GFP signal, with the latter colocalized with the tonoplast marker, VAC-RK (SI Appendix, Fig. S2). A clearer localization pattern was observed from tobacco protoplasts lysed by a mild osmotic shock, where MdERDL6-1-GFP fluorescence was seen only in the membrane of the vacuoles with the auto-fluorescent chloroplasts sitting outside (Fig. 2A). Further, the MdERDL6-1-GFP fluorescence was localized on membranes inside the plasma membranes of protoplasts from apple calli cells (Fig. 2B). These data collectively indicate that MdERDL6-1 targets to tonoplast in vivo.

![Tonoplast localization of MdERDL6-1 in tobacco leaves and apple calli and its transport prosperities. (A and B) Tonoplast localization of the MdERDL6-1-GFP fusion protein in the isolated vacuoles of protoplasts from tobacco leaves (A) and the protoplasts from apple calli (B). The green fluorescence represents MdERDL6-1-GFP fusion proteins. Note the chloroplasts showing red auto fluorescence are located outside the ring of GFP fluorescence (A). For the apple calli protoplast (B), the MdERDL6-1-GFP fluorescence exhibited a ring-like pattern inside the plasma membrane (black arrows), indicating that MdERDL6-1 is localized to the tonoplast. (C) The localization of MdERDL6-1 to plasma membrane in yeast, based on MdERDL6-1-GFP fusion expression, in comparison with that of pYST II-GFP control in the yeast mutant strain EBY.VW4000. (Scale bars = 2 μm.) (D) Growth of the yeast mutant strain EBY.VW4000 expressing MdERDL6-1 on culture medium with 2% of different sugars. The deficient yeast mutant carrying the empty pYST II vector (Control 1) or the MdERDL6-1-antisense recombinant plasmid (Control 2) could not grow normally on each monosaccharide culture medium, whereas the yeast mutant transformed with MdERDL6-1-sense construct grew normally on glucose but slowly on Fru, Gal, and Xyl. (E) The transport capacity of [14C] glucose was measured in yeast cells expressing MdERDL6-1 cDNA in the sense orientation (strain EBY.VW4000; blue circles) or the empty vector on 100 mM of glucose at pH 5.5. (Inset) The uptake rates with increasing concentrations of [14C] glucose were determined 2 min after substrate addition and used to calculate the Km value. The plot of a typical Km determination is presented with five independent measurements. Here, a Km value of 21.7 mM and a maximum uptake rate (V max) of 1.214 mmol ⋅ h−1 ⋅ ml−1 packed cells were determined for Glc uptake, driven by the MdERDL6-1 transporter. (F) Relative uptake rates of sugars at an initial concentration of 100 mM and the influence on glucose uptake by 50 μM uncoupler CCCP in MdERDL6-1–expressing yeast cells. Data represent average values of five independent transport tests (mean ± SD).](/dataresources/secured/content-1765841840853-77449c08-4b2b-4147-b206-aa5b7f067e4c/assets/pnas.2022788118fig02.jpg)
Tonoplast localization of MdERDL6-1 in tobacco leaves and apple calli and its transport prosperities. (A and B) Tonoplast localization of the MdERDL6-1-GFP fusion protein in the isolated vacuoles of protoplasts from tobacco leaves (A) and the protoplasts from apple calli (B). The green fluorescence represents MdERDL6-1-GFP fusion proteins. Note the chloroplasts showing red auto fluorescence are located outside the ring of GFP fluorescence (A). For the apple calli protoplast (B), the MdERDL6-1-GFP fluorescence exhibited a ring-like pattern inside the plasma membrane (black arrows), indicating that MdERDL6-1 is localized to the tonoplast. (C) The localization of MdERDL6-1 to plasma membrane in yeast, based on MdERDL6-1-GFP fusion expression, in comparison with that of pYST II-GFP control in the yeast mutant strain EBY.VW4000. (Scale bars = 2 μm.) (D) Growth of the yeast mutant strain EBY.VW4000 expressing MdERDL6-1 on culture medium with 2% of different sugars. The deficient yeast mutant carrying the empty pYST II vector (Control 1) or the MdERDL6-1-antisense recombinant plasmid (Control 2) could not grow normally on each monosaccharide culture medium, whereas the yeast mutant transformed with MdERDL6-1-sense construct grew normally on glucose but slowly on Fru, Gal, and Xyl. (E) The transport capacity of [14C] glucose was measured in yeast cells expressing MdERDL6-1 cDNA in the sense orientation (strain EBY.VW4000; blue circles) or the empty vector on 100 mM of glucose at pH 5.5. (Inset) The uptake rates with increasing concentrations of [14C] glucose were determined 2 min after substrate addition and used to calculate the Km value. The plot of a typical Km determination is presented with five independent measurements. Here, a Km value of 21.7 mM and a maximum uptake rate (V max) of 1.214 mmol ⋅ h−1 ⋅ ml−1 packed cells were determined for Glc uptake, driven by the MdERDL6-1 transporter. (F) Relative uptake rates of sugars at an initial concentration of 100 mM and the influence on glucose uptake by 50 μM uncoupler CCCP in MdERDL6-1–expressing yeast cells. Data represent average values of five independent transport tests (mean ± SD).
To characterize the transport properties of MdERDL6-1 protein, we cloned MdERDL6-1 into the vector pYES-DEST2-eGFP and expressed it in a yeast mutant deficient in hexose transport, which grows only on maltose (26). Analysis of the transformed yeast lines by fluorescent microscopy revealed that the MdERDL6-1-GFP fluorescence clearly localized to the plasma membrane but not to the internal compartments of yeast cells (Fig. 2C), consistent with a previous report that the tonoplast gene AtERDL10 from Arabidopsis was also targeted to the plasma membrane in the yeast (19). These observations indicate that the yeast system could be used to detect the transport properties of MdERDL6-1 protein.
We then cloned MdERDL6-1 into the vector pYES-DEST2, in both the sense and antisense orientation, and expressed them in the yeast mutant EBY.VW4000. The yeast transformed with pYES-DEST2-MdERDL6-1 in the sense orientation was used to assess complementation efficiency, with those transformed with MdERDL6-1 in the antisense orientation serving as the control. As expected, expression of MdERDL6-1 in the antisense orientation in this null mutant did not restore growth on sugar-containing medium. By contrast, the strain expressing MdERDL6-1 in the sense orientation regained the capacity to grow well on 2% Glc-containing medium but could not grow on medium containing Fru, galactose (Gal), or xylose (Xyl) (Fig. 2D). This indicates that MdERDL6-1 functions to specifically transport Glc into the cytosol of the yeast cells, thereby restoring the growth of the mutant. This notion was supported by 14C-labeled D-hexose transport assay, which showed the transport capacity by MdERDL6-1 was clearly specific to Glc as compared with Fru and Gal (Fig. 2 E and F). The Km of MdERDL6-1 for Glc was measured to be ∼21.7 mM (Fig. 2 E, Inset). Moreover, application of a proton uncoupler, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), significantly decreased Glc uptake (Fig. 2F), suggesting that sugar uptake via MdERDL6-1 is probably driven by a proton gradient across the membrane. Taken together, the data from the heterologous expression analyses in yeast suggests that MdERDL6-1 acts as a H+/Glc symporter.
To determine the role of MdERDL6-1 in sugar accumulation, we first constructed RNA interference (RNAi) and overexpression vectors and transformed both into the cultured calli derived from apple fruit. The fruit calli is a fast-tracking efficient system to assess the function of genes related to fruit metabolism in apples (27, 28). After evaluation by using qRT-PCR, we obtained two silencing (SL1 and SL3) and two overexpression lines (OL3 and OL6) in which the MdERDL6-1 expression was reduced to less than 30% of the wild-type (WT) level, but increased by more than 15 times relative to that in the WT, respectively (Fig. 3A). The growth rate of the fruit calli was clearly inhibited or increased in the silencing or the overexpression lines, respectively (Fig. 3B). Consistent with the finding that MdERDL6-1 encoded a tonoplast-localized Glc exporter (Fig. 2), silencing MdERDL6-1 increased Glc concentration by ∼2 times, with Fru, Suc, and Sor levels remained unchanged (Fig. 3 C and D). Surprisingly, however, overexpressing MdERDL6-1 did not lead to an expected reduction in Glc level, but rather doubled the concentrations of Glc and Fru, and increased Suc level by about 70 to 80% in the fruit calli on the 3% Glc- or Suc-containing medium (Fig. 3 C and D).

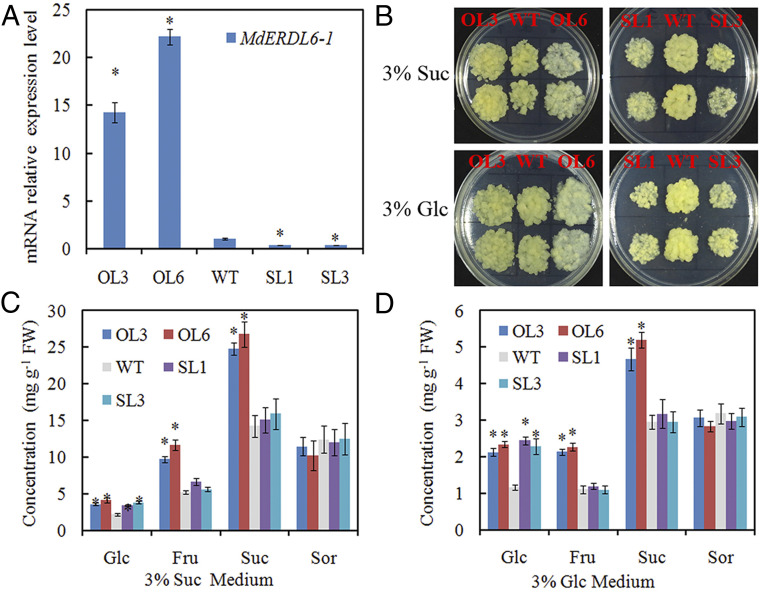
The impact of altering MdERDL6-1 expression on sugar (Glc, Fru, Suc, and Sor) concentrations in apple fruit calli. (A) MdERDL6-1 expression levels in the overexpressing (OL3 and OL6) and silencing (SL1 and SL3) calli lines were measured by using qRT-PCR. The transcript levels were normalized to those of MdActin. The relative expression level for MdERDL6-1 was obtained via the ddCT method, setting its expression in WT as 1. (B) The growth of the transformed calli on 3% Suc or 3% Glc medium. (C and D) The sugar concentrations of the transformed calli lines cultured on 3% Suc (C) or 3% Glc (D) medium. The bars represent the mean value ± SD (n ≥ 4). *P ≤ 0.05, a significant difference from the WT.
To further determine the impact of increases in MdERDL6-1 expression on sugar accumulation in planta, we generated stable transgenic apple and tomato lines overexpressing MdERDL6-1 driven by the CaMV35S promoter. Following PCR and qRT-PCR screening, as well as Western blotting detection using a specific antibody against MdERDL6-1, three transgenic apple lines (OEm-1, OEm-3, and OEm-4) and two tomato lines (OEs-1 and OEs-2) were obtained (SI Appendix, Fig. S3). The specificity of the antibody was indicated by the detection of a single band at the predicted size of MdERDL6-1 protein on Western blots loaded with proteins from apple leaves and tomato fruits (SI Appendix, Fig. S4). In the apple lines, the mRNA level of the MdERDL6-1 was increased 5 to 10 times in leaves (SI Appendix, Fig. S3C) without affecting the transcript levels of other MdERDL6 family members (SI Appendix, Fig. S5) or the growth and photosynthesis rates of the transgenic seedlings (Fig. 4A and SI Appendix, Fig. S6). The transgenic tomato plants showed expression of MdERDL6-1 mRNA and protein in ripening fruits (SI Appendix, Figs. S3 F and G and S4), increased plant height and fruit size with delayed flowering, and reduced seed and fruit number (SI Appendix, Fig. S3E and Table S2).

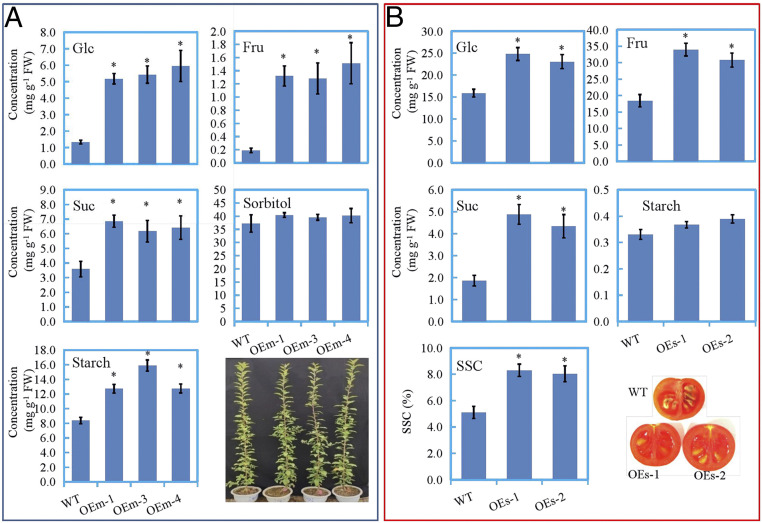
The carbohydrate levels of the transgenic apple (OEm-1, OEm-3, and OEm-4) leaves and tomato (OEs-1 and OEs-2) fruits overexpressing MdERDL6-1. (A) Glc, Fru, Suc, Sor, and starch levels in mature leaves of transgenic apple. (B) The carbohydrate levels and soluble solids content in ripening fruits of transgenic tomato and longitudinal sections of the fruits. The bars represent the mean value ± SD (n ≥ 4). *P ≤ 0.05, a significant difference from the WT.
To gain a clue as whether the overexpression of MdERDL6-1 altered the subcellular sugar levels, we measured the expression of the sugar-sensitive reporter gene chlorophyll a/b binding protein (CAB1) in the transgenic lines. The transcription of CAB1, encoding the chlorophyll a/b binding protein 1, is negatively controlled by the cytosolic Glc concentration (19, 29). The analyses revealed that CAB1 mRNA level was reduced by about 50 to 60% in the transgenic apple leaves and 40 to 50% in the tomato leaves and fruits overexpressing MdERDL6-1 (SI Appendix, Fig. S7), indicating enhanced Glc export from vacuole into cytosol in the transgenic lines.
Since it takes ∼5 y for any transformed apple plantlets to reach the adult reproductive phase for fruiting, we analyzed sugar levels in the leaves of transgenic apple seedlings. The assay revealed massive increases in the soluble sugar and starch levels, where the Glc content was increased by 3.9 to 4.5 times, while the Fru and Suc levels have risen by 6.9 to 8.0 times and 1.7 to 1.9 times of that in the WT, respectively (Fig. 4A). Similar results were observed in the leaves of two transgenic tomato lines (SI Appendix, Fig. S8). Strikingly, we distinctly smelled a sweet aroma from the tomato fruit expressing MdERDL6-1, and indeed, the total soluble sugar content in the mature tomato fruit was 1.6 times of that in the WT. Consistently, the concentrations of Glc and Fru were increased by 60 to 95%, with Suc levels being more than doubled in the transgenic tomato fruits as compared to that of the WT (Fig. 4B).
To explore the basis for the significant increase in the sugar levels in MdERDL6-1 overexpressing plants, RNA-seq was performed to examine the changes in gene expression in the leaves of MdERDL6-1 overexpressing apple lines, OEm-1 and 3. A total of 2,777 and 3,753 genes (log2 fold changes greater than 1, P value <0.01) were differentially expressed in the OEm-1 and OEm-3 lines, respectively, compared to the WT. Of these differentially expressed genes (DEGs), 2,631 genes were found in both lines (SI Appendix, Fig. S9, and Dataset S2A).
For the DEGs, the MdERDL6-1 expression levels in OEm-1 and OEm-3 were 4.9 and 10.1 times, respectively, of that in the WT, while other MdERDL6 orthologs remained unchanged, as confirmed by qRT-PCR (Fig. 5 A and B and SI Appendix, Fig. S5). The expression levels of three MdCAB1s were significantly decreased in the OEm-1 and OEm-3 lines (Fig. 5B). These expression results from RNA-seq were in agreement with those measured by using qRT-PCR (SI Appendix, Fig. S7), indicating that the RNA-seq data were credible. After annotation, 39 DEGs were found to relate carbohydrate metabolism and transport. Among them, 23 were down-regulated, including three beta-amylase genes (MdBMYs), five sorbitol transporter genes (MdSOTs), three hexose transporter genes (MdHTs), two MdSWEET2s, and seven genes related to Suc degradation (MdCWINVs, MdNINVs, MdvAINVs, and MdSUSYs), whereas ADP-glucose pyrophosphorylase genes (MdAPLs), chloroplast beta-amylase genes (MdBMY3), three fructokinase genes (MdFRKs), and two tonoplast sugar transporter genes (MdTST1 and MdTST2) were up-regulated in both transgenic lines (Fig. 5A).

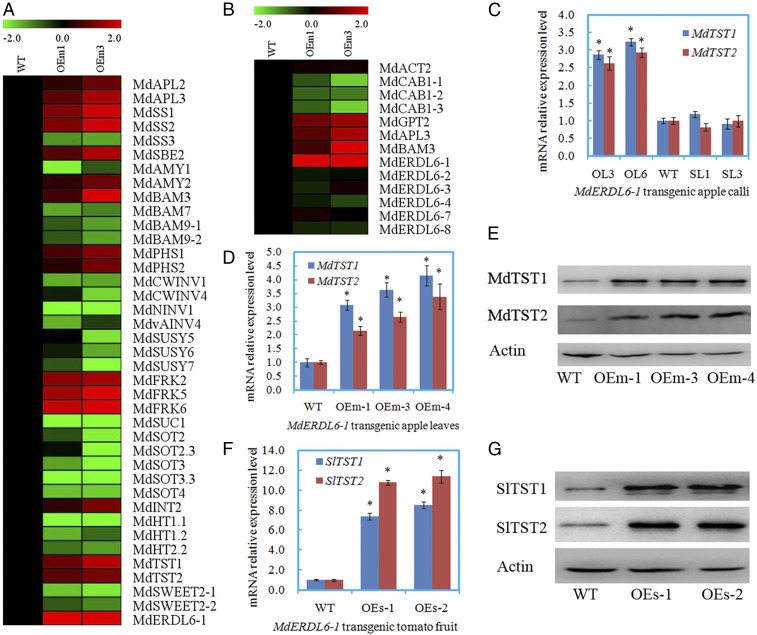
The expression of genes related to carbohydrate metabolism and transport in the transgenic apple or tomato lines overexpressing MdERDL6-1. (A) A heat map illustrating different expression genes related to carbohydrate metabolism and transport in the leaves of transgenic apple lines (OEm-1 and OEm-3) based on RNA-seq. APL, ADP-glucose pyrophosphorylase; SS, starch synthase; SBE, starch branching enzyme; AMY, α-amylase; BMY, β-amylase; PHS, α-glucan phosphorylase; CWINV, cell wall invertase; NINV, neutral invertase; vAINV, vacuolar acid invertase; SUSY, sucrose synthase; FRK, fructokinse; SUC, sucrose transporter; SOT, sorbitol transporter; INT, inositol transporter; HT, hexose transporter; TST, tonoplast sugar transporter; SWEET, sugars will eventually be exported transporter; ERDL6, early response to dehydration like six. (B) A heat map illustrating the expression of marker genes involved in sugar response and other members of MdERDL6 family in the leaves of transgenic lines based on RNA-seq. RPKM values measured with RNA-seq are shown in Dataset S2 B and C, respectively. The fold difference is designated as a log2 value, while the data in WT was set as 1 for each gene. ACT, actin; CAB, chlorophyll a/b binding protein; GPT, glucose-6-phosphate transporter. (C) Expression levels of MdTST1 and MdTST2 mRNAs in the transgenic apple calli overexpressing MdERDL6-1 (OL3, 6) in comparison with the WT and two silencing lines (SL1, 3). (D and E) The expression levels of MdTST1 and MdTST2 mRNAs (D) and proteins (E) in the transgenic apple (OEm-1, OEm-3, and OEm-4) leaves overexpressing MdERDL6-1. (F and G) The expression levels of SlTST1 and SlTST2 mRNAs (F) and proteins (G) in the ripening fruits of transgenic tomato (OEs-1 and OEs-2) overexpressing MdERDL6-1. For qRT-PCR, the transcript levels were normalized to those of MdActin (D) and SlActin (F), respectively. Relative expression levels for each gene were obtained via the ddCT method, with its expression in WT set as 1. The bars represent the mean value ± SD (n ≥ 3). *P ≤ 0.05, a significant difference from the WT.
Among these DEGs, TSTs are tonoplast H+/sugar antiporters and known to play an important role in the import of sugars to the vacuoles for storage (8, 9, 15, 16). Thus, we further examined MdTST1 and MdTST2 expression levels in all of the MdERDL6-1 transgenic lines by using qRT-PCR and Western blotting. The mRNA expression levels of MdTST1 and MdTST2 were significantly up-regulated in the three apple overexpression lines (Fig. 5D), leading to increased protein levels by 2.2- to 3.3-fold as compared to that in the WT (Fig. 5E and SI Appendix, Fig. S4). Similarly, in tomato fruit, the heterologous expression of MdERDL6-1 also resulted in massive increases in SlTST1 and SlTST2 mRNA levels (Fig. 5F), resulting in an increase in their protein abundance by 2.7- to 4.0-fold (Fig. 5G and SI Appendix, Fig. S4).
As shown above, MdTST1 and MdTST2 exhibited expression patterns that were similar to those of MdERDL6-1 and correlated with sugar concentrations in leaves and fruits. Given that MdERDL6-1 was characterized as a tonoplast H+/Glc symporter (Fig. 2) and MdTST orthologous genes AtTMT1 and 2 were strongly induced by Glc (8), it raised the possibility that sugar exported by MdERDL6-1 may up-regulate the expression of MdTST1 and MdTST2. As the first step to test this hypothesis, the apple leaves were fed with different sugars, followed by qRT-PCR measurement of the respective transcripts. In apple and tomato leaves, the mRNA levels of MdTST1 and 2 and SlTST1 and 2 were significantly induced by feeding with Glc, Fru, and Suc (SI Appendix, Fig. S10 A and B). The finding was further confirmed by the observed positive responses of MdTST1 and MdTST2 promoter activities to sugar feeding in tobacco leaves transiently expressing MdTST1P-GUS or MdTST2P-GUS reporter genes (Fig. 6 A and B), consistent with up-regulation of TSTs by sugars as previously reported (8). To further test the influence of MdERDL6-1 expression on the transcription of MdTST1 and MdTST2,35S:MdERDL6-1 was transiently coexpressed with MdTST1P: GUS and/or MdTST2P:GUS in tobacco leaves. The analyses revealed that coexpression of MdERDL6-1 increased the promoter activities of MdTST1 and/or MdTST2 (Fig. 6 C and D).

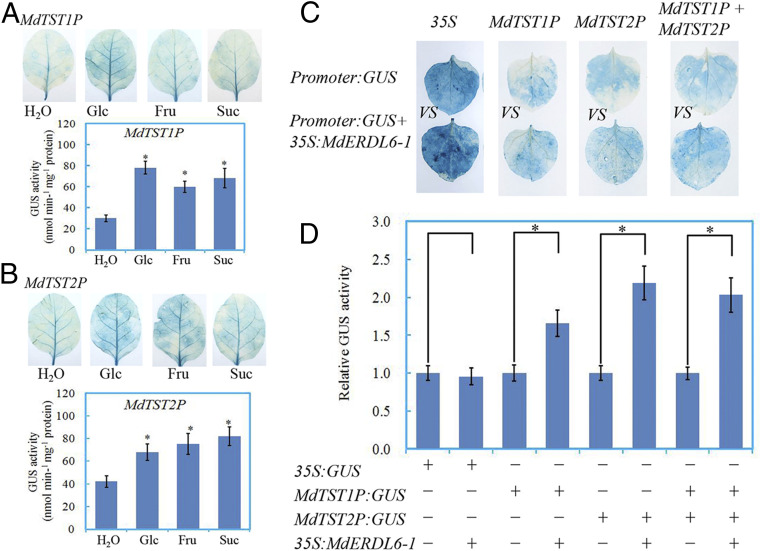
The influence of sugar feeding and coexpression of 35S:MdERDL6-1 on the promoter activities of MdTST1 (MdTST1P) and MdTST2 (MdTST2P). (A and B) The impact of sugar feeding on MdTST1 (A) and MdTST2 (B) promoter activities. Tobacco leaves were fed for 24 h with 2% different exogenous sugar, starting from 48 h after infiltrating with Agrobacterium harboring MdTST1P or MdTST2P plasmids. The treated and control leaves were harvested for GUS activity assay after 24 h feeding. (C) The impact of 35S:MdERDL6-1 coexpression on MdTST1 and MdTST2 promoter activities. The 35S:GUS infiltration was used as a positive control. (D) The relative GUS activities of different combinations of infiltration. The treated leaves were harvested for GUS activity assay at 2 d after infiltration. “+” and “−” represent the presence or absence, respectively, of the Agrobacterium containing corresponding plasmids in the mixture for infiltration. The bars represent the mean value ± SD (n ≥ 4). *P ≤ 0.05, a significant difference from control.
To determine the role of MdTST1 and MdTST2 in controlling sugar concentrations, we obtained overexpressing and silencing apple calli lines of MdTST1 and MdTST2, respectively (SI Appendix, Fig. S11). Compared with the WT, the overexpression lines of MdTST1 (OT1-1 and OT1-2) and MdTST2 (OT2-1 and OT2-2) accelerated the growth of calli, whereas the silencing lines (ST1-1 and ST1-2 for MdTST1; ST2-1 and ST2-2 for MdTST2) inhibited calli growth (SI Appendix, Fig. S11B), as observed for MdERDL6-1 transgenic calli (Fig. 3B). The MdTST1 overexpression lines exhibited significantly increased Glc level in the transgenic calli, with moderate increases in Fru and Suc concentrations. Opposing results for the sugar concentration were detected in the silencing calli lines of MdTST1 (SI Appendix, Fig. S11C). For MdTST2 overexpressed lines, Fru and Suc levels were also significantly increased with only slight effect on Glc, while the silencing lines displayed reduced Fru and Suc levels (SI Appendix, Fig. S11D), supporting the theory that MdTST2 played a more important role in controlling the accumulation of Fru and Suc than Glc in apple cells. Increased concentrations of the three soluble sugars in MdTST1- and MdTST2-overexpressing fruit calli concur with the notions that elevated sugar concentrations in MdERDL6-1–overexpressing apple calli and leaves are likely related to increased expression of MdTST1 and MdTST2.
To genetically determine whether the positive effect of MdERDL6-1 on sugar levels is dependent on TST1 and TST2 expression, we generated sltst1/2 double mutant in tomato using the CRISPR-Cas9 method, targeting the 5′ end of the SlTST1 and SlTST2 coding sequences. After sequencing verification, we obtained edited single and double mutant lines for SlTST1 and SlTST2 in the wild type background, where a series frame shifts of the target genes occurred (SI Appendix, Fig. S12A).
The single mutant lines for SlTST1 (tst1-1 and -7) and SlTST2 (tst2-6 and -13) exhibited no, or a slight but significant, decrease in hexoses and Suc concentrations, respectively, in the mature tomato fruits (SI Appendix, Fig. S12B) and leaves (SI Appendix, Fig. S13). The double mutant lines (tst1/2-4 and tst1/2-15) for SlTST1 and SlTST2 displayed much stronger reductions in the concentrations of the three soluble sugars than that in the single mutants (SI Appendix, Figs. S12B and S13). These results showed that there was a functional complementarity between SlTST1 and SlTST2 in modulating sugar accumulation in tomato, and SlTST2 appears to play a more important role. Significantly, for the double mutant lines in the MdERDL6-1 overexpressed background (OEs1-tst1/2-1 and OEs1-tst1/2-4), the concentrations of fruit Fru and Suc failed to be increased and remained at the same levels as that of SlTST1 and SlTST2 double mutant lines, whereas the concentration of Glc was lower than double mutant lines (Fig. 7), consistent with MdERDL6-1 acting as a vacuolar H+/Glc exporter (Figs. 2 and 3). Similar phenomenon was observed in tomato leaves (SI Appendix, Fig. S13), indicating the conserved response between tomato fruits and leaves.

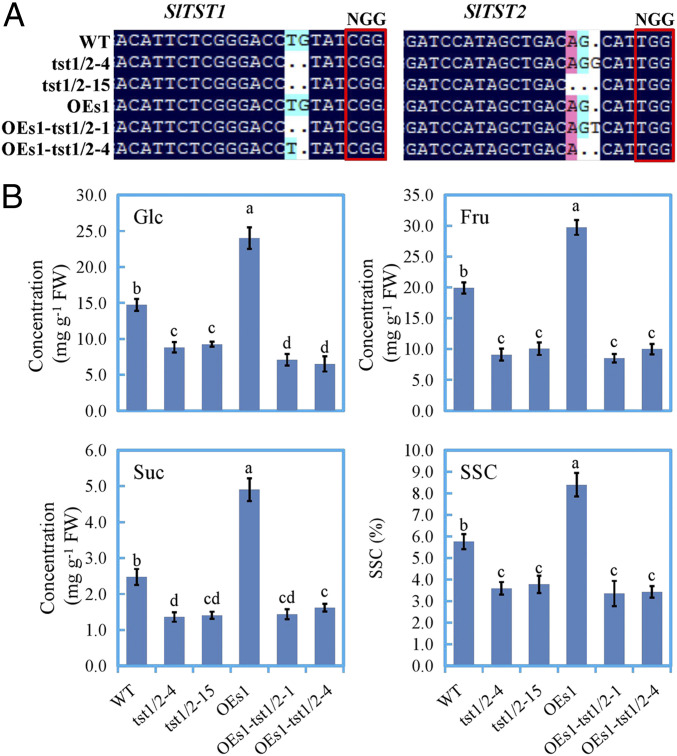
CRISPR-Cas9–mediated knockout of SlTST1 and SlTST2 abolished the positive effect on sugar levels exerted by MdERDL6-1 overexpression in tomato fruits. (A) The DNA sequence of the target region. SlTST1 and SlTST2 target sequences were chosen from CRISPR direct for gene editing. Sequences were aligned using DNAMAN. The dark region is the target sequence, and the other colored region is the difference in sequence among the lines indicated; the target sequences of WT and OEs-1 (transgenic line overexpressing MdERDL6-1) were the same as the control. The edited lines tst1/2-4 had two bases missing for SlTST1 and one base inserted for SlTST2, while the tst1/2-15 had two bases missing for SlTST1 and two bases missing for SlTST2. The OEs-1-tst1/2-1 had two bases missing for SlTST1 and one base inserted for SlTST2, whereas the OEs-1-tst1/2-4 had one base missing for each of SlTST1 and SlTST2. (B) The Glc, Fru, and Suc concentrations and soluble solids content in ripening fruit. The bars represent the mean value ± SD (n ≥ 4). Different letters indicate significant differences at P ≤ 0.05.
To gain further evidence if the dramatically increased sugar levels in the MdERDL6-1 overexpressing lines (Figs. 4 and 7) are dependent on a high expression of TST1 and TST2, we generated RNAi tomato lines with decreased expression levels of SlTST1 and SlTST2 by using virus-induced gene silencing (VIGS). Here, silencing of SlTST1 and SlTST2 in the WT tomato fruit reduced Glc, Fru, and Suc levels (SI Appendix, Fig. S14), confirming the role of SlTSTs in sugar import to the vacuoles (78–9 and SI Appendix, Figs. S12 and S13). A noteworthy observation is that in the MdERDL6-1 overexpression lines (OEs-1 and -2), silencing of SlTST1 and SlTST2 by ∼65% to ∼70% (OEs1-TRV #4, OEs2-TRV#5) led to a ∼30% and ∼20% reduction in fruit hexoses and sucrose levels, respectively, in comparison with that in the MdERDL6-1 overexpression controls, whereas a mild decrease of SlTST1 and SlTST2 mRNA levels by less than 10% (OEs1-TRV#1 and OEs2-TRV#2) did not reduce sugar levels in the overexpression background (SI Appendix, Fig. S14). This observation, together with the finding that the CRISPR-Cas9-mediated knockout of SlTST1 and SlTST2 decreased the sugar level further below the WT level in the MdERDL6-1 overexpression background (Fig. 7 and SI Appendix, Fig. S12), indicates that the positive role of MdERDL6-1 in sugar accumulation is achieved through MdTST1 and MdTST2 in apple or their orthologous genes, SlTST1 and SlTST2, in tomato.
Functional characterization of sugar transporters has mostly been performed in model plants, such as Arabidopsis and rice (7, 18, 30), where soluble sugar levels are low and rarely exceed 10 mM (31). Much less is known about roles of those transporters in organs that accumulate sugars to high concentrations, often more than 200 mM, such as in the mature fruits of apples (24), tomatoes (32), and watermelons (16). There is also a scarcity in understanding how different groups of transporters may work together to regulate growth and sugar homeostasis. Findings from the present work provide insight into the coordinated action of two classes of tonoplast sugar transporters that drives the accumulation of soluble sugar to high levels in apple and tomatoes.
In this study, we identified MdERDL6-1 from apples that shares a high sequence identity with AtERDL6 in Arabidopsis and BvIMP from sugar beets, both of which encode H+/Glc symporters, delivering Glc to the cytosol from the vacuole (18, 19). Consistently, MdERDL6-1 was characterized in the yeast system as a proton-coupled Glc symporter with a Km of 21.7 mM (Fig. 2 D–F). This Km value is higher than that of many sugar transporters reported from other species (33) but comparable with that of LeHT1 and LeHT2, two H+/hexose carriers in tomato fruit (21) and VvERDL6-13, a H+/Suc symporters isolated from grape berries (34). In apple fruits, Glc in the vacuoles could reach over 100 mM. Thus, sugar transporters such as MdERDL6-1 with high Km and Vmax values appear to be commensurate with the physiological context to transport sugars from the vacuole to cytosol during fruit development.
Further evidence on MdERDL6-1 functioning as a proton-coupled tonoplast Glc exporter comes from transgenic analyses. First, silencing of MdERDL6-1 in apple fruit calli significantly increased the Glc concentration (Fig. 3 C and D). This observation is consistent with that reported for the corresponding erdl6 mutant of Arabidopsis exhibiting high Glc level in the vacuole (18, 19). Second, in the transgenic apples and tomatoes overexpressing MdERDL6-1, the down-regulated expression of MdCAB1-1 and SlCAB1 indicates an increased concentration of Glc in the cytosol of the transgenic lines (SI Appendix, Fig. S7), since CAB1 expression is negatively controlled by the cytosolic Glc concentration (19). Third, all homologous genes encoding Glc-6-phosphate transporter 2, ADP-Glc pyrophosphorylase 3, and beta-amylase 3, that displayed a positive response to increased Glc in cytosol (35), were significantly up-regulated in the transgenic apple leaves overexpressing MdERDL6-1 (Fig. 5B). Collectively, these findings indicate that more Glc was exported into cytosol from vacuole in transgenic apples and tomatoes overexpressing MdERDL6-1, further supporting the conclusion that MdERDL6-1 functions as a vacuolar Glc exporter.
One remarkable observation from this study is that overexpression of MdERDL6-1 did not meet our expectation of decreasing Glc concentration in apple fruit calli and leaves and tomato fruits, as reported in transgenic Arabidopsis overexpressing a homolog of AtERDL6, BvIMP (19). On the contrary, it substantially increased the Glc, Fru, and Suc concentrations (Figs. 3 and 4), most likely stored in vacuoles that occupies the bulk of the cell volume (36).
Dynamic sugar storage in plant vacuoles is highly regulated by the balance between the import and export of sugars across tonoplast, mediated by different tonoplast sugar transporters (6). Among the DEGs identified from the MdERDL6-1-overexpressed lines, expression levels of TST1 and TST2, homologous to AtTMT1 and AtTMT2 encoding tonoplast H+/sugar antiporters in Arabidopsis (8, 9), were significantly up-regulated, resulting in an increased protein abundance for MdTST1 and 2 in apple leaves, and SlTST1 and 2 in tomato fruits (Fig. 5 and SI Appendix, Fig. S4). The expression of AtTMT1 and AtTMT2 is known to be strongly induced by Glc and with the latter also induced by Fru and Suc (8). Consistently, the promoter activities of MdTST1 and 2 were indeed significantly enhanced by sugar feeding (Fig. 6 A and B). Further, CRISPR-Cas9–mediated knockout or VIGS-induced suppression of SlTST1 and 2 in the MdERDL6-1 overexpressed tomato lines blocked or reduced, respectively, the increase in sugar levels in mature fruits (Fig. 7 and SI Appendix, Fig. S14). Collectively, these findings demonstrate that the enhanced Glc efflux to cytosol by overexpressing MdERDL6-1 up-regulates the expression of TST1 and TST2, leading to accumulation of sugars in the vacuoles in apples and tomatoes.
As discussed above, the transgenic lines overexpressing MdERDL6-1 likely exported more Glc into the cytoplasm to induce or enhance the expression of TST1 and TST2 to import sugars to the vacuoles in the MdERDL6-1–overexpressing plants (Figs. 4–7), a view also supported by the coexpression patterns of MdERDL6-1, MdTST1, and MdTST2 in developmental fruit of apple (Fig. 1). As with many other fleshy fruits, Suc, Fru, and Glc are stored in the central vacuoles of parenchyma cells in apple and tomato fruit pericarps, with their collective concentration reaching 300 to ∼400 mM at maturity (36), which is far more than that in other tissues, such as leaves (32, 37). It remains unclear how such a high level of soluble sugars could be accumulated in the vacuole from the cytosol, apart from vacuolar lumen in most fruit cells are highly acidic (38), hence favoring the activities of tonoplast H+/sugar antiporters. Our findings on the regulation of TST1/2 expression by MdERDL6-1 reveal a mechanism of regulating sugar status between the cytosol and vacuole.
Biochemical and evolutionary studies have established that cytosolic sugar homeostasis is critical for fundamental cellular function and needs to be tightly controlled at an optimum level (1, 39). We hypothesize that an increase in the Glc level in the cytosol would trigger an influx system to transport sugars to the vacuole, thereby maintaining sugar homeostasis in the cytoplasm. It is probably an effective and advantageous strategy to channel physiologically excessive sugars to the vacuole rather than to other subcellular compartments when the cytosolic Glc levels or signals are above a threshold value. Here, for a given amount of Glc exported from the vacuole, its concentration in the cytoplasm might be instantaneously increased by nearly eightfold, assuming the vacuole occupies up to 80%, with the cytosol occupying 10% (half of the cytoplasm) of the cell volume (36). In this way, the MdERDL6-1–derived cytosolic Glc could act as an effective signal to up-regulate TSTs to import sugars into the vacuole from cytosol (Fig. 8). To this end, increased cytosolic sugar levels, indicated by a decreased CAB1 expression, have been observed in Arabidopsis overexpressing BvIMP that encodes a tonoplast H+/Glc symporter (19). On the other hand, the role of AtTMT1 in importing sugars from the cytosol to the vacuole was shown by analyzing the tmt1 knockout mutant upon feeding with Glc, Fru, or Suc (8). More recently, vernalization-induced sink-to-source transition of sugar beet root was linked with increased expression of BvSUT4 but reduced expression of BvTST2.1 for Suc export from and import into vacuoles, respectively (7). In both cases, however, it was unknown if the tonoplast sugar exporters and importers were modulated by one or another.

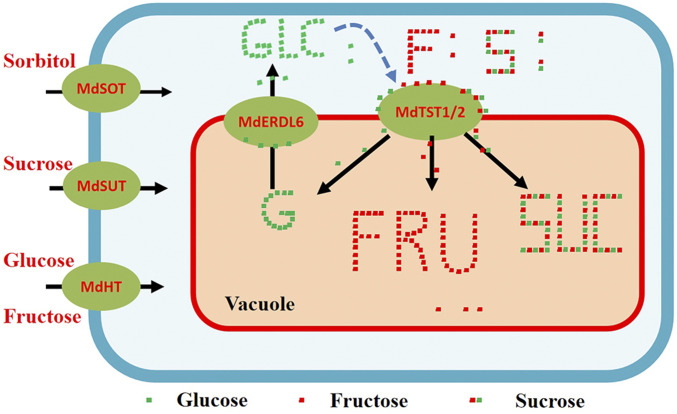
A model on how MdERDL6 could modulate sugar accumulation in vacuole through regulating MdTST1/2 or their orthologous genes. In apple fruits, sugars are unloaded from the phloem into the parenchyma cells via transporters MdSUT, MdHT, and MdSOT for sucrose, hexose, and sorbitol, respectively. Upon meeting the requirements for energy and carbon skeleton production, excessive soluble sugars are imported into the vacuoles for storage, mediated by a set of tonoplast sugar transporters including the H+/sugar antiporters, MdTST1 and MdTST2. On the other hand, MdERDL6-1 acts as an H+/sugar symporter to export glucose from the vacuole to cytosol. Findings from our study indicate that the MdERDL6-mediated glucose efflux to the cytosol activates or enhances the expression of MdTST1 and MdTST2 to import sugars into the vacuole, leading to the accumulation of high concentration of sugars. Our data also indicate that the model is likely applicable to other systems such as tomato fruits (see article text for details). G/Glc, glucose; F/Fru, fructose; S/Suc, sucrose.
The cytosolic sugar homeostasis is regulated by multiple metabolic and transport processes (1). Thus, it is not surprising that there are a number of sugar-related DEGs in the apple leaves overexpressing MdERDL6-1 (Fig. 5A). Here, in addition to the significant up-regulation of MdTST1 and 2, MdSWEET2, a homologous gene to AtSWEET2 for exporting Glc from vacuole to cytosol in Arabidopsis roots (40), was down-regulated (Fig. 5A), probably to avoid further rise of Glc level in the cytosol due to MdERDL6-1 overexpression. Interestingly, several predicted plasma membrane sugar transporter genes were also down-regulated in response to MdERDL6-1 overexpression. These include one MdSUC1 gene for Suc and three MdHT genes for Glc or Fru, as well as five MdSOT genes for sorbitol (Fig. 5A). We speculate that the down-regulation of these transporter genes may potentially contribute to the rebalancing of sugar homeostasis in the cytosol by reducing sugar import from extracellular space. It is also worth noting that genes for two cell wall invertases, one cytosolic invertase and three sucrose synthases were also down-regulated (Fig. 5A). This indicates a reduced sucrose cleavage in the cells overexpressing MdERDL6-1, likely to help alleviating the elevation of cytosolic Glc levels. Pertinently, a coordinated regulation between some plasma membrane sugar transporter and cell wall invertase genes has been recently reported in apple and tomato fruits (4, 41) and Arabidopsis floral organs (42).
Overexpression of MdERDL6-1 under the 35S promoter might result in too much Glc to be exported by the H+/Glc symporter to the cytosol, thereby generating an unusual situation affecting expression of TSTs. This scenario is unlikely, since there was only 40 to 60% reduction in the transcript level of CAB1 in apple leaves, tomato leaves, and fruits (SI Appendix, Fig. S7). Such a level of changes in CAB1 transcripts is within the range observed during plant development and in response to environmental changes (8, 19, 29), indicating that the increased cytosolic Glc from the MdERDL6-1-overexpressed plants is within the physiological levels. The two- to fourfold increase in MdTST1/2 and SlTST1/2 abundance in the transgenic apple and tomato (Fig. 5 E and G and SI Appendix, Fig. S4) also falls in range of fluctuation in protein levels during plant growth and development. Consistent with this view is the normal growth of the apple seedlings overexpressing MdERDL6-1 (SI Appendix, Fig. S3A). Heterologous expression of MdERDL6-1 in tomato promoted vegetative growth and generated fertile plants but delayed flowerings and reduced seed and fruit number (SI Appendix, Fig. S3E and Table S2). The latter finding indicates that reproductive development is sensitive to the MdERDL6-1–induced rise of cytosolic Glc level, consistent with the critical role of sugar signaling in ovule initiation and seed set, as recently shown in Arabidopsis (42). Collectively, the data indicates 1) the up-regulation of TST1/2 expression by MdERDL6-1-mediated Glc efflux likely occur in planta and 2) the abnormal flowering and seed set observed in the MdERDL6-1 overexpression tomato lines under the CaMV35S promoter is probably due to misexpression of the transgene in cellular sites that otherwise show no or little ERDL6 expression or the up-regulation of the SlTSTs is not sufficient to counteract the increased efflux of Glc from vacuole to cytosol in the reproductive organs.
In summary, we showed in this study that MdERDL6-1 encodes a tonoplast H+/sugar symporter to export Glc from the vacuole to cytosol. Significantly, overexpression of MdERDL6-1 substantially increased the Suc, Fru, and Glc levels in apple fruit calli and leaves, and tomato fruits and leaves, which was due to the up-regulation of the expression of the H+/sugar antiporter genes, TST1 and TST2. The finding offers insight into the regulatory mechanism of sugar accumulation, in which an increased cytoplasmic Glc level mediated by MdERDL6 activates the expression of TST1 and TST2 to import sugars into the vacuole, probably through sugar signaling effect of Glc (Fig. 8). High expression of homologous ERDL6 genes has also been observed in fruits of many other species, such as pineapple (20) and orange (22), indicating the ERDL6-regualted vacuolar sugar accumulation pathway (Fig. 8) may operate in a broad range of fruit crops. It remains to be determined as how the cytoplasmic Glc signals are sensed and transmitted to modulate the expression of TST1 and TST2 and if the high expression trait of ERDL6-TST nexus was selected during evolution and domestication of fruit crops.
The samples from the “Gala” apple (Malus domestica) were the same as those used in our previous report (23). Fruits were sampled between 3:00 PM and 4:00 PM at 16, 34, 55, 75, 98, and 122 (maturity) days after bloom (DAB), corresponding to the timing of major physiological events for “Gala” fruit (24). On each collection date, six apples per replicate were harvested from three trees, with five replicates in total. The fruits were immediately weighed, cut into small pieces after the core was removed, and frozen on-site in liquid nitrogen. Additionally, full flowers, roots, mature leaves, and shoot tips were also collected. All frozen samples were stored at –80 °C until use.
Tissue-cultured WT and MdERDL6-1–transformed “GL3” apple plantlets were initially grown on Murashige and Skoog (MS) medium supplemented with 25 mg ⋅ L−1 kanamycin, 0.2 mg ⋅ L−1 IAA, and 0.3 mg ⋅ L−1 6-BA for 4 wk. They were then transferred to a rooting MS medium supplemented with 0.5 mg ⋅ L−1 IAA and 0.5 mg ⋅ L−1 IBA. After rooting, plants of both genotypes were transferred to a culture room maintained at 23 °C with a 14-h photoperiod, supplemented with fluorescent light (60 μmol ⋅ m−2 ⋅ s−1). After these plants had grown for three months, the fourth to eighth leaves from the base of the stem (fully mature leaves) were sampled. The samples were immediately frozen in liquid nitrogen and stored at −80 °C for further analysis.
Candidate genes were identified by performing a BLASTp (protein-protein BLAST) analysis against the apple gene set (amino acids) in the Malus × domestica genome, GDDH13 v1.1 (25). As a query, we used sequences for Arabidopsis ERDL6, TST, and vGT obtained from The Arabidopsis Information Resource. An E-value of 1.00E-10 was set as the threshold. Putative candidate gene sequences were retrieved from the Malus genome GDDH13 v1.1. We then constructed neighbor-joining phylogenetic trees with 1,000 trials of bootstrap replicates using MEGA 6.
RNA-seq data were generated from a “greensleeves” apple fruit taken at five developmental stages (16, 41, 70, 94, and 128 DAB) and recently fully expanded leaves, with three biological replicates, to determine the expression patterns of the apple sugar transporter genes, as reported by Li et al. (24). Sequencing was performed using an Illumina HiSEq. 2000 sequencer with 50-base-pair (bp) single-end sequencing (Illumina, San Diego, CA) in 2012. However, RNA-seq for transgenic apple leaves in 2017 was sequenced using an Illumina HiSEq 2500 sequencer with 250-bp single-end sequencing (Illumina, San Diego, CA) at Biomarker Technologies, China.
The RNA-seq reads were processed by removing barcode and adaptor sequences first, followed by alignments to the rRNA database Silva using Bowtie, allowing up to three mismatches to remove potential contaminating reads. The clean reads were then aligned to the Malus Genome GDDH13 Version 1.1 (25) using Tophat, allowing one mismatch. After alignment, raw counts of mapped reads for each Malus gene model of GDDH13 Version 1.1 were derived and then normalized to the RPKM per million mapped reads .
Total RNA was extracted from “gala” fruit by using the cetyl trimethyl ammonium bromide method. A set of specific primers (SI Appendix, Table S1) were used to clone the complementary DNAs (cDNAs) of MdERDL6-1, MdTST1, and MdTST2.
To determine its subcellular localization, the full-length open reading frame (ORF) of MdERDL6-1 without the stop codon was cloned into the pMDC83-GFP vector under the control of the CaMV35S promoter, and GFP was attached to the C terminus of the target gene. To examine transient expression, we transformed both the MdERDL6-1 fusion plasmid and tonoplast targeting vector VAC-RK (vacuole marker) CD3–975 into Arabidopsis mesophyll protoplasts and apple calli protoplasts via polyethylene glycol-mediated transformation (43, 44). Furthermore, we also isolated the vacuoles of N. benthamiana leaves, which were infiltrated with Agrobacterium tumefaciens containing MdERDL6-1-GFP plasmid for 3 d, as described in (45), with minor modifications. The isolated protoplasts and vacuoles were examined for GFP signal and chlorophyll autofluorescence using a confocal laser-scanning microscope (LSM 710; Carl Zeiss) at excitation wavelengths of 488 nm for GFP and 633 nm for chlorophyll autofluorescence.
To determine the subcellular localization of MdERDL6-1 in yeast, we amplified the full-length ORF of MdERDL6-1 without the stop codon into the pYES-DEST2-eGFP vector for transformation into the hexose transporter-deficient yeast strain EBY.VW4000, which grows only on maltose (26). The GFP fluorescence of the transformed yeast was visualized under a fluorescence microscope (x 600 to 800 nm) (LSM 710; Carl Zeiss).
To determine the sugar transport properties of MdERDL6-1, its ORF was inserted into the pYES-DEST2 vector and transferred into the yeast strain EBY.VW4000 (26). For uptake experiments with 14C-labeled sugars to test H+/sugar symport across plasma membrane in yeast, single colonies of yeast strain EBY.VW4000, containing pYES-DEST2-MdERDL6-1 or empty pYES-DEST2 vector, were precultured on maltose-casamino acid medium (0.67% weight (wt)/volume yeast nitrogen base, 1% wt/volume casamino acids, 0.002% wt/volume Trp, and 2% wt/volume maltose) to an OD600 value of 0.8. The transport assay was then performed as described by Sauer and Stadler (46). For the inhibitor assays, a final concentration of 50-μM CCCP, as a proton uncoupler, was added to energized yeast cells 30 s before the addition of 14C-labeled Glc.
To construct the vectors for overexpression of MdERDL6-1, MdTST1, and MdTST2, we introduced the respective coding sequence into the pGWB402 binary vector under the CaMV35S promoter. The RNAi constructs were generated using the RNAi vector, pHannibal, and the binary vector, pCAMBIA2300. The specific PCR fragments of the target genes (MdERDL6-1: 301 bp, MdTST1: 313 bp, and MdTST2: 311 bp) were inserted into the pHannibal vector in antisense orientation. The pHannibal vectors containing target fragments were then subcloned into the binary vector, pCAMBIA2300. The recombinant plasmids were introduced into the Agrobacterium tumefaciens strain, EHA105.
Transgenic apple plants were generated from leaf fragments through Agrobacterium-mediated transformation, as previously described (38). Regenerated Kan-resistant buds were subcultured every 3 wk. The candidate resistant lines were subcultured every 4 wk and rooted using the two-phase method following the description of Dai et al. (47). A total of 25 rooting plants were grown in a greenhouse for at least 2 mo. MdERDL6-1 expression in mature leaves from three independently transformed lines was detected by qRT-PCR and Western blotting. Respective samples harvested were used for measurement of sugar concentrations and RNA-seq with at least three biological replications in each case.
The pGWB402-MdERDL6-1 construct was transformed into tomato (Solanum lycopersicum cv. Micro-Tom), as previously described (48). Tomato plants were grown at 25 °C with a 16-h photoperiod, supplemented with light at 120 μmol m−2 s−1. The transgenic lines were screened by Kan resistance and PCR analysis with homozygous lines identified at T3 generation.
For the CRISPR-Cas9 experiment, the target sequences for SlTST1 and SlTST2 were designed using CRISPR direct (http://crispr.dbcls.jp/), and the sequence specificity was confirmed by BLAST in National Center for Biotechnology Information. After the specificity was verified in Micro-Tom tomatoes, the target sequence was synthesized and cloned into the pHSE401 vector to construct the single target vector for SlTST1 or SlTST2, as described by Xing et al. (49). Moreover, a double target vector against SlTST1 and SlTST2 was produced. The single target vector for SlTST1 or SlTST2 was transformed into WT Micro-Tom tomatoes to identify their respective functions in controlling sugar concentration in tomato, while the double target vector was transformed into WT Micro-Tom tomatoes and the transgenic tomato line overexpressing MdERDL6-1. Following transformation, the edited lines were screened by hygromycin resistance and sequencing.
Specific partial sequences of SlTST1 (290 bp) and SlTST2 (285 bp) were cloned from tomato leaves using RT-PCR. The resulting PCR products were both inserted into the pTRV2 (tobacco rattle virus) vector in tandem under the control of the dual CaMV35S promoter. Then, the Agrobacterium strain EHA105 contained the resulting vector pTRV2-SlTST1/2 and pTRV1 were cultured to OD600 of around 1.0 respectively, and resuspended to OD600 of 0.4 to 0.6 at a 1:1 ratio with pTRV1 (with empty pTRV2 as a control), which were used for infiltration of the carpopodium of tomato fruit, attached to the plant by Agrobacterium-mediated transformation, as described previously (50). Ten days after infiltration, the tomato fruit were harvested for quantification of SlTST1 and SlTST2, and measurement of sugar concentrations.
Soluble sugars and sorbitol were extracted and derivatized sequentially with methoxyamine hydrochloride and N-methyl-N-trimethylsilyl-trifluoroacetamide, as previously described (51). Thereafter, the metabolites were analyzed with a Shimadzu GCMS-2010SE (Shimadzu Corporation, Kyoto, Japan). The tissue residue that remained after 75% methanol extraction for gas chromatography-mass spectrometry (GC‒MS) analysis was re-extracted three times with 80% (volume/volume) ethanol at 80 °C, and the pellet was retained for starch determinations (23). Additionally, the assimilation of CO2 in seedling leaves was monitored between 9:30 and 11:30 AM, using a LI-COR 6400 portable photosynthesis system (LI-COR, Huntington Beach, CA).
qRT-PCR was used to analyze the expression of all detected genes. After sequence similarities were examined based on Malus domestica or Solanum lycopersicum genome data, gene-specific primers (SI Appendix, Table S1) were designed using Primer 5 software. Primer specificity was determined by RT-PCR and melting curve analysis. qRT-PCR was performed on an ABI7300 Real-Time PCR System (Thermo Fisher Scientific). Transcripts of Actin (GenBank: CN938023 for apple, and BT013707 for tomato) served to standardize the cDNA from our test genes. For each sample, total RNA was extracted from three biological replicates before the qRT-PCR experiments were run. All data were examined by the delta-delta cycle threshold (ddCT) method.
To examine why MdTST1 and MdTST2 expression were up-regulated in the transgenic plants overexpressing MdERDL6-1, we designed primers to clone the promoters of MdTST1 and MdTST2 based on their genome sequences (SI Appendix, Table S1). About 1.8 kb of DNA sequence, upstream of MdTST1 or MdTST2 mRNA, was cloned from the genomic DNA of the “Royal Gala” apple. To examine transient gene expression in tobacco, the MdTST1 and MdTST2 promoters were introduced into the pC0390GUS vector to generate MdTST1P:GUS and MdTST2P:GUS. The p35S:GUS vector and the empty vector pC0390GUS were used as the positive and negative controls, respectively. Agrobacterium-mediated transient transformation was carried out on unfolded leaves according to the protocol previously described (52). Exogenous sugar feeding was conducted on tobacco leaves 48 h following agroinfiltration. Here, tobacco leaves were cut at the petioles and immediately inserted into different 2% sugar solutions or water for 24 h before being harvested for measurement of β-glucuronidase (GUS) activity.
To test whether MdERDL6-1 could regulate the expression of MdTST1 and MdTST2, we investigate the influence of MdERDL6-1 expression on activities of MdTST1 and MdTST2 promoters via transient coexpression of 35S:MdERDL6-1 and MdTST1P:GUS, and/or MdTST2P:GUS, in tobacco leaves. The leaves were sampled to measure GUS activity 48 h after the infiltration.
Total protein samples were extracted from apple leaves and tomato fruits as previously described (53), and the total concentrations were determined with protein assay kits (Bio-Rad, Hercules, CA) using bovine serum albumin as a standard. Specific monoclonal antibodies were raised in rabbits (Genscript, Nanjing, China) against a peptide from a highly conserved region in MdERDL6-1 (GSLSNVGAMVGAIAS), MdTST1 (FYLPESPRWLVSKGR, conserved sequences with SlTST1), and MdTST2 (VEGLIVAMSLIGAT, conserved sequences with SlTST2), respectively. Actin abundance was monitored with a monoclonal antibody (CWBIO, Beijing, China). The antigen-antibody complexes were detected using Clarity Western ECL Substrate (Bio-Rad, Hercules, CA), and the protein bands of Western blotting were quantified using Image J software according to the manufacturer’s instructions.
All data were analyzed via IBM SPSS Statistics 21 and graphed with Sigma Plot 10.0 software. Data were analyzed using independent t tests or one-way ANOVA tests, with a significance level accepted at P < 0.05.
This work was supported by the Program for the National Key Research and Development Program (2018YFD1000200), the National Natural Science Foundation of China (No. 31872043), the Training Program Foundation for the Young Talents of Northwest A&F University to M.L., and Australian Research Council (DP180103834) to Y.-L.R.
All study data are included in the article and supporting information.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52