
On global scale, the current situation of pandemic is symptomatic of increased incidences of contagious diseases caused by pathogens. The faster spread of these diseases, in a moderately short timeframe, is threatening the overall population wellbeing and conceivably the economy. The inadequacy of conventional diagnostic tools in terms of time consuming and complex laboratory-based diagnosis process is a major challenge to medical care. In present era, the development of point-of-care testing (POCT) is in demand for fast detection of infectious diseases along with “on-site” results that are helpful in timely and early action for better treatment. In addition, POCT devices also play a crucial role in preventing the transmission of infectious diseases by offering real-time testing and lab quality microbial diagnosis within minutes. Timely diagnosis and further treatment optimization facilitate the containment of outbreaks of infectious diseases. Presently, efforts are being made to support such POCT by the technological development in the field of internet of medical things (IoMT). The IoMT offers wireless-based operation and connectivity of POCT devices with health expert and medical centre. In this review, the recently developed POC diagnostics integrated or future possibilities of integration with IoMT are discussed with focus on emerging and re-emerging infectious diseases like malaria, dengue fever, influenza A (H1N1), human papilloma virus (HPV), Ebola virus disease (EVD), Zika virus (ZIKV), and coronavirus (COVID-19). The IoMT-assisted POCT systems are capable enough to fill the gap between bioinformatics generation, big rapid analytics, and clinical validation. An optimized IoMT-assisted POCT will be useful in understanding the diseases progression, treatment decision, and evaluation of efficacy of prescribed therapy.
Infectious disease refers to the functional impairment of health due to presence of array of pathogenic microorganisms (like bacteria, viruses, fungi, and parasites) inside the body. Pathogenic infection follows a linear pathway inside the host, e.g., first enters the human body, thereafter colonization, and causes disease (Libertucci and Young, 2019). Further, an infectious microorganism can overcome the host defence system through antigenic variation, latency, and suppression (Ortega-Prieto and Dorner, 2017). Anthropogenic activities driving continuous parasite evolution can cause new microbial threats to human health (Lebarbenchon et al., 2008). Jones & group reported emergence of 335 infectious diseases worldwide, which can be from unrecognized novel pathogenic strains (e.g., swine flu, Ebola virus, and coronavirus) or re-emergence of existing strains (e.g., malaria cholera, plague, dengue fever, and yellow fever) with increased virulence (Jones et al., 2008). Relatively, 1500 new unknown zoonotic pathogens of animal origin including Ebola virus (EBOV), human immunodeficiency virus (HIV), severe acute respiratory syndrome (SARS), influenza A (H1N1) virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and Zika virus (ZIKV) were discovered in the timeline of 1976, 1980, 2003, 2009, 2012, and 2015, respectively (refer to Fig. 1 ).

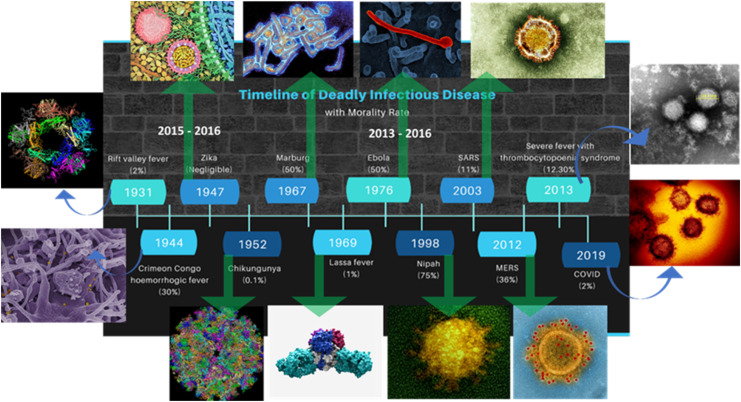
Timeline of emerging infectious diseases.
In the recent years, the geographical distribution of infectious diseases has enlarged rapidly and destroyed human life. Multiple factors (like human population, demographic migrations, and the global environmental changes) combinedly led to increased incidences of such pathologies (refer to Fig. 2 ). These epidemic diseases rapidly spread to different territories across the globe, resulting in thousands of cases of infection and deaths (Zhang et al., 2019). These infections can spread exponentially among community by multiple ways like direct contact, cough, or sneeze or even sometime by asymptomatic person. Onset of infectious diseases shows mild to moderate symptoms, e.g., fever, cough, sore throat, chilling, and fatigue. Whereas, severe complications include breathlessness, septic shock, heart failure, ventilation, organ dysfunction, and death (Verity et al., 2020). Determining the severity of infectious disease is important to make the appropriate plan and mitigation strategies for healthcare needs as epidemics unfold (Fig. 2).

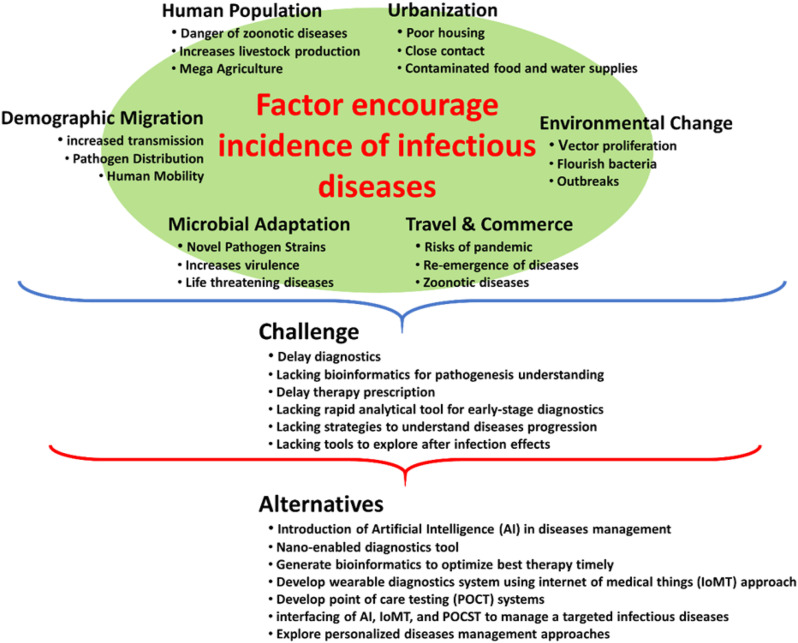
Schematic representation of different factors determining the higher incidence of infectious diseases.
In the present era, the infections are among the serious natural disasters that have severe impact on worldwide wellbeing (in terms of severe morbidity and mortality) and economies. There is a strong correlation between incubation period (time required for onset of symptoms) and severity of infections (Virlogeux et al., 2015). A shorter incubation period could be characteristic of a higher dose of pathogens, prompting quicker replication, and results in a more aggressive infection, followed by serious illness. Moreover, age significantly affect the severity of infection, e.g., school-age children found to be least affected age group than the young adults and older ones (Kang and Jung, 2020). Moreover, pregnant women also found to be more severely affected from viral infections related to physiological changes occurring during pregnancy (Rac et al., 2019). During epidemics including SARS, MERS, and recent new coronavirus infection pandemic (i.e., COVID-19), several surveillance studies confirmed more severe illness in pregnant woman than general population. Other high-risk group of contracting infectious disease includes obesity and diabetic patients due to the deleterious effects on host immunity, which primarily increased the risk for infectious susceptibility and severity. Obesity can worsen the infection due to lower respiratory function related to inflammatory aspect, and even can cause respiratory failure. Therefore, individuals with compromised or weakened immune system are at higher risk for such kinds of infectious diseases.
In order to manage the burden of pandemics, the detailed understanding of infectious disease dynamics is important to stop their further spread among people and overwhelm the health system (Kaushik et al., 2020; Paliwal et al., 2020). The pandemic diseases usually go through four phases, although not necessary for all the infectious diseases, e.g., (i) the first phase: the emergence of positive patients in a healthy community, (ii) the second phase: localized transmission, (iii) the third stage: outbreak in terms of community transmission, and (iv) the last phase: reduced transmission of the pathogen due to acquired immunity and clinical interventions. The economic risk in these long running epidemics is not insignificant. Outbreaks and epidemics overburden the existing health infrastructure and affect front line workers critically besides other human resources (Kaushik et al., 2020). The economic risk in these long running epidemics are significant and has resulted in a sharp decline in nations gross domestic product (GDP) during outbreaks (Bloom and Cadarette, 2019).
Complex interplay of complicating factors like vaccine, drugs, theranostics, diagnostic assays, and therapeutics coordinated response helps dealing with the danger of epidemics (Kaushik, 2019; Kaushik et al., 2020). In comparison to conventional laboratory-based methods, point-of-care (POC) tests have tremendous potential to improve the management of infectious diseases as these help in early diagnosis of infected population and thus allow timely monitoring and treatment that ultimately help in efficient management of disease. An overview of all aspects of POCT device, fabricated on the bases of artificial intelligence (AI) and internet of things (IoT), is presented with future perspectives in terms of clinical diagnosis (refer to Fig. 3 ) (Kaushik, 2019; Mujawar et al., 2020). Besides material innovation and biorecognition element investigation, the other important role of AI-based biosensing are i) signal acquisition and transportation, ii) data processing, and iii) decision system (Jin et al., 2020).

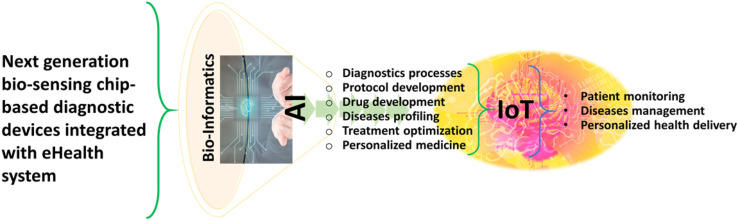
Schematic representation of next generation biosensing, AI and IoT to explore POCT diagnostics to manage a targeted infectious disease.
Keeping advancements and prospects into consideration with the AI to manage a targeted infectious disease, this review focuses on exploring IoMT-assisted advanced biosensor technologies for POCT of infectious diseases like malaria, dengue fever, H1N1, HIV, human papilloma virus (HPV), Ebola virus disease (EVD), ZIKV, and COVID-19 infection. The outcomes of this comprehensive review will be useful to understand the role and importance of IoMT-assisted POCT system in diagnostics. Along with the development of IoMT-assisted POCT, the discussion is further extended to cover the challenges and future aspects of these scalable and affordable devices to manage infectious disease outbreaks.
The concept of infection management comprises identification of bacterial pathogen, causes of infection, diagnostics, treatment, healthcare cost, and minimizing the effects of the disease (Bissonnette and Bergeron, 2012). Disease management begins with the assessment of microbial infection and then strategic decisions for medical care of chronic patients. The outbreak of infectious disease sometimes remains undiagnosed for a period of time that allowed unseen transmission of the virus, leading to large epidemics. Therefore, clinical awareness and the relevant knowledge about diagnostic tools are critically important to perform as effective detectors and first-line responders. Most importantly, in the fight against emerging infections, early and accurate diagnosis is the most effective way to break the chain of transmission and mitigate the impact of these diseases. The routine diagnostic approaches depend on type of microorganisms and infectious diseases (Hahn et al., 2020).
Typical disease biomarkers include aptamer, antibodies, whole pathogen (e.g., bacteria and viruses), and the molecular agents (e.g., nucleic acids and proteins) from the infectious agents. Readily available conventional methods like microscopy and culturing are well established and considered effective diagnostic for infectious diseases (Srivastava et al., 2018). However, these approaches face various challenges during viral markers detection such as i) low sensitivity in microscopy methods and ii) need of highly skilled personnel and techniques to carry out the reliable tests. Moreover, these conventional diagnostics also require more time for clinical interpretations due to cultivation and drug susceptibility testing. Further, molecular methods have increased laboratories throughput due to their higher sensitivity and ability to detect infections faster than the conventional approaches (Lee et al., 2015). Among these molecular methods, nucleic acid amplification (NAA) assays, enzyme linked immunosorbent assay (ELISA), colorimetric assay and other advance biotechnological methods are becoming more famous and are routinely conducted in many clinical microbiology laboratories. However, these healthcare techniques have several limitations such as too expensive for routine clinical diagnosis, time-consuming (in terms of several hours or days to complete), and labour-intensive. The continuous evolution of automated diagnostic tools like proteome chips, microfluidic, colorimetric assay, and other advance molecular methods allows diagnosis from laboratory to bedside and even recommended by the world health organization (WHO) for use in emergency and remote settings. The selection of an appropriate diagnostic technique relies on the several parameters like sensitivity, selectivity, cost, required time for diagnosis, and availability of the diagnostic tools. The rapid diagnostic procedures can help in more efficient management of transmission of infectious disease in an economic and reliable manner.
The real-time identification of infection helps in providing effective medical treatment, and most importantly offers control over epidemic outbreaks (Sin et al., 2014). Best clinical management and improved treatment of diseases rely on the development of appropriate diagnostic tools that are affordable, specific, rapid, sensitive, robust, and deliverable to end-users (Kosack et al., 2017). Demand of POC platform has led to the rapid evolution in biosensor technologies (Cruz et al., 2014; Kaushik et al, 2014, 2016b, 2016c; Kaushik and Mujawar, 2018). Biosensors integrated into analytical devices convert recognition events into readable output using a biological molecule (He et al, 2018, 2019; Prakash et al., 2020). The specificity and sensitivity of POC biosensors also depends on affinity reagents detecting target molecules (Nayak et al., 2017). Antibodies are oldest and still the most widely used affinity reagents in biosensors but cannot be a part of pandemic due to their slow and costly synthesis processes. Recently, synthetic single-stranded oligonucleotides (aptamer) scaffolds generated against different targets were reported as a promising alternative. Functionalization of aptamers with nanomaterials can further enhance the performance of POC diagnostics. Advances in POC systems involving nanotechnology improves signal enhancement and detection chemistries (Bhanjana et al., 2019; Kumar et al, 2019, 2021; Li et al., 2011). Moreover, the use of flexible substrate for integrating different components improves the capabilities of POCs as affordable, non-invasive, and sophisticated diagnostic devices.
The microfluidic platform integrates multistep assay (like amplification, sample preparation, and detection) into one automated miniature devices to enable true POCT (Vasudev et al., 2013). Several biosensing platforms have been reported for Lab-on-a-Chip devices, e.g., optical, electrochemical, piezoelectric, and surface-enhanced Raman spectroscopy (Ali et al., 2020; Pashchenko et al., 2018). To prevent the community transmission of infectious diseases, the portable polymerase chain reaction (PCR) systems can offer fast and early diagnosis. However, these systems require multiple times amplification to improve the detection sensitivity. A solution of this limitation is fluorescence-based quantitative PCR where amplification of target is directly proportional to quenching of initial fluorescence (Lee et al., 2021; Zhang et al., 2018).
Optical monitoring allows interpretation by naked eyes or fluorometers using fluorescence molecules. While electrochemical detection modalities (either amperometry, potentiometric, or impedimetric) provide highly sensitive platform using disposable microfluidic chips (Luka et al., 2015). Additionally, these electrochemical devices easily miniaturized using hand-held potentiostat that avoids the requirement of any complex instruments for detection (Cruz et al., 2014). The incorporation of fluorescent modalities in POC platforms offers a significant potential for customization. However, specific sample preparation is required in such POCs in order to eliminate the background disturbances from the intrinsic properties of the fluorescent molecules.
In POCT systems, real samples (such as blood, serum, urine, or saliva with minimal to no preparation) are mainly used to perform testing. In resource-limited settings, the benchtop technologies are not feasible due to their high cost and requirement of trained personal. The POC diagnostic technologies are focused on easy utilization of the device by untrained personnel as well in a portable manner. Recent advancement in technology has facilitated development efforts toward portable and wearable POCT frameworks. Uptake of versatile and wearable gadgets (smartphones, drones, and Bluetooth) transformed the entire POC system. Smart electronic devices also allow real-time communication between patient and the health experts that ensure effective diagnostic performance for healthcare management even in resource-limited settings (Jin et al., 2020). These attributes make POC diagnostics ideal for use as a rapid diagnostic device for infectious diseases (refer to Fig. 4 ) management via detecting a marker and monitoring viral infection progression.

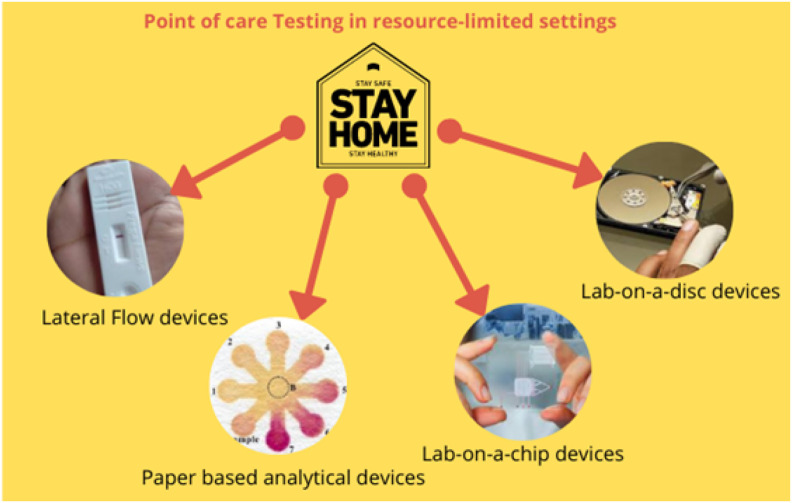
Schematic illustration of different POC tools to promote patient self-testing in resource-limited settings.
In case of inaccessibility due to lacking availability and affordability of commercial diagnostic tests, POC tests can sufficiently be utilized according to the accessibility of patients, even at home. Such features facilitate the self-monitoring and diagnostics of infectious diseases at early-stage of the viral infection (Pai et al., 2012). It is widely accepted that design of risk-reduction policies and primary prevention at initial stage of spread of any infectious disease can reduce the incidence rate, disease burden, and improve the health management. Therefore, POCT platforms have significant potential to impact medical care in challenging time of pandemics via rapid results, multimode sensing, easy to use, and home care helps (“Global Point-of-Care Diagnostics Market Outlook 2020,” 2020). Nowadays, several agencies are investing huge amount for developing new POC diagnostics in market for global health (Liu et al., 2019).
The internet-of-things (IoT) is a technological advancement in information technology (IT) to automate the processes and transfer data through a wireless network without human intervention (Joyia et al., 2017; Kaushik et al., 2020; Mujawar et al., 2020). The IoT has now become a part of numerous organizations to design and develop smart devices for easier and smarter solutions at workplace such as smart home, automobile sensor, smart farming, and smart meters to save energy and manpower. The concept of IoT technologies also invades health-tech sector to achieve brighter future and referred as internet-of-medical things (IoMT) (Al-Turjman et al., 2020). The IoMT is a computation of medical devices with wireless connectivity (Fig. 5 ). The IoMT devices increases efficiency and quality of real-time diagnosis using a sophisticated health-tech ecosystem (Mujawar et al., 2020).

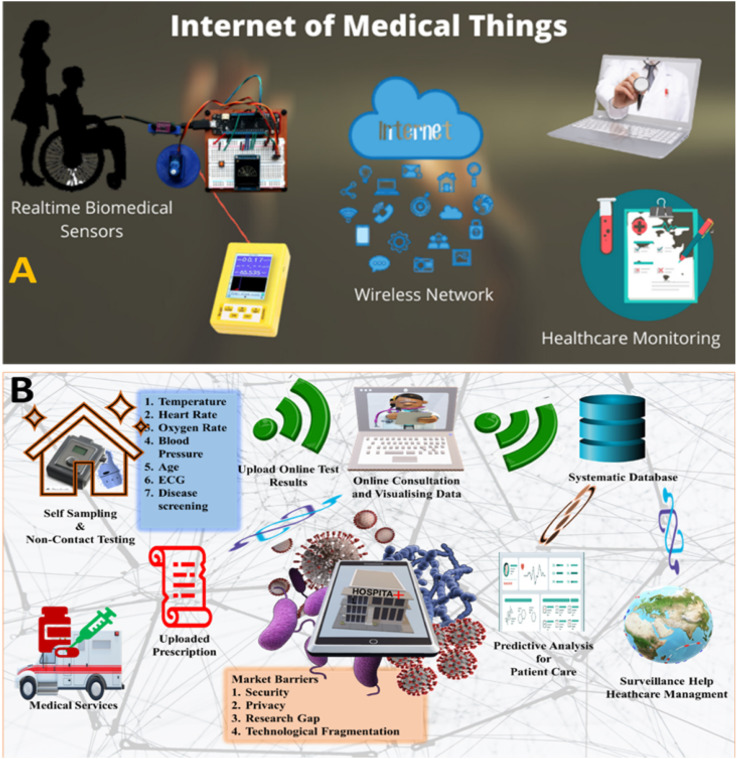
Illustration of the impact of IoMT on patient health management via connecting various steps or gaps associated POCT including diagnostics, bioinformatics collection, date sharing, rapid analysis, and timely therapy decision.
Numerous healthcare providers have started heavy investment in this domain to leverage the potential of IoMT to empower medical practitioners. This new era of digitalization creates smart environment through intelligent services (IoT and smartphones) for imaging, sensing, and diagnostics. Resolution of cell-phone camera lens can be effectively used for optical microscopy, flow cytometry, and other imaging readouts for better real-time results (Nayak et al., 2017). Bluetooth low energy (BLE) technology-equipped POCs manifest data connectivity up to few feet such as non-invasive mouthguard biosensor for salivary glucose and fitness trackers, to continuously monitor patient's physical activity and vital signs. (Christodouleas et al., 2018). Research efforts are directed towards development of smart fabrics having sensing capabilities to record blood pressure, electrocardiogram, heart rate, and body temperature. In 2020, a huge number of internet-connected devices are available in market like medical kiosks, fitness tracking devices (wrist band, smart watches, shorts etc), clinical grade wearables (smart belts, chest strap), remote patient monitoring devices, smart pills, and many more (Ding et al., 2019). Several non-invasive samples like sweat, saliva, faeces, tears, and breath could be used in eHealth diagnostic devices (eDiagnostics) to detect biomarkers of major diseases (viral infections, cancer, and HIV); however, these devices are still in developing phase as the pathological level of these biomarkers yet not established. At eHealth systems, several diagnostic needs could be covered by eDiagnostic wearable devices. Interestingly, smartphones ubiquity is widely adopted among modest and low-income peoples, promoting the use of POCT without additional costs. It will also increase medical facilities in remote areas and rural communities at lesser costs.
Major motivation leading to rapid adoption of smartphone-based computing devices in health system is the need of better communication, information resources, and social networking at the POC (Lee Ventola, 2014). Web browser gives interface for sending and receiving health information with no time delay. In addition, IoMT device also offers other advantageous features, like short message service, e-mail, global positioning systems (GPS), and interactive voice recordings that can help towards automation of POC diagnostics in data collection and further continuous transmission into a central database through satellite networks (Nayak et al., 2017). Patient's health information can be stored to develop large set of databases that help researchers to study complex diseases such as cancer, asthma, coronary, diabetes, and Parkinson's disease.
Smartphone based POCs envisioned can perform either end point or real-time tests on the basis of particular detection mechanism either colorimetric, fluorescence emission, reflection, current, or turbidity (Ding et al., 2019; Gupta et al., 2021). For example, development of color change in ELISA-based assay can be identified by smart POCs, and captured images can be further analysed using customized applications to quantify the pathogens. While to achieve sensitive smartphone fluorescence detection, a variety of optical accessories need to be added. Similarly, IoT-based POC devices have been reported with easy read-out platforms for detection of different infectious diseases, with the help of different molecular assays including lateral flow, PCR, and loop-mediated isothermal nucleic acid amplification (LAMP) for detection of emerging pathogens, refer to Table 1 . Further, electrochemical IoMT consists of fabricated integrated circuits to acquire clinical signals and enable real-time detection of pathogen.

| S. No. | Device | Detection principle | Target | Limit of detection | Time (min) | IoMT integration Feasibility | Ref. |
|---|---|---|---|---|---|---|---|
| (a) Malaria | |||||||
| 1 | NAAT | LAMP | kelch 13 | 1 c/r | <20 min | – | Malpartida-Cardenas et al. (2019) |
| 2. | Gazelle | Magneto-optical detection | haemozoin crystals | 50 parasites/mL | ~1 min | Yes | Kumar et al. (2020) |
| 3. | MCFA | Chemiluminescence-based ELISA | PfHRP2 | 8 ng/mL | – | Yes | Ghosh et al. (2020) |
| (b) Dengue fever | |||||||
| 4. | NAAT | QUASR multiplexed RT-LAMP assay | DENV RNA | 108 to 103 PFU/mL | <40 min | Yes | Priye et al. (2017) |
| 5. | Portable PCR | RT-PCR | DENV RNS | 1 × 108 genome copy equivalents/mL | – | – | Mehta et al. (2019) |
| 6. | IoT PCR | PCR | DENV cDNA | – | 34 min | Yes | Zhu et al. (2020) |
| (c) Influenza A | |||||||
| 7. | Fluorescent probe based POC | Fluorescence and light guiding | H1N1 | 138 pg mL−1 | 40 min | Yes | Lee et al. (2018) |
| 8. | Genosensor | Electrochemical | Hemagglutinin gene | 0.002 ng/6 μL | 30 min | Yes | Ravina et al. (2020) |
| (d) Human immunodeficiency virus | |||||||
| 9. | Label-free fluorescence biosensor | TMSDRs combined with non-enzymatic target recycling amplification | HIV-1 gene | 1.9 pM | 120 min | – | Li et al. (2019) |
| 10. | EGOFET | – | HIV-1 p24 capsid protein | 1 fM | – | Yes | Sailapu et al. (2020) |
| (e) Human papilloma virus | |||||||
| 11. | DNA biosensor | Electrochemical | HPV16 DNA | 1 fM | – | – | Shariati et al. (2019) |
| 12. | Combined device with isothermal RPA with LFD and reverse dot blot | – | HPV genotypes | 103 copies/μL | 60 min | – | B. Ma et al. (2019) |
| (f) Others | |||||||
| 13. | Immunochromatographic strip | LFA | EBOV-GP1−649 | 200 ng/mL | 15 min | Yes | Brangel et al. (2018) |
| 14. | Impedimetric micro-immunosensor | – | ZIKV protein | 10 pM | 40 min | Yes | Kaushik et al. (2018) |
| 15. | Supersandwich-type Electrochemical biosensor | – | SARS-CoV-2 RNA | 200 copies/mL | – | Yes | Zhao et al. (2021) |
cDNA: Complementary deoxyribonucleic acid.
EGOFET: electrolyte-gated organic field-effect transistor.
ELISA: Enzyme-linked immunosorbent assay.
IoT: Internet-of-things.
LAMP: Loop-mediated isothermal amplification.
LFA: Lateral flow assay.
LFD: Lateral flow dipstick.
MCFA: Microchannel capillary flow assay.
NAAT: Nucleic acid amplification tests.
QUASR: Quenching of unincorporated amplification signal reporters.
RNA: Ribonucleic acid.
RPA: Recombinase polymerase amplification.
RT-LAMP: Reverse transcription loop-mediated isothermal amplification.
SARS: Severe acute respiratory syndrome.
TMSDRs: Toehold-mediated strand displacement reactions.
In recent years, POC systems integrated with machine learning (ML) algorithms, i.e., artificial intelligence (AI) resulted in a new paradigm shift towards data-analytics-based decision making (McRae et al., 2016). AI provides an additional option to medical practitioner in terms of specific treatment to individuals and observation of their outcomes. Using these AI systems, health care officials capable of assessing risks of different diseases like heart attack, cancer, and trauma in patients. The AI sensors initially perform the multistep assay using portable analyser which further automatically digitize the biomarker concentrations. With the help of acquired, the spectrum of diseases can be predicted using numerous algorithms, e.g., classification, cluster, pattern, and features of diseases to make treatment decisions. M-Health is a WHO global observatory used to display results to the patients and supported by different wireless devices, such as cell phones and personal digital assistants (Wang et al., 2017). Thus, health analytics provide patients with personalized care for active management and prevention of disease risks. The real time POC diagnostics and ML combinedly revolutionized the healthcare sector. The IoMT market growth is expected to pace up more with the integration of AI into the connected ecosystem for automated prescription possible (Shrivastava et al., 2020a).
Vector borne, malaria is a fatal infectious diseases resulted in millions of patients every year in tropical countries (Chen et al., 2019). All five species of malarial parasite Plasmodium falciparum, Plasmodium knowlesi, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax are known to cause the severe infections. Management of malaria relies heavily on diagnosis at early stages and treatment with artemisinin-combined therapy (ACT). Despite of many safety measures to prevent the disease spread, the high malarial mortality is attributed to the multidrug resistant (MDR) parasite (Ragavan et al., 2018). Established gold standard protocols for malaria diagnosis are Giemsa-staining and PCR, which require well-trained operators for instrument handling. The available methods are not adequate for detection and treatment of this parasite-born infection. However, WHO supported rapid diagnostic tests (RDTs) for malarial screening, promoting commercial evaluation of various biosensing strategies for POC malaria detection (Dutta, 2020). Recently developed POCT offered unexplored potential in elimination and reduction of mortality in malaria-endemic regions (Fig. 6 ). For example, chip-based portable PCR system offer real time differential identification of malarial parasites (C. B. Nair et al., 2016). The specifically designed algorithms automatically calculate the pathogen load, followed by display of results on the remote screen through general packet radio service (GPRS)/Wi-Fi/bluetooth networking. Flash memory of the device allow user to store results of nearly 5000 tests. Most of the currently used malaria RDT are immunodiagnostic assays, which imply higher economical costs and stability issues.

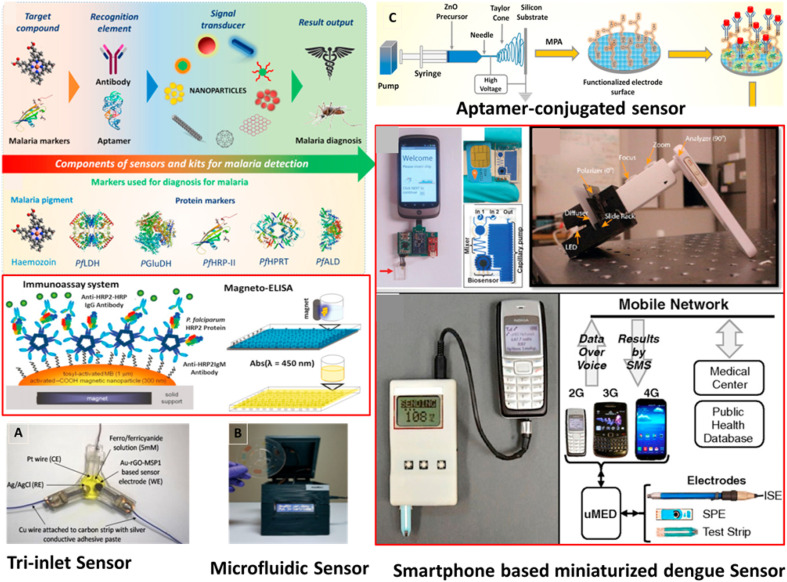
Schematic diagrams illustrating the recently developed POC settings for detection of malarial parasites. Reprinted with permission from ref (Choi et al., 2016; Ragavan et al., 2018; Singh et al., 2021).
Several research groups are working on development of aptamer-based assay as a promising alternative to the antibodies in terms of low immunogenicity, economically viability, and less variability (Dunn et al., 2017). Paper microfluidics (μPADs) technique-based printed biochips have successfully been developed for rapid prototyping of malaria parasites in blood (Dirkzwager et al., 2016). The method used specific aptamer sequence to capture Plasmodium falciparum Lactate Dehydrogenase enzyme (PfLDH). Moreover, aptamers have also successfully applied to design electrochemical impedance POCT against PfLDH, glutamate dehydrogenase (GDH), HRP, and aldolase (Figueroa-Miranda et al., 2018; Singh et al., 2018). Recently, another aptamer-tethered enzyme capture (APTEC)-based next generation POCT was developed to quantify PfLDH from minute blood sample (Fraser et al., 2018). Imaging of malarial parasite in blood smears using optimized cell-phone lens is also of great interest due to its ultimate performance (Agbana et al., 2018).
Choi et al. designed field deployable standalone LAMP-based molecular diagnostic system (AnyMDx) for real time monitoring of target parasite DNA (Choi et al., 2016). Here, microfluidic discs, preloaded with reaction mixture, were integrated with the small footprint analysers. Malpartida-Cardenas et al. demonstrated LAMP-based fully electronic device for real-time detection of malarial parasite (Malpartida-Cardenas et al., 2019). This lab-on-chip (LOC) platform utilized ion-sensitive field-effect transistor (ISFET) sensing technique. Apart from this, LAMP amplification technique also explored to design lateral flow device, offering a straightforward endpoint assessment (Hartmeyer et al., 2019). Besides this, instrument-free detection can be easily performed in rural areas to avoid expensive molecular biology equipment e.g., paper-based microfluidics (Reboud et al., 2019). Further, Kumar et al. conducted successful field evaluation of battery-operated magneto-optical malaria diagnostic device (Kumar et al., 2020). In comparison to detection of P. falciparum histidine rich protein-2 (Pfhrp2), this device was based on detection of hemozoin (by-product of HB) due to its prevalent nature in resistant parasites. Most of the existing diagnostics detect HRP-2/PfLDH malarial antigens specific for P. falciparum. However, P. vivax (Pv) diagnosis is challenging due to low parasitaemia in blood. In a recently developed electrochemical POCT device based on “Tri-inlet” strategies, Pv-avid antibody (MSP1) was integrated to rGO-gold nanocomposite electrode (Singh et al., 2021). The sensor showed higher sensitivity with ease of detection of P. vivax. Several studies confirmed that pathogen biomarkers are also sequestered into non-invasive biofluids, like urine and saliva.
Mature gametocytes of malarial parasite potentially aggregate in gum capillary beds during stage V. Based on this, Tao et al., developed saliva-based LFA for gametocyte carriers in subclinical patients (Tao et al., 2019). LFA strips contain monoclonal antibody conjugated to Europium chelate (EuChelate) microparticles with visible fluorescence in handheld ultraviolet flashlight. The speed performance of the assay ranges between 5 and 30 min depending on the biomarker abundance in saliva. Besides these advancements, very limited literature is available on IoMT integration of POC devices for detection of the biomarkers of malaria (Mora et al., 2017). Ahn & group successfully employed microfluidic platform for the development of hand-held, inexpensive, and disposable IoMT device for detecting malarial biomarkers (Ghosh et al., 2020). Microfluidic chip autonomously performs chemiluminescent ELISA eliminating the need of manual handling (sample storage and pipetting) and the analysis can be easily performed by detachable optical detector connected via smartphone or inbuilt USB-OTG port. The developed platform with full networking capability can be easily customized for different infectious diseases and biomarkers. The researchers at Makerere University, Uganda developed AI-based mobile application to detect malaria infection in almost 2 min (Masud et al., 2020). Cell phone application analyses the photos of the blood smear and marks a red circle on malaria parasites using ML algorithms.
Dengue fever is a vector borne RNA virus belongs to the Flaviviridae family with four different serotypes DENV1-DENV4 based on surface antigen (“Dengue and severe dengue,” 2020). The self-limited infectious diseases generate severe outbreaks and illness in many tropical and subtropical countries. Moreover, until now there is no specific antiviral treatment to cure dengue fever. A single licenced dengue vaccine, named ‘Dengvaxia, is utilized to treat patients having DENV infections history particularly in endemic areas. However, accurate and early detection of dengue virus (Fig. 8) seems to be especially important to avoid unnecessary treatment during misdiagnosis, in addition to timely management of severe dengue fever (Darwish et al., 2018). Eivazzadeh-Keihan et al. explained various symptoms and biomarkers used as targets for DENV detection with the biosensor's methodology (Eivazzadeh-Keihan et al., 2019).
Currently, the clinical laboratories use NS1 antigen-based ELISA and RT-PCR for DENV infection. However, expensive instruments and need of skilled operators, limiting the facilities for rural communities. User friendly, rapid, accurate, and affordable POCTs is desirable to carry out DENV testing in suspected cases. Dengue Duo RDT is a sensitive immunochromatography method simultaneously detect both NS1 antigen, and IgM/IgG antibodies (Kikuti et al., 2019). Moreover, higher sensitivity and early diagnosis with Duo RDT can offer prevention of severe complications, helping in ruling out dengue infection during endemics. The clinical symptoms of dengue infection share extensive similarity with the emerging ZIKV, consequently increasing the chances of misinterpretation and false positive results.
In view of above, different immunochromatographic tests have been evaluated for the correct diagnosis of NS1 from DENV serotypes and ZIKV virus during pandemics (Andreata-Santos et al., 2020). Results indicated the presence of cross reactivity with ZIKV NS1 for ‘On Site Dengue Antigen Rapid Test’ and ‘Dengue Duo Rapid Test’ POCT. LFA-POCT based on DyLight-800 (a near-infrared (NIR) fluorescent dye) to detect anti-DENV1 IgG antibodies (Chen et al., 2018). However, NIR-LFA had low limit of detection (LOD) when compared to existing Pan bio and the Dengue Duo test. The NIR POCT device was rapid, easy to use, and highly sensitive for application to clinical diagnosis of dengue fever. Paper-based analytical devices (PAD) are also used by scientific community for highly sensitive ELISA molecular assay. A novel magnetic paper–based ELISA POCT developed by Ortega et al. to detect IgM-dengue antibodies (Ortega et al., 2017). In this immunoassay platform magnetite@polydopamine core-shell nanoparticles were immobilized on cellulose paper. The system found to be 700 times more sensitive and accurate compare to conventional ELISA. In the quest, Cecchetto et al. evaluate the efficiency of capacitive label-free POC system to detect dengue NS1 protein directly in neat serum (Cecchetto et al., 2017). Capacitive biosensing has shown excellent detection sensitivity and suitability for designing of multiplex POCs. Similarly, Meagher & group employed smartphone-based device for high performance diagnostics. They integrated quenching of unincorporated amplification signal reporters (QUASR) technique with the ‘LAMP box’ to improve the signals between negative and positive by many fold (Priye et al., 2017). Another LAMP-based POCT was designed using paper and microfluidic control platform against ZIKV, DENV, and chikungunya virus, combinedly (Seok et al., 2020). Whole amplification process was carried out on all-in-one 3D paper chip (engineered properly to have both lateral and vertical flow) using dried reagents. The proposed bioassay platform was able to detect 5–5000 copies of viral RNA in human serum within 1 h. Recently, microfluidic paper-based POCT based on sandwich immunoassay was fabricated for detection of dengue NS1 (Prabowo et al., 2020). The results were visible to the naked eye, while intensity of signal was analysed using portable scanners or a smartphone camera.
Early diagnosis is important for both patient management and to differentiate infections with similar symptoms, such as malaria, chikungunya, and ZIKV. Many RT-PCR-based POC diagnostics have been reported to detect viral RNA; but molecular diagnostic tests for DENV in field settings are still underdeveloped. Yanow et al. validated a RT-PCR POCT to detect DENV directly from plasma samples without RNA extraction (Mehta et al., 2019). The assay was able to differentiate between serotypes of DENV effectively, and do not cross-react with pathogens like dengue infection. Recently, Chang & group validate non-optical RT-PCR chip for selective detection of all DENV serotypes (Z. Yin et al., 2020). The diagnostic platform (Fig. 7 A) utilized ion-selective membrane sensor to quantify the amplification of a template DNA in real time. Here, sensing modules are functionalized with specific oligoprobes that produce shift in ionic current upon target selection. The key attributes of current technology include insensitiveness to variation in pH and ionic strength as well as elimination of non-specific binding. Here, primer dimerization is considered as a major barrier in the current electrochemical sensor arrays. The rapid detection (90 min), low resource setting, and sensitivity favours scalability of the platform. In addition to aptamers, chemically-modified peptides are also known to be used as molecular receptor in electrochemical biosensor (J. H. Kim et al., 2019).

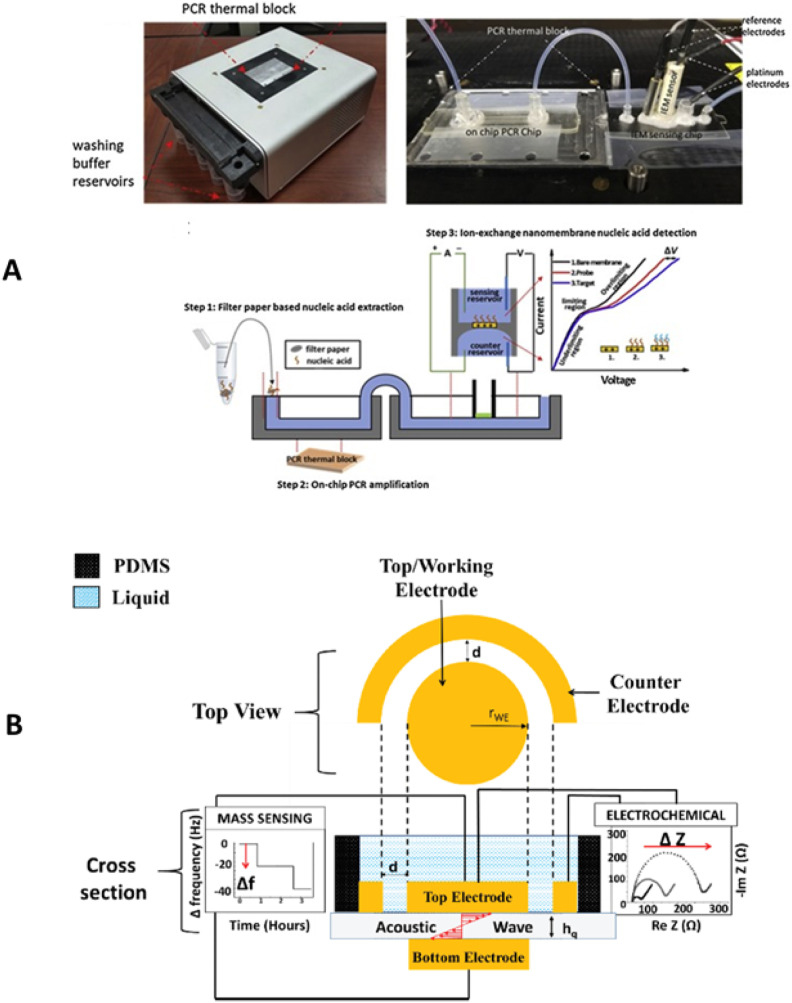
A) Illustration of non-optical multiplex PCR system (consisting a washing buffer component, microfluidic platform, and a paper based nucleic acid sensor) developed for DENV diagnostics. B) an integrated QCM and electrochemical sensing based DENV diagnostics system.

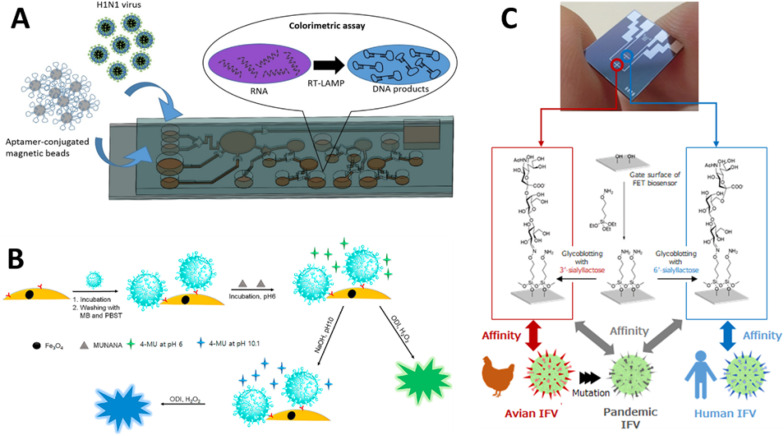
A) Illustration of integrated self-driven microfluidic system to detect H1N1 virus based on reverse transcription loop-mediated isothermal amplification (Y. D. Ma et al., 2019). B) presentation of a self-enzyme chemiluminescence based immunoassay for rapid detection of H1N1 virus (Kyme et al., 2019). C) A dual channel glycin functionalized FET based biosensor developed for selective identification of pandemic influenza viral particles (Hideshima et al., 2019).
In a recent study, gold electrode chemically functionalized with synthesized peptides used for the detection of dengue virus NS1 via electrochemical impedance spectroscopy (EIS) and square wave voltammetry (SWV). The developed sensor showed good specificity against all four dengue serotypes and low LOD up to 1.49 μg/mL in spiked culture broth. Moreover, POCT devices detected the presence of several infectious agents within a specimen. Recent report validated new analytical device for screening of multiple infectious agents in a single sample within a single PCR reactor chip. The recombinase-aided amplification (RAA) is a new isothermal methodology that progresses in the range of 35–45 °C. The RAA assay exploits four enzymes: recombinase UvsX & UvsY, a single-stranded DNA-binding protein (SSB), and a DNA polymerase to initiate DNA synthesis. Xiong et al. developed reverse transcription (RT)-recombinase-aided amplification (RAA)-based lateral-flow dipstick (LFD) POCT for the early detection of DENV RNA (Xiong et al., 2020). The excellent sensitivity of assay offered reliable detection of dengue virus in resource-limited settings.
Quartz crystal microbalance (QCM) is extremely sensitive towards mass changes on the surface of sensor. Deposition of analytes onto the surface causes damping effects that lead to shifts in oscillation frequency, generating output signal in terms of alternating current (AC) through the piezoelectric quartz substrate. The integration of QCM (Fig. 7B) and electrochemical measurement on the same crystal has recently been proposed as a promising avenue for early detection of dengue fever (Zainuddin et al, 2019, 2020). Integrated devices have dual-measurement capabilities (mass and impedance), allowing extremely low detection limit up to 10 ng/mL of DENV NS1 protein in serum sample. During epidemics, differentiating uncomplicated dengue fever from severe form of the disease needs intensive care for proper clinical management. Interestingly, thrombocytopenia (“drop” in platelet level) is strongly correlated with severity of DENV infection in patients. In this matter, POCT devices detecting platelet aggregation has been developed. Multiplate™ multiple-electrode biosensor potential was evaluated for platelet aggregation (De Jong et al., 2020). Platelets become adhesive upon activation with reagents and got deposited onto the metal sensor wires that led to increase in electrical resistance. POC Device potentially provide additional decision-making benefit on the length of hospital stay according to severity of disease. Zhu et al. developed portable IoT integrated miniaturized PCR device (having a weight of ~170 g) for detection of DENV based on amplification of cDNA or RNA (Zhu et al., 2020). The results of the PCR device were further uploaded to an Android wireless tool (such as a tablet or smartphone) via a bluetooth interface. From the Android-based smartphone, the results were further sent to global network to ensure the availability of test results anywhere in the world. Such tools can help in mapping of spread of disease with the GPS information which is sent along with the test results.
H1N1 virus with segmented RNA produces a hemagglutinin (HA) and neuraminidase (NA) glycoproteins, contributing to disease transmission. Based on different antigenic epitopes, virus can be classified into 15 HA and 9 NA serotypes, respectively (Qiu et al., 2017). Among all, H1N1 and H3N2 are more virulent and prevalent serotypes causing severe pandemics. In particular, emergence of swine flu from novel influenza strain (S-OIV) H1N1, marked the first global pandemic of 21st century (Ginsberg et al., 2009). It is an infectious respiratory disease spread to all continent as reported by WHO (WHO, 2018). According to a report of National Centre for Disease Control (NCDC), a total of 29,930 with 1236 swine flu death cases were reported in India (Ravina et al., 2020). Symptoms of Swine flu are different from seasonal influenza like high grade fever for more than three days, nausea, vomiting, dehydration, headache, coughing, and sore throat (Fig. 8).
With advancements in POCs, an electricity free one-step nucleic acid dipstick assay with a microfluidic reactor is adopted to design POC against H1N1 virus. The device helps to detect the amplification products with the naked eye (Qiu et al., 2017). Lee et al. proposed a portable setup for the H1N1 virus detection via combined effects of quantum dot (Q dot)-aptamer conjugates and light guide in3D photonic crystals (PC) for signal enhancement (Lee et al., 2018). Such technologies allow end users to collect sensitive data without waiting for long time. Further, the acquired information can be exchanged using IoT devices. Similarly, bai et al. developed an EIS aptasensor specifically against inactivated H1N1 virus (Bai et al., 2018). The gold electrode was functionalized with thiolated aptamer probe for binding virions that significantly change the impedance property of electrode.
Moreover, recently self-driven microfluidic chip-based POCT (Fig. 8A) was developed for influenza (Y. D. Ma et al., 2019). Here, reaction substituents were transferred through capillary action for LAMP amplification, and the results were analysed by hydroxy naphthol blue (HNB) colorimetric assay (Fig. 8B). Another example of biosensor-based POCT was demonstrated by Hannah et al. (Kyme et al., 2019). Hemagglutinin (HA) specific antibodies were immobilized on nanoconjugate (Au–Fe3O4), and another glycoprotein NA was utilized as self-enzyme, producing chemiluminescence with fluorogenic substrate. Another study reported the detection of H1N1 HA in nanomolar concentration using Fab-type Quench body (fluorophore-labelled antibody) (Jeong et al., 2018). Basically, internal Trp residues of antibody molecules are responsible for quenching while the binding reaction leads to fluorescence of dye.
Rapid antibody-based assay was extensively used for diagnosis of pandemic H1N1 virus; but the availability of specific antibodies for emerging infectious diseases is time consuming. In view of this, Hai et al. (2017) used poly (3,4-ethylenethiophene (EDOT)) (PEDOT) conducting polymer-modified QCM crystal for grafting a biosensor (Hai et al., 2017). These sensing elements were further activated with H1N1 specific 2,6-sialyllactose ligand. The biosensor allowed label-free monitoring of H1N1 viruses up to a detection limit of 0.12 HAU by QCM and potentiometry. In another work, a label-free field effect transistor (FET, Fig. 8C) biosensor was designed for the rapid identification of avian influenza virus (IFV) in biological fluids (Hideshima et al., 2019). Sialylated glycan, particularly prominent on human epithelial cells, was found to mediate influenza viral infection. Hereafter, sialic-acid-containing glycans functionalized electrochemical sensor was designed to recognise human H1N1 virus directly in nasal mucus. In addition, POCT was provided with smartphone to provide user interface for sharing sensing data in order to discover pandemic influenza infection at the early stages of an outbreak. Another ion sensitive FET based biosensor for the detection of H1N1 was fabricated using microfabrication technique by Lee & group (Park et al., 2019). Highly sensitive silicon nanonet FET device was modified with biological receptor molecule (monoclonal antibody) for the quantification of H1N1 virus. The BioFETs POCT showed excellent signal response within the range of 10 pg/mL-100 ng/mL viral load at room temperature. In addition to high specificity to H1N1 by the nanonet sensor, negligible false positive results were also reported for influenza B virus.
Recently, a genosensor employing immobilized HA gene-specific oligoprobe was developed for sensitive POC detection of H1N1 (swine flu) in human nasal swabs (Ravina et al., 2020). The complementary ssDNA probe immobilized on cysteine-functionalized screen-printed gold electrode was able to generate electrochemical differential pulse voltammetry (DPV) signal upon hybridization to target genome in the presence of redox indicator methylene blue (MB). The developed POCT analysed the H1N1 with higher sensitivity, no cross reactivity, and a detection limit of 0.002 ng/6 μL within 30 min. Optical biosensor based on surface plasmon resonance (SPR) technique was successfully applied for the real-time and label-free monitoring of biomolecular interactions. Herein, reusable SPR sensor chip was designed for multiple times usage against target analytes (such as H1N1 virus) without any refreshment (Yoo et al., 2020). Here, ferromagnetic patterns on a SPR sensor was able to trap a layer of fluorescence-tagged magnetic particles as a solid substrate for immobilization of H1N1 nucleoprotein (NP)-specific antibodies. After sensing, the used layer of magnetic particles was removed by applying external magnetic fields in opposite direction and fresh fluorescence magnetic particles was trapped on the SPR chip for further measurements. The optical signals found to be stable even after repeated SPR measurements.
HIV infection impairs the immune system of individuals. Virus targets hosts CD4+ T-lymphocytes, resulting in shaping adaptive immune responses (“HIV/AIDS,” 2020). HIV has been found to be most infectious during its early stages, due to asymptomatic behaviour (M. Nair et al., 2016). Eventually, serious infection occurs when CD4 + count shows a sharp decrease, and patient body succumb to opportunistic infections (e.g., pneumonia). Hence, early detection of HIV infection prevents transmissions risk and prompt treatment using antiretroviral therapy (ART) (Kaushik et al., 2016a). Next generation standalone p24 antigen immunoassays (EIA) significantly narrowed the diagnostic window to 2 weeks from the time of transmission (Stone et al., 2018). Commercially available RDTs for HIV includes Bio-Rad ‘Multispot HIV-1/HIV-2, ABON triline HIV 1/2/O device, Alere combo immunochromatographic test ‘Determine HIV 1/2’, OraSure 20 min ADVANCE Rapid HIV-1/2 qualitative test and patented Chembio DPP HIV 1/2 POC (Chen et al., 2019). However, beside these commercial RDTs for viral load quantitation, there is an urgent demand for POCT platform for sustainability in resource-limited countries.
Metal-organic frameworks (MOFs) provide promising quenching platforms for the development FRET-based biosensing system for the virus detection (Nehra et al., 2020). Pan et al. employed zeolitic imidazolate frameworks (ZIF-8) to design oligo FRET sensing platform to detect 20-bp HIV proviral DNA (Pan et al., 2018). Fluorophore-tagged ss-DNA got adsorbed on ZIF-8 through Π-Π stacking and electrostatic interactions, resulting in quenching of FAM fluorescence due to the photoinduced electron transfer (PET) process. Thereafter, desorption of probe DNA from ZIF-8 occurs due to complementary base pairing with target DNA leading to fluorescence regeneration. The formed ss-DNA@ ZIF-8 biosensing system can be used effectively to design optical POCT device for early diagnosis of HIV-1 DNA. Another promising approach is the functionalization of electrode with graphene-based nanocomposites for the improvement of sensing sensitivity.
For example, Gong et al. reported an impedimetric method for the determination of HIV virus through direct electron transfer (Gong et al., 2017). In this strategy, L-cysteine modified graphene oxide (GO) was drop casted on the glassy carbon electrode (GCE) to provide better electron-transfer kinetics. On the GO-modified GCE electrode, the capture probe was immobilized for direct determination of different concentrations of HIV-1. The result demonstrated good selectivity and successful detection HIV-1 gene from 1.0 × 10−13 to 1.0 × 10−10 M. Zou et al. developed novel DNA probe-stabilized silver nanoclusters (AgNCs)-based fluorescent device to detect two HIV oligonucleotides (1 & 2) at the same time (Zou et al., 2019). Here, HIV-1 was detected using green emitting AgNCs probe lighted up by guanine (G)-rich sequence while HIV-2 detection relies on the dimer of AgNCs generating orange fluorescence signals (Fig. 9 A).

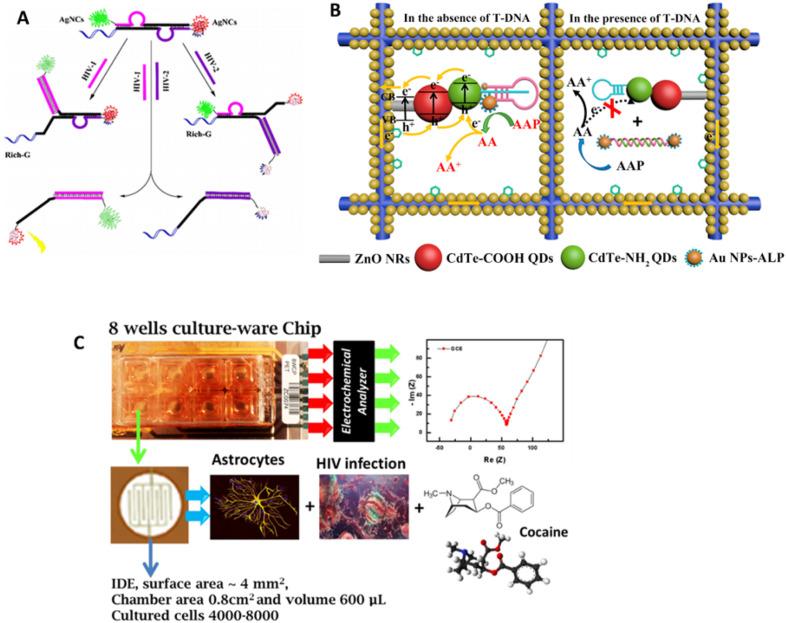
A) Schematic representation of DNA-programming multicolor silver nanoclusters for detection of two HIV DNAs simultaneously. Reprinted with permission from Ref (Zou et al., 2019). B) A nano-enabled photoelectrochemical (PEC) biosensor for HIV-1 virus detection (Wang et al., 2019). C) Lab-on-a-chip approach to evaluate electrophysiology of cell during HIV infection and treatment using a Food and Drug Administration (FDA)-approved drug.
In another study, toehold-mediated strand displacement reactions (TMSDRs)-mediated fluorescent biosensor was designed against HIV gene (Li et al., 2019). In this system, target gene (HIV-1) binds to the toehold region of the triplicate-stranded DNA substrate probe (SP) through complementary base pairing and initiate the enzyme free amplification reaction, resulting in production of plentiful G-quadruplex. This G-quadruplex sequences selectively show binding affinity towards N-methyl mesoporphyrin IX (NMM) complex. Formation of G-quadruplex/NMM complex dramatically increased the intensity of fluorescence signal that leads to high sensitivity for the HIV-1 gene detection. The designed system meets all the need of POCT like cost-effectiveness, isothermal operation, and flexibility.
Photoelectrochemical (PEC) technique also found to be a promising tool for fabrication of portable biosensors. Wang et al. used QDs-functionalized ZnO nanorods to form photoactive interface on gold paper electrode, offering increased charge transfer rate, tunable emission spectra, and biocompatibility (Wang et al., 2019). This PEC biosensor (Fig. 9B) further modified with triple helix structure for better specificity and binding affinity towards DNA sequence of HIV virus. A hairpin structure DNA (H-DNA) as capture probe was conjugated onto the photoactive interface through amide bond, and then a single-stranded DNA modified with gold nanoparticles labelled alkaline phosphatase (ALP-Au NPs-DNA) at each end was introduced to hybridize with the H-DNA that forms a triple-helix conformation. The T-DNA detection was based on the photocurrent response change that results from conformation change of the triple-helix molecule after hybridization with T-DNA. Alkaline phosphatase present in triple-helix molecular switch specifically catalysed the conversion of ascorbic acid 2-phosphate (AAP) to ascorbic acid (AA) that effectively generate photocurrent response in absence of target DNA through rapid electron transfer process. The proposed system offered a wide detection range of 1 fM-1 nM with excellent specificity and stability. To meet the clinical needs, new mPIMA analyser was reported for automated quantification of HIV-1/2 with a concordance of 88.9% (Mariani et al., 2020). In this system, the detection was based on competitive reporter monitored amplification (CMA), requiring only 50 μL of plasma sample in a single use.
Besides HIV diagnostics analytical tools, the monitoring of HIV progression has significant importance to evaluate the efficacy assessment of a therapy, establishing a pathological correlation between viral load and patient medical condition (Shafiee et al., 2013). In this direction, Kaushik et al. developed a miniaturized system which can monitor the variation in electrophysiology of the cell during HIV infection along with pre/post treatment. This research demonstrates the application of LOC-based approach wherein cells were infected with HIV and then treated with FDA-approved drug, as illustrated in Fig. 9C (Kaushik et al., 2016d). The outcomes of the research suggested that variations in electrical properties on cell can be detected at every step using EIS. This electrophysiology variation detection was also performed in setting of drug abuse and specific treatment. Such systems can be promoted for clinical and POCT application; however, a systemic research-based on real sample is suggested as a future research. Further, integration of these POCT devices with IoT technology will enable the disease prevention and health promotion.
HPV is infectious disease of reproductive tract and can be sexually transmitted. Some of the HPV serotypes are oncogenic and cause cancer for lower genital tract, especially HPV16 infection. Here, every women is at risk for lifetime as HPV infection can be chronic and pre-invasive malignant lesions progress to different types of cancer, prominently to cervical cancer (refer to Fig. 10 A) (Chen et al., 2019). Global mission to prevent cervical cancer in poor countries require concerted efforts to improve screening and access to treatment. HPV screening, during cervical intra-epithelial neoplasia (CIN), reduced the progression to cervical cancer and mortality. Mainly, the HPV16 E6 and E7 sequences are conserved and thus can be utilized as an significant indicator for screening of cervical cancer. HPV-DNA testing and Pap smears are standard protocols for screening of cervical cancer in developed countries. However, these tests need manpower and are also non sustainable.

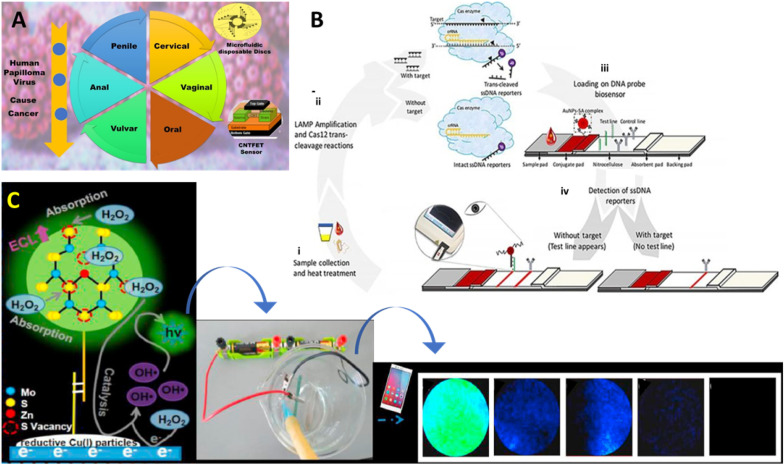
A) HPV POCT screening reduced progression to different cancer and mortality. B) illustration of a CIALFB bioassay developed using CRISPR/Cas12a for the selective detection of HPV 18 and HPV 18 (Mukama et al., 2020). C) A Zn-dopped MoS2 QD based ECL biosensor developed for detecting HPV-16 DNA (Nie et al., 2020).
GeneXpert is currently the only validated HPV POC device (Sayed et al., 2020). GeneXpert, developed by Cepheid is the only POCT endorsed by WHO for rapid diagnosis of HPV. The system integrates microfluidic cartridge-based sample preparation with RT-PCR to perform isolation and amplification in just 35 min. Grant et al. validated paper-based immunoassay amenable to POCT(Grant et al., 2016). Here, LFA detects antibodies in a finger prick blood sample against HPV16 to determine HPV immunization status. Furthermore, The use of self-collected vaginal specimens is more feasible for large scale HPV testing in remote areas and low income countries (Toliman et al., 2018). Integration of LFA methodology in biosensor provides inexpensive and rapid means for determining isothermal amplification reaction.
Peng's group fabricated a fluorescent biosensor with extremely high selectivity and sensitivity for the detection of HPV-18 (Peng et al., 2019). In this work, dye-labelled ssDNA probes were anchored on ultrathin MXene nanosheets. MXene Ti3C2 nanosheets (NSs) is of particular interest due to ultrahigh quenching efficiency for a range of fluorescent dyes. Sensing strategy includes the binding of probe with its complementary HPV DNA and forms dsDNA, which ultimately weakens the interaction of dsDNA with MXene nanosheet, resulting in the fluorescence recovery. The sensitivity of MXene-based sensor sensitivity is enhanced using Exo III enzyme cleave protruding dye-labelled probe DNA. Liberated fluorophore leads to remarkable amplification of the fluorescence emission, offering detection of HPA DNA at the level of 100 pM. Mukama et al. validated a novel fluorescence diagnostic biosensor (Fig. 10 B) based on isothermal LAMP amplification and the trans cleavage property of CRISPR/Cas system, which allowed selective detection of HPV16 and HPV18 with the LOD of 3.1 amol (~1.8 copies) (Mukama et al., 2020). During detection, HPV sequence bind to a complementary capture oligo immobilized on the LFA test line that lead to reporter sequences free for cleavage. Cas12a enzyme trans cleave all the ssDNA reporter DNA labelled with both fluorescent and a quencher.
Teengam et al. postulated a paper-based electrochemical sensor to detect the HPV virus on the surface of modified graphene-polyaniline (G-PANI) electrode (Teengam et al., 2017a). The electrode was prepared by printing conductive G-PANI nanostructures on to the paper through inkjet printing process that further indicates the potential of POCT commercialization for viral diagnosis. Later, anthraquinone-labelled pyrrolidinyl peptide nucleic acid (acpcPNA) probe (AQ-PNA) was immobilized onto the electrode surface to detect 14 bp synthetic oligonucleotide sequence like HPV DNA using square-wave voltammetry. In another study, Gopinath and colleagues developed a carbon nanotube FET (CNTFET) based biosensor for the detection of HPV infections in early stages (Gopinath et al., 2018). Here, HPV DNA probe was deposited onto the CNT thin film via 3-aminopropyltriethoxysilane (APTES) to improve the current ratings. Biopotential generates in the presence of antibody on CNTFET surface that interacts with the target antigen. Moreover, an array of sensors is designed to increase the sensitivity of POCT against analyte. Another impedimetric biosensor for HPV POCT was demonstrated by Karimizefreh & group (Shariati et al., 2019). In this POCT, the nano-porous polycarbonate template decorated with gold nanotubes using electrodeposition method. The electrical interfaces are than functionalized with oligo probes for the determination of HPV. Biosensor was highly stable up to 6 weeks and offered a low LOD of 1 fM in the linear range of 0.01 pM–1 μM. Recently, Nie et al. designed Zn-doped MoS2 QDs based electrochemiluminescence (ECL) sensor (Fig. 10 C) (Nie et al., 2020). Reductive Cu(I) particles enhanced the ECL activity of QDs up to fivefold. Furthermore, the system is sensitized with an enzyme-assisted DNA walker specific for HPV 16 DNA. The methodology offered efficient amplification of ECL signal on the electrode surface for POC HPV determination. For the determination of the HPV16 E6 and E7 gene, Hong and colleagues proposed label free electrochemical biosensor that offered an extremely low LOD of 18.6 amol (Y. He et al., 2020). In this study, amplification strategy like entropy-driven amplification and rolling circle amplification were required to amplify the signal in the low concentration scrape sample. In the absence of target HPV gene sequence, MB dye can easily approach the surface of electrode, resulting in the changes of signal. Conversely, the presence of target gene in test sample triggered the amplification reaction and produced numerous DNA molecules that embed MB into it. This further allows only minimal amount of MB to reach surface of electrode, leading to a drop in signal intensity. The integration of these quantitative detection platforms with smartphones can help in spatiotemporal mapping of disease and pathogen tracking by utilizing web-based surveillance (K. Yin et al., 2020).
EBOV (Fig. 11 A–C) is another deadly zoonotic virus emerges from fruit bats that led to largest outbreak in West Africa during 2014–2016. Syndromes of Ebola infection overlap substantially with other viral fevers (like dengue fever) that greatly complicate the health management and decision-making process. Presently, five different EBOV serotypes have been discovered, named as Bundibugyo, Sudan, Reston, Tai Forest, and Zaire. The incubation period estimated to be 2–21 days for the emerging EVD (Fig. 11 D). Asymptomatic patients are not infectious and even infected human remain contagious as long as their blood contains the virus. EVD has not been reported to be transmitted by aerosols. Rapid isolation of infected patient, tracing of contact, and early diagnosis of suspected cases is the best way to stop transmission (Key facts about major deadly diseases Managing epidemics, 2020). Real time RT-PCR is among the most widely accepted method for EBOV detection with high sensitivity and specificity. Epidemics and outbreaks emphasize the need for POC diagnostic tools. For example, Baca & colleagues demonstrate potential of surface acoustic waves (SAW) technology in designing label-free biosensor (Baca et al., 2015). Such sensors detect EBOV directly in raw specimens like blood, serum, faeces, and saliva, thereby eliminating the need of sample preparation. Moreover, the technology is beneficial in designing portable and rapid POCT, allowing viral detection within 2 min (refer to Fig. 11E). The piezoelectric sensor chips patterned with inter-digital transducers were coated with antibodies specific for EBOV. The surface of sensor has a characteristic resonant frequency, and the binding event results in a phase shift of the signal wave that can be quantified through interface device. The SAW POCT revolutionized the diagnostic platform via enabling early treatment in pandemic responses. Broadhurst et al. performed field validation of the ReEBOV Antigen dipstick test that detects EBOV VP40 matrix protein against a gold standard RT-PCR assay. The study indicates 100% sensitivity (Broadhurst et al., 2015). These thermocycler platforms can be miniaturized to reduce the burden of laboratories along with increased sensitivity of the assay which could potentially allow for faster turnaround testing (Coarsey et al., 2017).

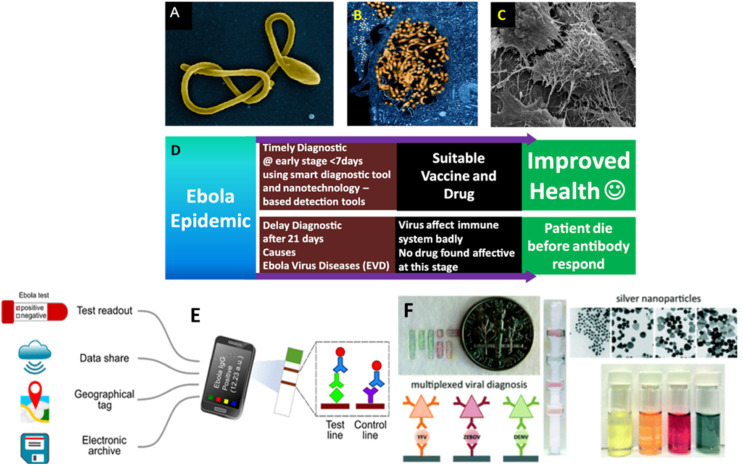
Microscopic illustration of EBOV (A-C; Source NIAID of NIH) and strategy (D) along with its impact to manage EVD outbreak. It is predicted that use of advanced POCT approach, EBOV infection can be managed to reduce mortality via diseases management approach. E) illustration of a IoMT-assisted biosensing system developed for POCT of IgG antibodies for selective diagnostics of EBOV (Brangel et al., 2018). F) illustration of AgNPs of multicolor feature developed for multiplexed diagnostics of dengue fever, yellow fever, and EVD (Yen et al., 2015). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Another study developed portable cell phone sized and battery-powered PCR device to detect EBOV RNA using single step RT-PCR (Ahrberg et al., 2016). This POCT quantify viral load in small sample volume by performing four RT-PCRs concurrently: two samples, one positive control containing RNAs from both with EBOV and human transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and one negative control. A virtual reaction chamber (VRC) was formed by a sample covered with an oil located on the cover slip of the disposable glass microslide. Further this biosensing system is integrated with a miniaturized fluorescence detector to monitor the progress of amplification reaction. The viral load in the simulated sample of patient was determined by relative quantification of EBOV to GAPDH expression. The entire process was conducted in a very short time span (less than 37 min). In addition, isothermal amplification (LAMP) methodologies also allow designing of POCT performed outside of centralized laboratories. Here, Benzine et al. employed RT-LAMP molecular diagnostic for rapid detection of EBOV directly from whole blood (Benzine et al., 2016). In this case, reaction formulation was freeze dried and amplified EBOV specific glycoprotein gene in a battery-operated isothermal instrument. The results indicated specificity of POCT for EBOV, avoiding any kind of non-specific amplification.
LFA-based biosensing technology is most widely preferred for designing number of POCT for infectious diseases (R. Kumar et al., 2018). Previously, a few LFA biosensors for diagnosis of EBOV infections have been fabricated, but with a low sensitivity. New nanomaterials-based fluorophore molecules may increase the sensitivity of LFA-based POCT. Hu & colleagues investigated novel fluorescent nanosphere RNs@Au to increase the sensitivity of LFA against EBOV (Hu et al., 2017). This nanoplatform generated dual signal, i.e., one is fluorescent for quantification and other is colorimetric for visual readouts. The assay enables smart detection of virus using antibody and streptavidin (SA)-labelled nanospheres where reaction of SA with biotin amplify the signal intensity. The sensitivity of assay successfully tested in field work to detect EBOV in spiked samples and found to be a capable candidate for early diagnosis of EBOV in endemic areas. An IoMT sensor integrating LFA (Fig. 11 E) with computational abilities is designed specifically to detect recombinant Ebola viral protein. Customized smart phone-integrated POC collect both test results and geographical information with high sensitivity and specificity (Brangel et al., 2018). Ilkhani et al. explored highly specific thiolated DNA probe to design electrochemical sensor to detect Ebola in endemic countries (Ilkhani and Farhad, 2018). In this POCT, hybridized DNA was fabricated on screen printed electrode for DPV sensing of viral strain with a detection limit of 4.7 nM oligonucleotides.
Ebola is among the highly feared infectious disease due to its contagious nature and the rapid development of acute infection. Therefore, while selecting POC technologies, it is important to consider different practical aspects for intensive care of patients. Recently, there is a huge demand of multiplex diagnostics to detect multiple marker specific for different pathogens in a single strip to increases the speed and lowering the cost of screening. Yen & group exploited optical properties of silver nanoparticles (Ag- NPs) for fabricating LFA devices (Fig. 11F) against DENV, EBOV, and yellow fever virus (YFB), simultaneously (Yen et al., 2015). Based on the size dependent emissive behaviour of Ag-NPs, three different range of AgNPs (e.g., orange, red, and green) were conjugated with YFV-Ab, EBOV-Ab, and DENV-Ab, respectively. These AgNPs were easily distinguishable from one another when applied to paper. In this case, the color of the test lines distinguishes the different viral biomarkers.
The ZIKV (Fig. 12 A), dengue fever, chikungunya, and yellow fever all belongs to a common family of flaviridae (Kaushik et al., 2017). These emerging infectious diseases were seen after a decade in brazil and then spread rapidly in whole America. More recently, travel-associated cases of ZIKV infection have also been reported. ZIKV is also an arbovirus mainly propagated through arthropods like mosquitoes, most commonly from the Aedes species (Kaushik et al., 2017). ZIKV has been first of its kind that shows unusual symptoms like sexual transmission, microcephaly, and other severe neurological defects (Fauci and Morens, 2016). However, ZIKV-infection caused classical symptoms like fever, headache, and chills have resemblance to many other febrile diseases (Kaushik et al., 2017). But the continuing reports on infectious diseases pandemics need IoMT based diagnostics to monitor patients. To detect ZIKV, the emergency use of the IgM antibody capture ELISA (Zika MAC-ELISA) and Trioplex rRT-PCR laboratory test has recently been authorized by the FDA (Chen et al., 2019). To detect ZIKV, LFA technology was also applied to integrate molecular diagnostics. In this work, distinguished features of QDs (like broad excitation, tunable emission spectra, and high quantum yield) were exploited for fluorescent LFAs (Rong et al., 2019). ZIKV RNA-specific antibody was conjugated to QDs, forming an immune complex in the presence of target antigen on the test line of LFA strip. Later, the test strip was illuminated using handheld ultraviolet (UV) LED lamp to excite QDs microsphere. The intensity of fluorescent signal was readout using smartphone based optical device.

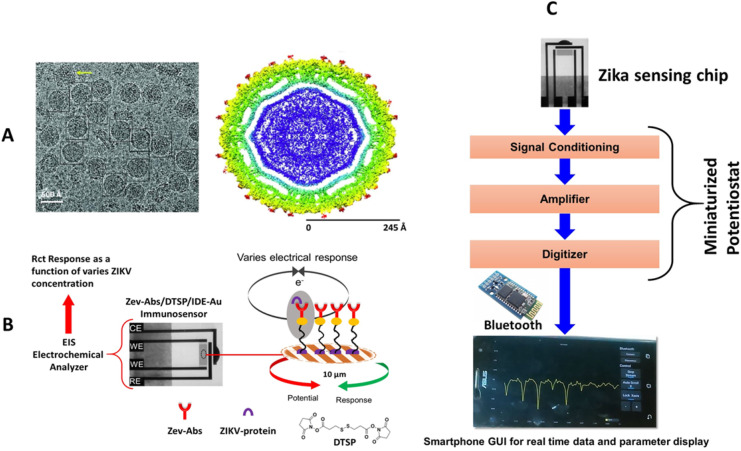
A) Illustration of ZIKV protein (Cyro-TEM imaging a & b), B) interdigitated electrode and electrochemical immunosensing approach for detecting ZIKV protein at 10 pM level, C) a strategy to develop a miniaturized sensing system to POCT of ZIKV protein needed for personalized ZIKV infection in personalized manner.
Besides, highly sensitive amplification systems (RT-PCR, LAMP, and recombinase polymerase amplification), pioneered POC technologies were also demonstrated for many emerging infectious diseases and recently for ZIKV. For example, Mendes Duarte & colleagues optimized biosensor for direct detection of ZIKV while maintaining high sensitivity using specific RT-LAMP amplification (Estrela et al., 2019). The amplification was isothermally progressed for 10 min at 72 °C. RT-LAMP reaction directly on a serum sample, without prior RNA extraction, provides opportunity for early diagnosis with a detection limit of 1 fM. Here, visual detection of amplification was based on phenol red acid-base indicator. POCT found significant application in the differential diagnosis of ZIKV from DENV and chikungunya. The similarity of clinical symptoms by these diseases poses a critical clinical challenge. To this end, RT-qPCR method was used by Broeders & team to detect ZIKV and chikungunya (CHIKV) viruses in a single test (Broeders et al., 2020). A new fourplex RT-qPCR assay was thoroughly validated directly for non-invasive samples like urine and saliva. Most importantly independent sequences for each virus have been used to overcome false-negative results. The obtained data is further analysed using the bioinformatic tool that help to validate the performance of the existing molecular diagnostic approaches.
Label-free electrochemical sensors simplify the detection of genetic materials, offering a great advantage in clinical analyses. Direct detection of virions in samples reduces the time and cost of analysis. Thus, label-free genosensors found their way in diagnosis of infectious diseases. Likewise, impedimetric electrochemical DNA biosensor has been designed for label-free detection of ZIKV (Faria and Zucolotto, 2019). For this, disposable electrode is fabricated through metallization where vapours of gold layered get deposited on polyethylene terephthalate (PET) substrates by thermal evaporation. The electrode surface was further modified with complementary capture DNA probes hybridized with the ZIKV genomes in sample. The hybridization event offered readout at room temperature with a 1.5 h response time along with a LOD of 25.0 ± 1.7 nM. Similarly, another genosensor was suggested in the pioneer work of Sotomayor & group (da Fonseca Alves et al., 2019). The electrochemical sensor was designed using pencil carbon graphite electrodes. Here, for the intact immobilization of oligoprobes without losing activity, polymeric film derived from 3-amino-4-hydroxybenzoic acid (3-4-AHBA) was coated on electrode surface. Hybridization with Zika oligo sequence resulted into higher electrostatic repulsion due to the increase of phosphates by addition of the complementary target sequence. Disposable, three-contact biosensor on a gold-PET electrode has been developed for the label-free detection of ZIKV oligo sequences at concentrations above 54 nM in a drop of sample within 90 min (Faria and Zucolotto, 2019). The results demonstrated high reproducibility and allowed differentiation among the oligonucleotide sequence of ZIKV and the closely associated dengue virus.
One study reported the direct identification of ZIKV from whole blood sample of an infected individual by smartphone-integrated POCT using nanoparticle-conjugated antibodies (Hsu et al., 2020). Here, platinum nanoparticles were successfully deposited onto the Au seeds to synthesize Pt@Au core-shell nanoparticles. Subsequently, these nanoparticles uniformly decorated on glassy chip via polyethyleneimine functionalization. The developed system can suitably meet the urgent needs of POCT in endemic regions with stressed resources for ZIKV detection. In this study, prepared chip was fitted inside the lid of a vial, which can be used specifically to capture ZIKV. The serum spiked with variety of antigens introduced into a chip containing vial and remained incubated for 15 min at room temperature. The results of ZIKV sensor were determined using optical analyser.
An electrochemical POCT approach for selective detection of ZIKV protein at 10 pM level was also proposed by Kaushik et al (Fig. 12 B - C) (Kaushik A, 2018). In this research, author functionalized interdigitated electrode using self-assembled monolayer to immobilize an anti-ZIKV antibody for the selective detection of envelop zika-protein virus. This sensor was selective with reference to major flavivirus protein and detected ZIKV selectively at 10 pM level within 45 min. Due to capabilities of miniaturization and desired sensing performance, this sensor is projected for POCT application after integrating this sensing chip with a miniaturized electrochemical analyser and optimization of smartphone-based operation. However, further research studies have also been carried out on ZIKV detection from real samples (Kaushik et al., 2018; Kaushik A, 2018)).
Besides above discussed infectious diseases, this century witnessed many other pandemic diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). February 2003, marks the emergence of SARS coronavirus (SARS-CoV) in mainland China and subsequently transmitted to global boundaries (de Wit et al., 2016). SARS-CoV infect humans from their reservoir host bats hints they can re-emerge. later in 2015, another coronavirus MERS emerged and spread to Arabian Peninsula. Now, COVID-19, still present in our community, revealed in Wuhan, China, and spread to 90% of the countries on our planet within 2 months. It is grouped into SARS, as infection result in serious illness of upper respiratory tract and named SARS-CoV-2 (Yamamoto et al., 2020). Early diagnosis is only solution to manage COVID-19 pandemic (Kaushik et al., 2020; Mujawar et al., 2020). Hence, all emerging corona virus (CoV) (Table 2 ) cause acute illness and are fatal with lung injuries, multi-organ, and heart failure in certain cases, especially the older people or individuals having co-occurring diseases (Ezhilan et al., 2021).

| Novel coronavirus (SARS-CoV-2) WHO reported COVID-19 infection as a pandemic and experts are thinking to declare it as an endemic | •First reported in December 2019 (Wuhan city of China) and causes COVID-19 infection pandemic •Virus is associated with an outbreak of Pneumonia •Some victims were older males with pre-existing conditions. •Cases: > 55 M confirmed; 1.3 M (Nov. 15, 2020). •In USA, > 15 M confirmed; 251 K (Nov. 15, 2020) |
| Middle East respiratory syndrome (MERS-CoV) | •First reported in 2012 in Saudi Arabia. •Cases: 2494 confirmed cases; 858 deaths (as of Nov. 30, 2019). Mortality rate of 34%. |
| Severe acute respiratory syndrome (SARS-CoV) | •First reported in 2002 in southern China. •Cases: 8098 cases; 774 deaths. Mortality rate of about 10%. |
| Common cold caused by coronavirus | •Four human coronavirus strains are thought to be responsible for 15–30% of common colds. ○HCoV-229E (1966), origin Bats and causes mild symptoms ○HCoV-OC43 (1967), origin cattle and causes mild symptoms ○HCoV-NL63 (2004), origin civets' bats and causes mild symptoms ○HCoV-HKU1 (2005), origin mice, and causes mild symptoms •Close contact with infected humans or touching a surface that carries the virus. •Millions each year. Generally nonlethal with rare exceptions •Circulates year-round, but more common in fall/winter. |
It has been demonstrated that COVID-19 infection pandemic is not only a single respiratory disease, but it creates a complex medical condition where organs, affected by SARS-CoV-2, exhibited alteration in biological function. Moreover, this pandemic is emerging more seriously due to its easy human to human transmission (though aerosol droplets) which depends on various factor, as summarized in Fig. 2. Significant efforts have been made to manage COVID-19 pandemic such as: 1) developing rapid testing kits, 2) exploring POCT of targeted biomarker, 3) investigating therapeutics among available viral infection related drugs, 4) investigating new therapeutics, and 5) making people aware to motivate them to understand pandemic consequences and use mask for avoiding infection transmission (Fig. 13 B) (Z. Huang et al., 2020; Kaushik et al., 2020; Paliwal et al., 2020). As it has been declared that combinational approaches are required at the same time to eradicate COVID-19 infection. To avoid errors, an optimized solution can be projected by analysis of risks and prediction of the effects of a therapy along with consequences associated with population of every race and age, AI is emerging as a very supporting component, as illustrated in Fig. 13 C. Kaushik et al. summarized the significance of developing efficient and effective nano-enabled sensing systems, electrochemical SARS-CoV-2 virus detection as one of the examples, which can detect a COVID-19 selectively at pM level (Fig. 13 D) (Kaushik et al., 2020). Such low scale virus detection capabilities are required to understand diseases progression and also to evaluate efficacy of prescribed therapy. These bio-sensing techniques are well supported by AI and IoMT to perform diagnostics at POC site and to analyse generated bioinformatics as per the individual diseases profiling. Considering significance of outcomes into consideration, these diagnostics system are recommended for early-stage viral infection identification and to manage viral infection in personalized manner for a overall intelligent healthcare (Fig. 13E) (Kaushik et al., 2020).

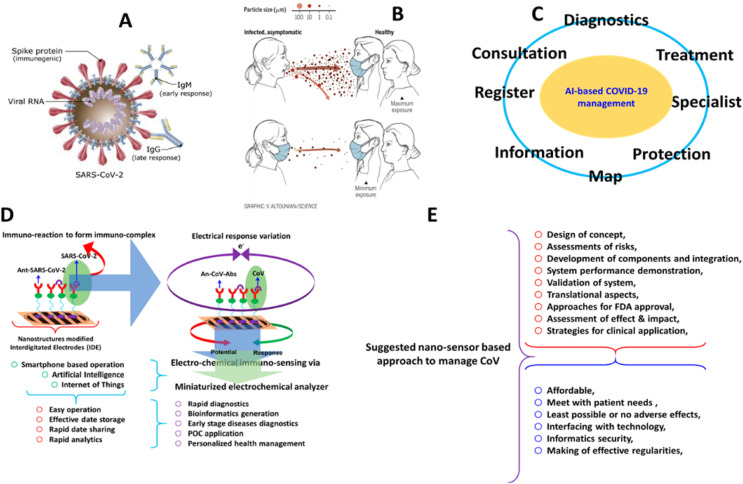
A) Illustration of SARS-CoV-2 virus structure sites useful for targeting to achieve COVID-19 diagnostics, B) schematic presentation of usefulness of using mask to avoid human-to-human SARS-CoV-2 virus transmission, C) proposed role of AI to manage COVID-19 infection successfully, D) electrochemical immunosensing of SARS-CoV-2 virus protein at pM level needed for selective diagnostics at early-stage, E) projected nano-enabled COVID-19 infection diagnostics supported by AI and IoMT.
SPR technique has important implications in diagnostic POCT due to its ability to measure biomolecular interactions directly without labelling. Hence, Guo & colleagues suggested SPR analysis for SARS-CoV in which sensor chip carrying streptavidin captures the biotinylated DNA (Yang et al., 2008). It is then used as interface to study the interactions between RNA and corona virus nucleocapsid (N) protein. The proposed diagnostic tool ensures the highly specific affinity of interactions within one chip. Kim et al. designed label-free colorimetric MERS-CoV biosensor (H. Kim et al., 2019). Here, surfaces of AuNPs capped with thiolated ss-DNA target two genomic regions of MERS-CoV virus. Hybridization reaction led to changes in localized SPR signal and color of AuNPs when subjected to UV light. The fabricated sensing element is also suitable for the on-site diagnosis of MERS-CoV up to the level of 1 pM/μL. The sensitivity of proposed sensors signifies the integration of biotechnology with nanotechnology, revolutionizing the fabrication of biosensors for the diagnosis of viral infections in non-invasive fluid samples.
Recently, low cost SPR sensor was reported for rapid detection and quantification of COVID-19. Nano-plasmonic sensor chips were designed specially using periodic nanostructures functionalized with SARS-CoV-2 monoclonal antibodies (mAbs) (L. Huang et al., 2020). Next, the chips were integrated into microwell plate or microfluidic cuvette, and the assay was analysed using handheld optical equipment controlled by a smartphone application. A dual functional approach (i.e., nano-plasmonic and photothermal features), based on biosensing protype was designed for the detection of SARS-CoV-2 virus protein (Fig. 14 A)(Qiu et al., 2020). In this research, nano islands of Au were functionalized with gene receptors (complimentary DNA) for the detection of targeted SARS-CoV-2 virus gene sequence selectively based on in situ nucleic acid hybridization, as a function of heat generated by Au nanoisland on stimulation. Such plasmonic photothermal effects-based sensing prototype (i.e., localized SPR) is selective and offered a detection limit of 0.22 pM (Qiu et al., 2020).

A) Au-Island based nano-system for selective detection of SARS-CoV-2 virus detection based on stimuli-responsive of plasmonic-photothermal effect. B) graphene modified FET immunosensor to detect SARS-CoV-2 virus protein, C) Nano-enabled LFA for detection of SARS-CoV-2 protein selectively within 10 min, D) toroidal plasmonic metasensors (magneto-plasmonic) for the detection of SARS-CoV-2 virus at femtomolar level (Ahmadivand et al., 2021).
Recently, Layqah & Eissa developed an electrochemical immunosensor based on AuNPs-modified interdigitated carbon electrodes for MERS-CoV detection (Layqah and Eissa, 2019). The sensing mechanism includes array of eight electrodes for detection of different CoV antigens simultaneously in the spike nasal fluid. Along with good linearity (0.001–100 ng/mL), the fabricated interdigitated carbon electrode offered a low LOD of 1 pg mL−1. In this direction, An FET, fabricated using graphene, was explored to develop an immuno-sensing platform for the detection of SARS-CoV-2 virus protein (Fig. 14 B)(Seo et al., 2020). This sensor exhibited SARS-CoV-2 virus detection at 1.6 × 101 pfu/mL in culture media and 2.42 × 102 copies/mL in real sample collected form nasal swab. Due to scaling up capability and selective virus detection at low level, such FET based SARS-CoV-2 virus sensor projected for POCT application to manage COVID-19 infection pandemic.
In another study, Zhao et al. developed a reliable electrochemical detection technology for COVID-19 using functionalized graphene oxide. The proposed technology detects RNA of SARS-CoV-2 without amplification by using smartphone equipped with DPV workstation (Zhao et al., 2021). Early detection seems to be the best way to stop the pandemic, which would not be possible using conventional analytical techniques. Owing to the advantages of paper-based POCs, LFA based POCTs are also proposed for emerging respiratory corona viruses. For example, Henry & group coupled PADs with AgNPs colorimetric assay (Teengam et al., 2017b). When pyrrolidinyl peptide nucleic acid (acpcPNA) linked AgNPs nanoparticles hybridized effectively with complementary MERS-CoV DNA in human blood sample, it led to dispersion of AgNPs and consequently change in color. The proposed sensor was used as multiplex POCT for simultaneous detection of MERS-CoV, HPV, and Mycobacterium. Recently, developed LFA against SARS-CoV-2 exploited the binding affinity of COVID-19 structural protein S1 to angiotensin-converting enzyme 2 (ACE2) domain (Lee et al., 2020). LFA identify matched pair at 1.86 × 105 copies/mL LOD and specifically without any cross reactivity to other corona viruses like SARS and MERS. To improve sensitivity of LFA, Chen et al. introduced lanthanide-doped polystyrene nano-platform in designing to achieve SARS-CoV-2 detection within 10 min (Chen et al., 2020)(Fig. 14 C). This IgG-based sensitive fluorescent bioassay captured recombinant nucleocapsid phosphoprotein of SARS-CoV-2 on self-assembled nano-system. This nano-enabled LFA was validated by testing COVID-19 infected 12 patients and compared with 51 normal samples. Due to desired sensing performance and selectivity, this LFA diagnostic platform can be prompted for clinical application. However, more testing based on real sample is suggested prior to promote it for public use.
NAA-based POCT has been reported as a promising diagnostic tool due to their ability to detect ultralow levels of human viral pathogens along with good specificity. Further, one step isothermal amplification of viral RNA bypassing thermal constraints was also reported for rapid diagnosis of MERS-CoV using silicon-based bio-optical sensor (Koo et al., 2017). The detection limit of iROAD assay was estimated to be 10-times higher than that of conventional RT-PCR method. NAA-based molecular diagnostics can be integrated to LFA technique to design portable colorimetric POCTs. Wang & group tracked two SARS-CoV-2 targets, open reading frame 1a/b and the nucleoprotein gene (N) using nanoparticle based LFA integrated with isothermal LAMP amplification. The advantage of this POC technique was the short analysis time (1 h), economical, and ease of access. Another isothermal amplification technique employed for the early diagnosis of infectious diseases is formation of DNA hydrogel by isothermal amplification of complementary targets (DhITACT) (Jung et al., 2016). For instance, Lee & colleagues proposed an advanced fluorescence DhITACT biosensor for rapid diagnosis of MERS-CoV. Moreover, instead of PAD, DNA primers and templates can be hybridized on microfluidic channels to achieve further increase in sensitivity of the system. Moreover, ELISA is also a highly sensitive molecular assay that motivates researchers to investigate ELISA-based POC devices to improve global healthcare. Hoy et al., illustrated rapid immunoassay methodology using ESPS microfiber mats as absorbent platform for different MERS-CoV antigen (Hoy et al., 2019). Capture membrane were patterned to create hydrophilic zones for immobilization of different antigens. These membranes were than installed in a compact cassette device to perform multiplex immunoassay. This microfiber based multiplex POCT allowed rapid preparation amounting to 5 min as well as high sensitivity, and specificity as down as 200 μg/mL.
Another highly sensitive assay using specific luminex microsphere beads detect IgG antibodies in plasma to a wide diversity of SARS-CoV-1, CoV-2, and MERS-CoV viruses (Ayouba et al., 2020). In brief, fluorescent magnetic beads covalently coupled to two different recombinant antigens that is spiked and nucleocapsid structural proteins through amine-carboxyl linking. Later, plasma samples incubated with coupled beads for 16 h at 4 °C. Reactions with a biotin-labelled anti-human IgG and streptavidin-R-phycoerythrin conjugate resulted in generation of fluorescent signal. The novel diagnostic assay is more sensitive and specific than EIA.
Due to tuneable properties and guided approach, magnetic systems are also emerging as choice of developing sensitive and selective biosensor for detecting SARS-CoV-2 virus protein. Recently, Ahmadivand et al. developed a toroidal plasmonic sensor for the effective and efficient detection of SARS-CoV-2 virus protein at femtomolar (fM) level, for the first time. In this research, magneto-plasmonic was produced by nano-Au modified magnetic platform on applying terahertz (THz) as stimulation, and further monitored as a function of monoclonal antibody against SARS-CoV-2 virus protein (Fig. 14 D) (Ahmadivand et al., 2021). Such plasmonic immunosensor functions on the basis of toroidal electrodynamics concept and can detect the spiked protein of SARS-CoV-2 virus at 4.2 fM. This development is at early stage and authors have projected its POCT application after validation using real samples. A magnetic beads assisted electrochemical immunosensing approach was developed by Fabiani et al for the selective detection of SARS-CoV-2 protein in saliva samples (Fig. 15 A) (Fabiani et al., 2021). In this electrochemical assay fabricated using screen-printed electrodes, magnetic beads support immunological chain and suitable bindings needed between selected antibody and spike protein (19 ng/mL) or nucleocapsid protein (8 ng/mL) of SARS-CoV-2 virus structure. This sensor detected SARS-CoV-2 virus protein selectively within 30 min and validated using saliva of COVID-19 infected patients.

A) illustration of magnetic bead based electrochemical SARS-CoV-2 immunosensor for the selective detection of S and N terminal of virus protein (Fabiani et al., 2021). B) Schematic presentation of a graphene-based electrochemical sensing platform for detecting SARS-CoV-2 virus (projected as telemedicine platform i.e., SARS-CoV-2 RapidPlex) in saliva and blood, a) detection platform (with reference antibodies (IgG and IgM), and inflammatory biomarker C-reactive protein) and wirelessly data sharing, b) laser-engraved graphene sensor arrays, c) disposable and flexible graphene array, d) sensor array connected with a circuit board needed for signal processing and wireless communication (Torrente-Rodríguez et al., 2020).
Besides these advancements in coronavirus detection, the bioinformatics needed to be analysed by medical experts for suggesting an appropriate therapy. In this direction, IoMT are playing crucial role and experts have established sensing methodologies with the feature of wireless communication, a foundation of telemedicine (Torrente-Rodrígueaz et al., 2020). Recently, IoT based biosensor ‘RapidPlex’ was grafted for ultra-rapid assessment of COVID-19 biomarkers (Torrente-Rodríguez et al., 2020). This is also a multiplex electrochemical platform to quantify four different COVID-19 antigens: nucleocapsid protein, inflammatory C-reactive protein (CRP), IgM, and IgG antibodies in test sample. Laser-engraved graphene (LEG) electrode functionalized with antigen specific antibodies detects target molecules, and the hybridization reaction reflect through current intensity. Rapid target binding capability of proposed platform is a major advantage for remote functionality.
As per the market reports 2020, the global POC diagnostic market size was $18.45 billion in 2019 which was estimated to reach $23.36 billion by 2027 at a compound annual growth rate of 7.3% (“Point-of-care (POC) Diagnostics Market Size, Share | Report, 2027,” 2020). The novel biosensors based POC can diagnose the emerging infectious disease in seconds that is the need of the hour. The demand of POC devices for self-diagnostics is likely to increase among patients. Several industries or research organizations are working on development of off-the-shelf or customized POC devices, e.g., ChipShop Dolomite, Abbott Laboratories, Cellix Ltd., Bio-Rad Laboratories, Agilent Technologies, and many more (Mejía-Salazar et al., 2020). Race towards technological advances in POC devices provide beneficial insight into opportunities, trends, and challenges. POC devices based on LFA are clinically more successful due to user-friendly operation. In contrast, microfluidics and biosensor based POC diagnostic are still under development, facing challenges in terms of both implementation and interpretation along with clinical validations. In this era of pandemics, POCT devices will significantly contribute towards economics due to robust and low-cost diagnosis (Ahmad et al., 2020). POC biosensors are portable devices hence can be available in remote locations and resource-limited settings. Moreover, early detection of infectious diseases benefits the government in controlling the outbreak. Rapid and accurate testing contributes significantly to epidemiologic studies and surveillance to estimate health status and future outbreaks of such infectious diseases.
However, development and commercialization of advanced biosensor technology is a bigger challenge considering its technical aspects (Everitt et al., 2020). Several factors work together in preventing translation of academic research to commercially viable devices. Primarily, biosensors for infectious diseases need sample collection and preparation that limit biosensors capability from qualifying as POCT. Besides, reagents sometimes need refrigeration for long shelf-life forms that is another barrier for POC testing. Therefore, researchers start utilizing microfluidic platforms that carry out all the required operational step but, in a cost-prohibitive bulky setup.
Furthermore, validation of biosensors for a reproducible result often requires substantial quantity of the purified biomolecule from a biological source, concerning the industrial production of biosensor (Rodrigues Ribeiro Teles et al., 2010). Moreover, sometimes purification compromised sensitivity of biomolecule, as in the case of polycolonal antibodies that are more specific to target antigens. Furthermore, immobilization of biomolecules on transducer surface in addition to interaction with matrix compounds may lead to loss in activity hence compromised selectivity, and sensitivity of the biosensor. Another most important issue is biosafety not as stringently regulated as in conventional diagnostics. Although advanced biosensors switching from reusable testing to one-time disposable devices due to undeniable advantages like avoid infection spreading, cleaning, and sterilization, but the investment in reusable devices comply with affordability conditions. Hence, disposable POCT sometimes fails in resource-depleted regions and settings as eventually dominate the production costs. Besides, most of the POCT biosensors robustness checked with target molecules-spiked samples while real specimen may relate to the usually low operational performance (Cammann et al., 1991). Presence of interfering substances in complex real samples can lead to nonspecific interfering signals at the level of the transducer element and hence low sensitivity. The modest stability of biomolecules for biosensing under working conditions (e.g., room temperature) may limit the performance of the biosensor after some months. Importantly, reported end-users does not rely on POC devices because of overestimation and error in results.
Besides all these technical aspects, POCT biosensors still need improvement in LOD limits to achieve detection in the amol (10−18 M) range necessary for diagnostic assays with real and nonamplified biological samples, whereas this is often beyond the fundamental limits of common sensing devices. Further, IoMT-based POC faces some challenges like electrode fouling, electrode drift, optical transducer blockage, probe degradation, and many. Despite being implemented in medical devices validations, the regulatory requirements, privacy, and risk assessment remain the main obstacle in IoMT-based POCs. Further, amplification-based diagnostics avail high-sensitivity biosensors, but their designing is still too complex and costly for front line POC diagnosis. Moreover, qualitative diagnostics is not suitable in these pandemic eras. Further, the quantification of serological level of biomarkers is needed to know the pathological states of diseases. Many POCT devices provides only qualitative outputs, indicating the presence or absence of biomarker in sample based on “yes/no” basis. Therefore in recent times LFA test strips also extended to quantitative measurement by incorporating small reader devices like charge-couple devices (St John and Price, 2014). This area grows well in near future with many devices being developed and likely to reach the commercial market in the coming years. Multiplexed diagnostics, analysing many targets in one frame of time, is very important tool for prompt and adequate case management in endemic areas (X. He et al., 2020). These layouts need much improvement in terms of sensitivity and cross reactivity, hence are under continuous research to accomplish the needs for high-throughput analysis. Rapid multiplexing diagnostics are of significant use, particularly in cases of co-infections. However, several questions remain unresolved in case of these multiplexed biosensing devices such as proper evaluation, validation, and standardization.
Once POCT device has been established for diagnostics application, a timely validation of such system based on standard and FDA approved protocols is always recommended for better public usage and clinical application. Clinical accuracy is one of major challenges in front of these IoMT-integrated POC systems due to lack of understanding of quality control as well as quality assurance practices related mainly with IoMT (Shaw, 2016). To ensure the accuracy of results, the implementation of quality assurance program is essential in POCT. Thus, it requires the establishments of standards with help of government, institutions, and experts. Usually most of the academic development fails on the part of patients and only few qualifies the first evaluation stage (McRae et al., 2020). This is mainly because of discrepancy between perception and reality as laboratory testing are carried out indoors rather than in the health care settings. Researchers are working on to address these drawbacks and developing new diagnostic tools via wide range of unprecedented opportunities in diagnostic biomarkers, detections methodology, and advanced nanomaterials (Califf, 2018). Moreover, computer-aided diagnosis systems or IoMT requires integration of big data analysis where different tools need to be explored like visualization tools, ML, and data mining. Data transmission and processing device play a major role in eHealth diagnostics, involving several challenges in terms of monitoring of patient's activities and health (Vitabile et al., 2019). In comparison to analogue approaches, computer-based or digital tools are more efficient and usually preferred by physicians. Beyond these technical challenges, clinical barriers also limit their performance. The main challenge is suitable disease biomarker that typically enable early diagnosis using simplified matrices in addition to allow testing at low level. Novel clinically relevant biomarkers from the advanced genomics and proteomics technique can be used to increase diagnostic accuracy. Predictive biomarkers facilitate early diagnosis as it can help identify individuals in early stages of diseases. Moreover, biomarker that can be detected in a non-invasive specimen is preferred for real time POCT devices. Hence, diagnostic biomarkers development together with sensing technology are reshaping diagnostic and therapeutic sciences as it prioritizes the quality and reproducibility of the POCT. Better understanding of progression of disease, virulence factors, and alteration in gene expression are also helpful in clinical research or POCT development.
Significant advancement in materials science and nanotechnology exhibit several advantages in context of detection technology, e.g., higher sensitivity and lower detection limits (Mishra et al., 2020; Rani et al., 2020; Syedmoradi et al., 2017). Wide range of nano-systems and their composites materials such as polymers, CNT, graphene, metal nanoparticles, metal oxides, hybrids, liquid crystals etc., have been investigated for designing smart nano-platforms for biomolecule immobilization, miniaturization, surface functionalization, and tuning of optoelectronic properties (Kumar et al., 2015; S. Kumar et al., 2018a; 2018b; Nehra et al., 2019; Samaddar et al., 2018). Predictably, such uniform fabrication will be useful to develop a sensing device of desired features wherein reproducibility and reliability considered as keys.
Presently, efforts are being made to support such POCT by the technological development investigated in the field of IoMT to achieve wireless based operation and connectivity with health expert and medical centre (Sadoughi et al., 2020). These IoMT devices are a viable alternative to bulky and expensive laboratory systems. Keeping advancements and prospects into consideration, current research focuses on exploring IoMT-assisted advanced biosensor technologies for POCT of infectious diseases associated with HIV/HPV, malaria, dengue fever, H1N1, HIV, HPV, Ebola, ZIKV, and COVID-19 infection. An optimized IoMT-assisted POCT will be useful to manage a targeted infectious disease via understanding diseases progression, decide treatment, and evaluate efficacy of prescribed therapy. POC devices must fulfil all the standard evaluation criteria put forth by the WHO using the acronym ASSURED-affordable, sensitive, specific, user friendly, rapid and robust, equipment-free, and deliverable to those in need (Everitt et al., 2020). The application of new advancements to the enormous medical needs of developing countries is a major challenge for the public sector. Moreover, the healthcare diagnostic under IoMT-assisted network faces huge security challenges. The attack on healthcare system can cause damage beyond imagination via losing control over the life supporting equipment (Shrivastava et al., 2020b). Thus, securing protocols and health data, and bioinformatics has emerged as a very serious concern. To secure the healthcare system from vulnerability exploitation, several options have been explored, e.g., blockchain-based IoT environment (Moin et al., 2019).
Expected economic revenues from capital investment in the designing, production, and marketing of novel POC for use in tropical developing countries are unattractive. Mostly, governmental organizations have financially supported commercial endeavors and research organization working on novel biosensor point of care technologies. Additional efforts for wider promotion of rapid diagnostic tests are expected from international public health organizations. Therefore, it is much more challenging to deal with barriers and develop a market for POCT assay supported by AI and IoMT (Fig. 16 ) In the coming years, widespread advancement of these bioanalytic tools is expected. This will ultimately be revolutionizing the world of clinical diagnostics to ease the disease burden in remote areas and even low-income countries.

Illustration of a combinational diagnostics approach consisting a nano-sensor, AI and IoMT projected for intelligent healthcare management.
Synergetic approaches involving IoMT and POCT based biosensing system is an interdisciplinary research that cover wide expertise of various fields to offer benefits to a wide range of communities including microbiology, immunology, pathology, clinical biology, electronics, material chemistry, and other applied field of sciences. Presently, the research efforts are focused on exploration of novel and smart biosensing technology using novel nano-systems, bio-active molecules, computational analytics, and hardware integration to achieve easy operation and performance assessment that are required for timely therapy decision. Therefore, this article served as an appeal to a wide range of stakeholders including scientists, clinical practitioners, engineers, medical professionals, industrialists, educators, and students devoted to the development of affordable IoMT-assisted POCT biosensing system for intelligent healthcare to serve the humanity in a better way.
In summary, wide accessibility, real-time screening, specificity, and data computation in POC devices pave the way for prompt diagnosis of infectious diseases. POC helps effective patient-centric treatment and management during pandemics. Although numerous challenges still associated with the POCT testing, the rapid advancements hold a huge potential for implementation of POCT programs to prevent the spread of infectious diseases. Currently technological research inclined most towards the next generation standalone POCT devices. Several smart applications are already commercialized for screening the basic health parameters, such as blood glucose, blood pressure, weight, and many more. Security and privacy are major concern for establishing the market of these POCTs. The global efforts will effectively tackle these challenges in the coming years. The framework, proposed in this article, can help in shaping many of the ongoing efforts to develop POC tests for pandemics.
Shikha Jain: Visualization, Writing - review & editing. Monika Nehra: Writing - review & editing, Visualization. Rajesh Kumar: Writing - review & editing, Visualization. Neeraj Dilbaghi: Writing - review & editing, Visualization. TonyY. Hu: Writing - review & editing, Visualization. Sandeep Kumar: Writing - review & editing, Visualization. Ajeet Kaushik: Writing - review & editing, Visualization. Chen-zhong Li: Visualization, Writing - review & editing.
There are no conflicts of interest declared by the authors.
S.K. thanks DBT, Govt. of India, for a research grant vide letter No. BT/PR18868/BCE/8/1370/2016 dated 31-01-2018. Shikha Jain would like to thank the University Grants Commission, India, for providing financial assistance in the form of a Senior Research Fellowship [award F.16–6 (Dec. 2016)/2017 (NET), 12-09-2017]. Monika Nehra thanks