
- Altmetric
- Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial
- Which causal link between vascular calcification and bone resorption in chronic kidney disease?
- Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T-cell exhaustion
- Genetic variation in MANBA and kidney disease development
Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial
Cohen et al. (Lancet Respir Med. [e-pub ahead of print] https://doi.org/10.1016/S2213-2600(20)30558-0. Accessed February 16, 2021.)
Since the beginning of the coronavirus 2019 (COVID-19) pandemic, the medical community has wrestled with the question of whether using angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) is beneficial or detrimental to patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). ACEi and ARBs may increase the expression of angiotensin-converting enzyme 2 (ACE2), which also happens to be a receptor for SARS-CoV-2 and facilitates viral entry into host cells. This raised the concern that COVID-19 may be worse in patients on these medications. At the same time, arguments were made suggesting ACEi and ARBs may decrease the hyperinflammatory response to SARS-CoV-2 and ameliorate COVID-19. Although observational data suggest that COVID-19 patients receiving renin-angiotensin system (RAS) inhibition do no worse than those not getting ACEi or ARB, there have been no prospective studies. The Randomized elimination and prolongation of ACE inhibitors and ARBs (REPLACE) COVID trial (NCT04338009) was undertaken to fill this gap.
REPLACE COVID was an open-label, randomized, multicenter study of adult patients receiving ACEi or ARBs who were hospitalized with COVID-19. The participants were from high- and low-income countries, increasing generalizability of the results. RAS blockers were continued or withdrawn in a 1:1 ratio. Of note, the adjudicators who verified end points were blinded as to study arm.
The primary end point of the trial was global rank outcome that considered, in hierarchical order, days to death (lowest to highest), days on mechanical ventilation or extracorporeal membrane oxygenation (highest to lowest), days on renal replacement therapy or blood pressure support (highest to lowest), or the area under the sequential organ failure assessment (SOFA) score curve. Each patient was ranked against all others, and overall, the lower the rank the worse the COVID-19 infection. Analyses were conducted on an intent-to-treat analysis.
A cohort of 152 patients was randomized, and this sample size had 80% power to detect a 25% difference in median global rank score between study arms. The mean age of the cohort was 62 years, with 45% women. Comorbidities were frequent; 52% had diabetes, 16% had cardiac disease, and all had hypertension. All but one patient had a positive polymerase chain reaction test for SARS-CoV-2 (patient expired before testing).
The global rank score was not different between patients who continued or discontinued ACEi or ARBs. This did not change after adjusting for age, sex, race/ethnicity, ACEi or ARB use, or preexisting lung or cardiovascular disease. There were also no differences in secondary outcomes, including death, length of overall hospital stay or need for an intensive care unit, need for mechanical ventilation, SOFA score, or serum creatinine. Acute kidney injury occurred in 4% of each group.
In summary, the REPLACE COVID trial provides randomized, controlled evidence that patients who are receiving RAS inhibitors and become infected with SARS-CoV-2 can continue these medications unless/until something other than COVID-19 requires their discontinuation. The question of whether de novo ACEi/ARB therapy may provide added benefit to patients with COVID-19 remains unanswered, but ongoing trials are currently testing this hypothesis.
—Brad H. Rovin
Note: Look for further discussion of this trial in an upcoming Nephrology Digest.
Which causal link between vascular calcification and bone resorption in chronic kidney disease?
Mace et al. (Chronic kidney disease-induced vascular calcification impairs bone metabolism [epub ahead of print]. J Bone Miner Res. https://doi.org/10.1002/jbmr.4203. Accessed January 27, 2021.)
Observational studies showed an association between low bone mineral density (BMD) and the presence of vascular calcification in various disease states, including aging, diabetes, osteoporosis, and chronic kidney disease (CKD). This inverse correlation is suggestive of a role of skeletal calcium and phosphate resorption in the development of vascular calcification as a plausible mechanistic link. The possibility of an inverse mechanism (namely, a causal role of vascular calcification in bone mineral loss) apparently has not been tested so far. This hypothesis has now been examined by Mace et al. in a novel experimental model of isogenic aorta transplantation (ATx). The authors transplanted severely calcified aortas from uremic rats into healthy rats (uremic ATx). Transplantation of normal aortas into healthy rats (normal ATx) and age-matched rats without aorta grafts (control) served as control groups. Trabecular tissue mineral density, as measured by micro–computed tomography, was significantly lower in uremic ATx rats compared with both control groups. Uremic ATx rats showed a significant upregulation of the mineralization inhibitors osteopontin and progressive ankylosis protein homolog in bone. In addition, there were significant changes in bone mRNA levels of several genes related to extracellular matrix, bone turnover, and Wnt signaling in uremic ATx rats, with no difference between normal ATx and control rats. Figure 1 shows changes in bone expression of genes involved in bone mineralization and formation in uremic ATx rats compared with the 2 other groups. Bone histomorphometry analysis showed lower osteoid area in uremic ATx rats compared with normal ATx rats along with a trend toward fewer osteoblasts and more osteoclasts. Uremic ATx and normal ATx had similar trabecular number and thickness, and comparable bone formation rate. Culture media of uremic rat aortas ex vivo exhibited >30 times higher concentrations of Wnt inhibitor sclerostin than media of control aortas. Plasma biochemistry did not show any difference, including plasma sclerostin levels. Thus, uremic aorta calcification associated with lower BMD and impaired bone metabolism, probably involving excessive vascular sclerostin secretion and suggesting a vasculature to bone tissue crosstalk in CKD. One could imagine the presence of a vicious circle, with vascular calcification inducing bone mineral loss, which, in turn, would aggravate vascular calcification.

![Bone expression of genes involved in bone mineralization and formation. (a–d) Rats transplanted with a calcified aorta from uremic rats (uremic ATx) had significantly increased mRNA levels of osteopontin (a), progressive ankylosis protein homolog (ANKH) (b), alkaline phosphatase (ALPL) (c), and collagen type I α2 (collagen I) (d) compared with rats transplanted with a normal aorta (normal ATx) and control rats. There was no difference in the expression of these genes between normal ATx and control. The mRNA levels of genes are normalized to reference genes Arbp and Rpl13 and shown as the ratio to the mean of control group. Data are shown as box plots with median, interquartile range, and all data points. Control, n = 6; normal ATx, n = 9; uremic ATx, n = 16. Reprinted from Mace ML, Gravesen E, Nordholm A, et al. Chronic kidney disease-induced vascular calcification impairs bone metabolism [epub ahead of print]. J Bone Miner Res. https://doi.org/10.1002/jbmr.4203 under CC BY-NC-ND 4.0 license. Accessed January 27, 2021.](/dataresources/secured/content-1765998832424-b5ab9b25-35d6-43e1-a039-0a1c9c9d13d6/assets/gr1_lrg.jpg)
Bone expression of genes involved in bone mineralization and formation. (a–d) Rats transplanted with a calcified aorta from uremic rats (uremic ATx) had significantly increased mRNA levels of osteopontin (a), progressive ankylosis protein homolog (ANKH) (b), alkaline phosphatase (ALPL) (c), and collagen type I α2 (collagen I) (d) compared with rats transplanted with a normal aorta (normal ATx) and control rats. There was no difference in the expression of these genes between normal ATx and control. The mRNA levels of genes are normalized to reference genes Arbp and Rpl13 and shown as the ratio to the mean of control group. Data are shown as box plots with median, interquartile range, and all data points. Control, n = 6; normal ATx, n = 9; uremic ATx, n = 16. Reprinted from Mace ML, Gravesen E, Nordholm A, et al. Chronic kidney disease-induced vascular calcification impairs bone metabolism [epub ahead of print]. J Bone Miner Res. https://doi.org/10.1002/jbmr.4203 under CC BY-NC-ND 4.0 license. Accessed January 27, 2021.
—Tilman B. Drüeke
Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T-cell exhaustion
Scharping et al. (Nat Immunol. 2021;22:205–215.)
Mechanisms that can potentially blunt immune responses are critical for our understanding of a number of immune-mediated conditions relevant to nephrology. In transplantation, the ability to dampen cluster of differentiation (CD) 8 T-cell responses against the allograft is a potential strategy to induce tolerance. Scharping et al. have uncovered a mechanism of T-cell exhaustion mediated through a loss of mitochondrial function under hypoxic conditions. Continuous activation of murine CD8 T cells by CD3/CD28 stimulation under hypoxic conditions induced an exhausted dysfunctional state in CD8+ T cells. This dysfunctional state was driven by mitochondrial reactive oxygen species (ROS) formation and resulted in the exhausted T-cell phenotype. Strategies to mitigate ROS formation or reversal of hypoxic environment were able to prevent T-cell exhaustion. A key exhaustion-related transcription factor identified in this study was Blimp-1, which appears to continually repress the plasticity of the exhausted cells. Gene Set Enrichment Analysis (GESA) identified clear differences in the transcriptional profile of continuously stimulated T cells under either hypoxic or normoxic conditions (Figure 2 ). The role of hypoxia in generating exhausted CD8 T cells appears to be functioning as an accelerator of the process that is mediated by Blimp-1. Thus, this article defines, for the first time, critical mechanisms that are implicated in immune exhaustion, an old concept in tumor and infectious immunology. These findings are of significant interest to the transplant immunologist as renal allotransplantation is a classic situation where persistent antigen stimulation occurs after the transplant. In this study, persistent antigenic stimulation leads to T-cell mitochondrial dysfunction and produces T-cell exhaustion. Thus, manipulation of mitochondrial pathways within T cells, potentially via Blimp-1, may be a promising strategy for induction of hyporesponsive states.

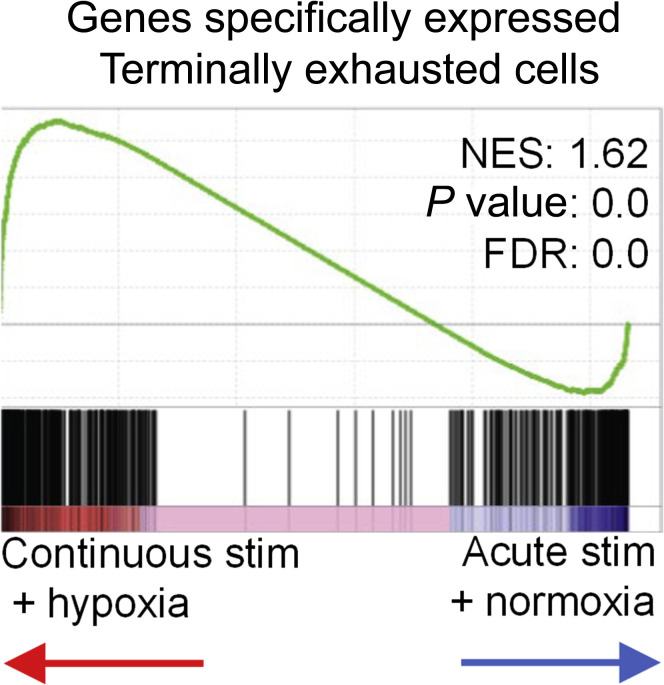
Continuous activation of T cells under hypoxia induces distinct intracellular programs from either stressor alone. GSEA of transcriptional profiles of exhausted T cells under either hypoxia or normoxia. FDR, false discovery rate; NES, normalized enrichment score; stim, stimulation. Reprinted by permission from Springer Nature, Nature Immunology, Scharping NE, Rivadeneira DB, Menk AV, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22:205–215. Copyright © Springer Nature 2021.
—P. Toby Coates
Genetic variation in MANBA and kidney disease development
Gu et al. (Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci Transl Med. 2021;13:eaaz1458.)
Identifying genetic factors associated with kidney disease may provide critical insights into disease mechanisms and indicate new therapeutic targets for the treatment of chronic kidney disease (CKD). Genome-wide association studies (GWAS) are used as an unbiased approach to discover genomic regions associated with kidney function parameters and the risk of CKD in large cohorts. In an elegant study, Gu et al. identified MANBA, a gene coding for the lysosomal enzyme β mannosidase, as a potentially causal gene included in a chromosome 4 GWAS locus associated with estimated glomerular filtration rate based on creatinine and CKD. The CKD risk genotype was associated with lower MANBA expression in human glomerular and tubule kidney samples. They next showed that rare loss-of-function variants of MANBA are associated with increased incidence of end-stage kidney disease in 2 cohorts including >40,000 subjects. They further characterized the role of MANBA in mouse: although Manba knockout mice had no obvious kidney phenotype at baseline, mice with homozygous or heterozygous deletion of Manba showed increased susceptibility to kidney damage and fibrosis when exposed to folic acid or cisplatin. Mechanistic studies in isolated mouse kidney tubular cells revealed that the deletion of Manba led to lysosomal dysfunction, impaired autophagy, and defective endocytosis, all features confirmed in the kidneys of Manba-knockout mice. These changes were paralleled by an activation of the NOD-like receptor family, pyrin domain-containing (NLRP) 3 inflammasome and downstream inflammatory cell death, resulting in a more severe fibrosis in Manba-knockout kidneys. Of note, human samples harboring the MANBA risk genotype showed an increase in lysosome number and size (immunostaining) and increased expression of immune activation genes (RNA sequencing). Taken together, these multilevel studies identify the lysosomal enzyme MANBA as a potential risk factor for CKD, supporting earlier evidence from GWAS. They also stress the functional importance of lysosomal function and autophagy in the regulation of tubular function and modulation of inflammatory response to injury in the kidney.
—Olivier Devuyst
 Journal Club
Journal Club