
Competing Interests: The authors have declared that no competing interests exist.
Versican is a large proteoglycan in the extracellular matrix. During embryonic stages, it plays a crucial role in the development of cartilage, heart, and dermis. Previously, we reported that Prx1-Vcan conditional knockout mice, lacking Vcan expression in mesenchymal condensation areas of the limb bud, show the impaired joint formation and delayed cartilage development. Here, we investigated their phenotype in adults and found that they develop swelling of the knee joint. Histologically, their newborn joint exhibited impaired formation of both anterior and posterior cruciate ligaments. Immunostaining revealed a decrease in scleraxis-positive cells in both articular cartilage and ligament of Prx1-Vcan knee joint, spotty patterns of type I collagen, and the presence of type II collagen concomitant with the absence of versican expression. These results suggest that versican expression during the perinatal period is required for cruciate ligaments’ formation and that its depletion affects joint function in later ages.
The joint is formed by apoptosis of undifferentiated mesenchymal cells in the cartilage primordium of long bones. Mesenchymal cells remain pre-differentiated chondrocytes and go through apoptosis, where hyaluronan (HA) is accumulated, forming a cavity named joint interzone. The marginal cells retain pre-chondrocytic nature and serve as articular chondrocytes. In this process, versican (Vcan) [1], a chondroitin sulfate (CS) proteoglycan of the extracellular matrix (ECM) type, is expressed with dynamic patterns. Its expression initiates in mesenchymal condensation areas. During the differentiation of mesenchymal cells into chondrocytes, it remains in pericondensation areas. When the joint formation occurs through the accumulation and lining up of mesenchymal cells, Vcan expression is restricted to the joint interzone. After the joint cavity formation, Vcan is localized in the articular cartilage and synovial tissue, lining the inside margin of the cavity [2, 3].
Vcan contains a core protein consisting of an N-terminal G1, CSα, CSβ, and C-terminal G3 domains. The N-terminal G1 domain consists of A, B, and B’ subdomains and binds to both HA and link protein (LP) [4]. Both CSα and CSβ domains are attached with CS chains. The C-terminal G3 domain binds different ECM molecules such as fibrillin-1 [5], fibulin-1 and -2 [6, 7], tenascins [6, 8], and heparan sulfate proteoglycans [9]. Vcan exhibits four spliced variants; V0 (G1-CSα-CSβ-G3), V1 (G1-CSβ-G3), V2(G1-CSα-G3), and V3(G1-G3) [10–13]. Whereas V0 and V1 are widely expressed, V2 expression is restricted to the nervous systems. Thus, the number of CS chains necessary for the function of Vcan may vary among tissues.
Previous cell culture studies have revealed various effects of Vcan on cell behavior [14]. For example, Vcan inhibits the adhesion of osteosarcoma cells and aggravates their malignant phenotype [15]. It inhibits neural crest cell migration and the outgrowth of motor and sensory axons [16]. The function of Vcan appears to be through the G1 domain regulating HA-mediated signaling, the G3 domain mediating TGFβ- and BMP-signaling, CS chains binding cytokines and growth factors such as midkine and pleiotrophin, and epidermal growth factor (EGF)-like motifs in the G3 domain binding to EGF receptors [14].
In addition to developing cartilage and forming joints, transient Vcan expression is observed in various developing tissues [17], including the brain [18], developing heart [19, 20], hair follicles [21, 22], and tooth and periodontal tissues during tooth eruption [23]. In the brain, Vcan promotes presynaptic maturation [24]. Vcan contributes to cardiac jelly formation and septal formation [25, 26]. Vcan in dense aggregates of dermis-derived stromal cells suggests its involvement in hair follicle formation [21]. These studies suggest that Vcan plays the central role in the formation of a provisional matrix [1, 27].
Previously, we generated and analyzed Prx1-Cre/Vcanflox/flox (herein Prx1-Vcan) mice, which lack Vcan expression in mesenchymal condensation areas of cartilage primordium. Whereas these mice are viable and fertile, they exhibit delayed chondrocyte differentiation and joint malformation in digits [28]. Further analysis revealed decreased TGFβ accumulation and down-regulation of its signaling required for chondrocyte differentiation, indicating that Vcan is necessary for the local concentration of TGFβ and its adequate signaling.
While maintaining Prx1-Vcan mice, we found the laxity of knee joints, which can be observed as early as six months. As Vcan is expressed in tendons and ligaments under physiological conditions [29, 30], and in tendinopathy [31, 32], lack of Vcan expression in tendons and ligaments may cause joint destruction. Here, we investigated abnormalities in their joint and found malformation of cruciate ligaments in newborn mice. These results suggest that Vcan expression in the perinatal period is necessary for the normal formation of jointed appendages and that its impairment causes joint laxity later.
All the experiments were approved by the animal care committee at Aichi Medical University. Prx1-Cre/Vcanflox/flox (Prx1-Vcan) and Prx1-Cre /Vcan+/+(Prx1-Vcan+/+) mice were maintained, and their genotyping was performed as reported previously [28]. Prx1-Vcan and Prx1-Vcan+/+ mice were crossed several times with mT/mG reporter mice, and Prx1-Vcan: mT/mG and Prx1-Vcan+/+: mT/mG mice were generated and maintained under physiological conditions. Usually, two~three mice were in a cage.
Mice were sacrificed at four~six months. Their limbs were fixed in 10% buffered formalin for 24 h and decalcified using KCX (Falma, Tokyo) overnight. Newborn mice were sacrificed, and their limbs were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) for 24 h. After embedding the samples in paraffin, deparaffinized sections (3–5 μm) were prepared for hematoxylin and eosin (H&E) and immunostaining. Step-wise section slides of every five slices and serial sections for four~six-month-old joints and newborn joints were prepared, respectively. The primary antibodies used were as follows: rabbit polyclonal anti-Vcan GAGβ(×500 dilutions; Millipore), goat anti-enhanced green fluorescent protein (EGFP, ×200; Rockland), anti-type I collagen (×200; LSL, LB-1102), and anti-type II collagen (x200; LSL, LB-1297). For secondary antibodies, anti-rabbit or anti-goat IgG conjugated with Alexa594 (Molecular Probes) and Alexa488 (Molecular Probes) were used. For observation, the BZ 9000 (Keyence) was used as light and immunofluorescence microscopy.
Limbs at five months were scanned for baseline microarchitecture in a randomized sequence, using a CosmoScan RmCT2 microCT machine (Rigaku, Tokyo). The joint region, including femur, tibia, and fibula, was evaluated.
Skin was collected from Prx1; mT/mG newborn hindlimbs, washed with PBS ten times, treated with 0.05% trypsin-EDTA overnight at 4 °C, and then with 0.35% collagenase type I (Roche) for 2 h. The sample was centrifuged at 1,000 ×g for 5 min. Cells were resuspended in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and analyzed using FACS Canto II™ (BD Biosciences).
Prx1-Cre/Vcanflox/flox (Prx1-Vcan) mice are viable and fertile, although they exhibit distorted digits in both fore and hindlimbs compared with Prx1-Cre/Vcan+/+ (Prx1-Vcan+/+) as control. We examined whether they have additional abnormalities during their growth or not. By gross appearance, Prx1-Vcan mice were slightly smaller than the Prx1-Vcan+/+. Their knee joint capsule was larger than Prx1-Vcan+/+ (Fig 1A), although it did not show inflammatory responses. It was discernible as early as ~five months, but not at ~four months. Then, we investigated the individual components of the joint region. By microCT analysis at five months, the bones of Prx1-Vcan mice did not show any abnormalities (S1 Fig).

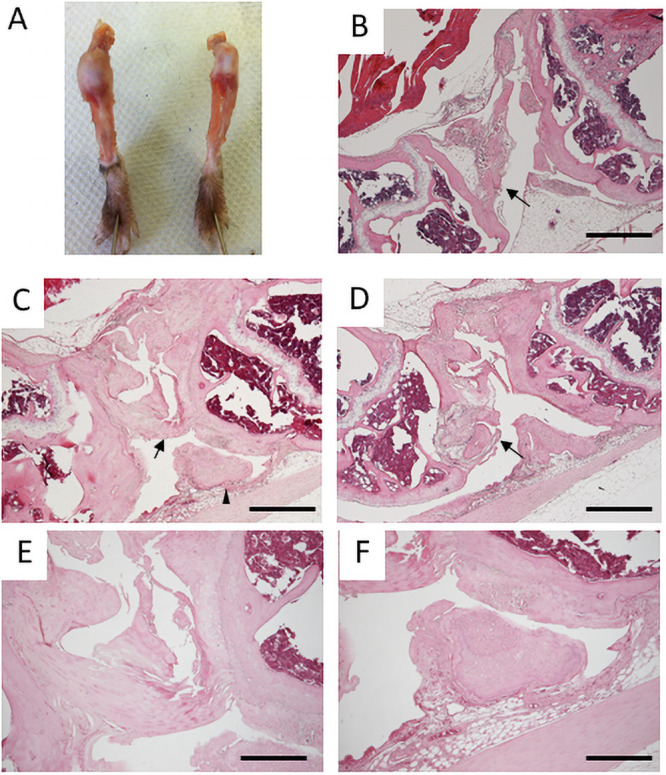
Knee joints of six-month-old mice.
A. Gross appearances of knee joint capsules of Prx1-Vcan (left) and Prx1-Vcan+/+ (right). B. Histological Analysis of Prx1-Vcan+/+ knee joint (H&E). Note that the PCL is normal (arrow). C. Prx1-Vcan knee joint (H&E). PCL is disorganized (arrow), and joint mouse is observed (arrowhead). D. Prx1-Vcan knee joint (H&E). A false joint is observed on the distal end of the femur (arrow). E. High power view of the arrowhead region in Panel C (H&E). Note degeneration of the ligament with calcification assessed by the tidemark. F. High power view of the region of the arrowhead in Panel C (H&E). Note the presence of a small free body composed of cartilaginous tissue. Bar = 500 μm in B-D, 200 μm in E, F.
Next, we performed histological analysis of six-month-old mice. Whereas articular cartilage of Prx1-Vcan+/+ remained smooth, that of Prx1-Vcan was severely disorganized, with fibrillated surfaces (Fig 1B–1D). Whereas the posterior cruciate ligament (PCL) appeared normal (Fig 1B, arrow) in Prx1-Vcan+/+ joint, the PCL of Prx1-Vcan joint was tortuous and fused (Fig 1C, arrow). Besides, a small free body composed of cartilaginous tissue, the so-called “joint mouse,” was observed (Fig 1C, arrowhead, and Fig 1F). Another Prx1-Vcan joint exhibited marked tissue degeneration (Fig 1D). The distal femoral region formed a “pseudojoint” (Fig 1D, arrow) presumably due to repeated fracture, and the boundary of bones and connective tissue was unclear (Fig 1D, arrowhead). The apparent anterior cruciate ligament (ACL) was disorganized (Fig 1E). Taken together, Prx1-Vcan mice at six months of age showed disorganization of cruciate ligaments and formation of a free body.
As Vcan is expressed at high levels in the joint of the perinatal period and rapidly disappears from the joint, we asked whether abnormalities were already initiated in that period. The newborn Prx1-Vcan joint exhibited disorganized PCL (Fig 2B, arrow) compared with Prx1- Vcan+/+ joint (Fig 2A and 2C). In Prx1-Vcan joint, the patella fat pad and anterior femoral region were connected with broader connective tissues (Fig 2D), whereas Prx1- Vcan+/+ joint barely exhibited connection. In the Prx1-Vcan joint, both ACL and PCL were disorganized and indiscernible (Fig 2E), and the ligamental cells showed different sizes and orientations (Fig 2F).

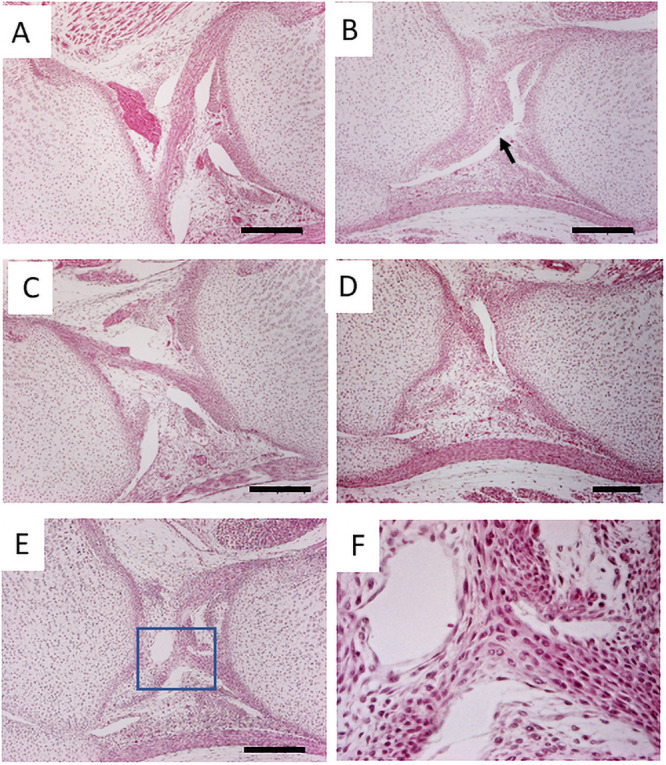
The histological analysis of newborn mice.
A, C. The knee joint of Prx1-Vcan+/+. B. Prx1-Vcan joint. Note disorganized ACL (arrow). D. Prx1-Vcan joint. Note that the patella fat and anterior femoral region are connected with broader tissues. E. Prx1-Vcan joint. ACL and PCL are disorganized and indiscernible (Bar = 200 μm). F. High-power view of the rectangle in E. Cells in the ligament are in different sizes, oriented to different directions.
In Prx1-Vcan mice, Vcan expression is depleted only in the cells with cre promoter activity. To identify them, we performed immunostaining for EGFP on Prx1-mT/mG mice. EGFP was detected in articular cartilage, synovia, cruciate ligament, and tendon (Fig 3A), but not in the dermis (Fig 3C). When we isolated and cultured dermal fibroblasts from Prx1-mT/mG mice and sorted by FACS, no green fluorescent fibroblasts were detected (S2 Fig), indicating that Prx1 promoter was and had been inactive in dermal fibroblasts. By immunostaining, Vcan was expressed in the articular surface, synovia, and cruciate ligaments at high levels (Fig 3D) in newborn Prx1-Cre/Vcan+/+ mice, whereas it was barely detected in Prx1-Vcan mice (Fig 3E). As the regions where both EGFP and Vcan are colocalized are likely affected in Prx1-Vcan mice, these observations implied that the abnormalities initiate in the ligament.

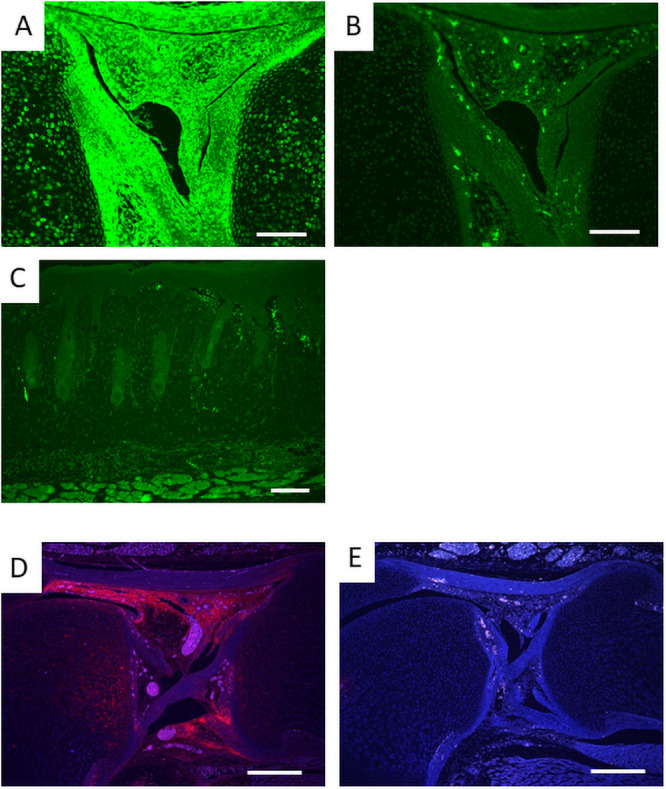
Immunostaining of EGFP and Vcan.
A. EGFP of newborn Prx1-Vcan: mT/mG mouse knee joint. B. Negative control with non-immune IgG. C. EGFP of newborn mouse skin. Note little fluorescence indicating that dermal fibroblasts lack or lacked Prx1 promoter activity. D. Vcan of Prx1-Vcan+/+ newborn mouse knee joint. E. Vcan of Prx1-Vcan knee joint. (Bar = 100 μm in A, B; 200 μm in C, D, E).
As the cruciate ligaments appeared as the initial site of abnormalities, we hypothesized that the number or the localization of cells that form ligaments are altered in Prx1-Vcan mice. The cells that contribute to ligament formation express scleraxis (scx) [33]. Whereas scx was immunostained in most articular chondrocytes and cells in the ligaments of Prx1- Vcan+/+ mice (Fig 4A), it was immunostained only in some chondrocytes and ligamentocytes in Prx1-Vcan joints (Fig 4B–4D).

Scx immunostaining of newborn mouse knee joint.
A. Prx1-Vcan+/+ mouse. Note that Scx is observed in articular chondrocytes and cells in the ligaments. B, C, D. Prx1-Vcan mouse. Note different immunostaining patterns. Scx is weakly immunostained at different levels (B, C, D) in articular chondrocytes (Bar = 200 μm).
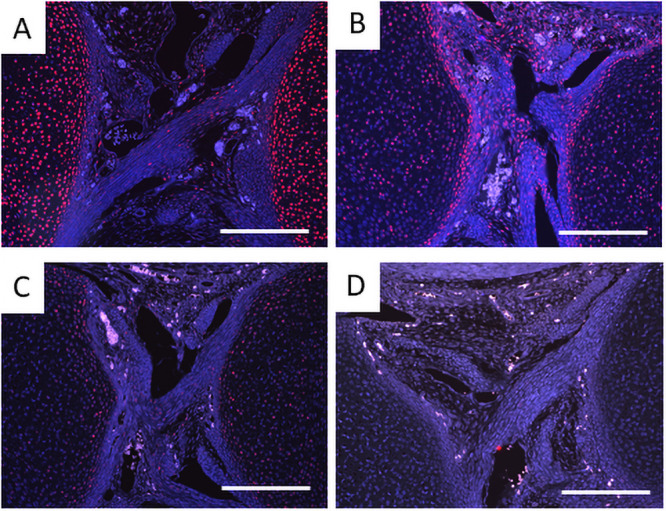
Progenitor cells of cruciate ligaments are localized in the joint interzone, and TGFβ shifts the balance of the cells from chondrogenesis to fibrogenesis [34]. Whereas type I collagen was immunostained associated with fibers in the Prx1-Vcan+/+ ligament, it was immunostained with spotty patterns in the Prx1-Vcan ligament (Fig 5A and 5B). Some cells were immunostained with type II collagen in Prx1-Vcan ligament, whereas type II collagen was barely detected in Prx1-Vcan+/+ ligament (Fig 5C and 5D). These results suggest that the Prx1-Vcan ligament contains cells with a chondrocytic phenotype.

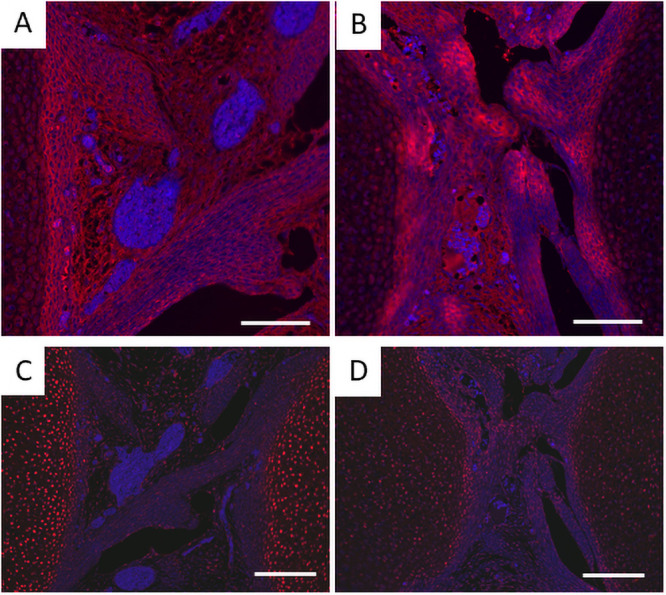
Immunostaining for type I and type II collagens of Prx1-Vcan+/+ (A, C) and Prx1-Vcan (B, D) mice. A, B. Whereas type I collagen is diffusely immunostained in the ligament of Prx1-Vcan+/+ (A), it is observed in spotty patterns in Prx1-Vcan mouse (B). Type I collagen is stained red, and the nuclei are counterstained blue. C, D. Whereas type II collagen is localized in articular chondrocytes of Prx1-Vcan+/+ joint (C), it is immunostained in some cells in the ligament of Prx1-Vcan joint (D) Type II collagen is stained red, and the nuclei are counterstained blue (Bar = 100 μm).
In this study, we have demonstrated that Vcan expression in the joint of the perinatal period is required for normal development of the jointed appendages, especially of the cruciate ligaments. Although Vcan expression is limited to embryonic and perinatal periods in mice, its absence has profound effects on the joint function in the later ages. Immunohistochemical analysis of scx and collagens strongly suggests that Vcan contributes to the differentiation of the progenitor cells toward ligamentocytes and organization of type I collagen fibers of the ligaments.
We reported that Prx1-Vcan mice reveal impaired joint formation during development, which was likely limited to digits [28]. Here, our further investigation has revealed abnormalities in large joints, which become grossly apparent at five~six months. Our histological analysis has detected the disorganization of both ACL and PCL already in newborn joints. This is probably the cause of instability of the joint, leading to its destruction in later ages. In contrast, we found no apparent abnormalities in bone, joint capsules, and surrounding connective tissues. These results indicate that Vcan expressed in the jointed appendages generally plays an important role in joint formation and maintenance.
In the tendon and ligament cell lineage, scx, a transcription factor, is persistently expressed throughout differentiation and is known as a marker for the lineage [34]. Scx is observed in the primordia of the rotator cuff in the shoulder joint of E13.5 embryos and the collateral and anterior cruciate ligaments of the knee joints at E16.5 [33]. Analysis of double knockout mice of Scx and Sox9 has revealed that the Scx+ cell population can be subdivided into two distinct subpopulations of Scx+/Sox9+ and Scx+/Sox9– cells and that Sox9 expression later disappears from tendons and ligaments as differentiation proceeds [35]. These cells give rise to Scx–/Sox9+ chondrocytes and Scx+/Sox9– tenocytes/ligamentocytes. We have found a decrease in Scx+ chondrocytes in Prx1-Vcan joint cartilage, suggesting that it may lead to the impaired or delayed formation of tendons, ligaments, and cartilage. This is in agreement with the malformation of the ACL and PCL, and delayed cartilage development [28].
Vcan facilitates TGFβ-signaling by increasing the local concentration of TGFβ. TGFβ has dual effects on the development of cartilage and jointed appendages. Whereas TGFβ promotes chondrocyte differentiation in the early stages, it shifts the differentiation from chondrogenesis to fibrogenesis [36]. The local absence of Vcan may attenuate TGFβ-signaling, leading to a smaller progenitor population and less differentiation toward ligamentocytes. Whereas type I collagen was diffuse in the ligaments of Prx1-Vcan+/+ joints, it was immunostained in spotty patterns in those of Prx1-Vcan joints. Interestingly, type II collagen was immunostained in the ligaments of Prx1-Vcan joints, whereas it was not in the ligaments of Prx1-Vcan+/+ joints. The cruciate ligaments develop from cells that previously expressed Col2a1, which disappears in the joint interzone [37]. Our observations suggest that the chondrocytic cells expressing type II collagen remain in the ligaments of Prx1-Vcan joints, which may interfere with the organization of type I collagen fibers. Besides, local depletion of Vcan may affect them, as Vcan contributes to collagen fiber formation in developing dermis [38] and cancer stroma [39].
Vcan is expressed in ACL and the extra-articular medial collateral ligament in the normal adult canine joint [30]. Vcan is present among collagen fibrils and fibers, serving as a lubricant [40]. Although we could detect Vcan expression only in newborn joints, mechanical loading may induce Vcan expression in adult mice.
In humans, congenital absence of cruciate ligaments shows similar defects, and the patients acquire osteoarthritis at later ages [41, 42]. As Vcan complete knockout mice are embryonic lethal due to cardiac defects, Vcan is unlikely to be associated with this disease. Our study suggested that local TGFβ signaling is required for differentiation of Scx+/Sox9+ cells toward Scx+/Sox9- ligamentocytes. This congenital disorder may be associated with this process.
We thank H. Okitani for her technical assistance.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42