
Competing Interests: The authors have declared that no competing interests exist
- Altmetric
Adolescent psychostimulant abuse has been on the rise over the past decade. This trend has demonstrable ramifications on adolescent behavior and brain morphology, increasing risk for development of addiction during adolescence and in later adulthood. Neuroimmune substrates are implicated in the etiology of substance use disorders. To add to this body of work, the current study was developed to explore the role of a chemokine receptor, CXC Chemokine Receptor 4 (CXCR4), in the development of amphetamine (AMPH) sensitization. We targeted CXCR4 as it is implicated in developmental processes, dopaminergic transmission, neuroimmune responses, and the potentiation of psychostimulant abuse pathology. To evaluate the role of CXCR4 activity on the development of AMPH sensitization, a CXCR4 antagonist (Plerixafor; AMD3100) was administered to rats as a pretreatment variable. Specifically, adolescent Long Evans male rats (N = 37) were divided into four groups: (1) AMD3100 (IP, 4.0 mg/kg) + AMPH (IP, 4.0 mg/kg), (2) saline (SAL; 0.9% NaCl) + AMPH, (3) AMD3100 + SAL, and (4) SAL + SAL. Animals were first habituated to locomotor activity (LMA) chambers, then injected with a pretreatment drug (AMD3100 or SAL) followed by AMPH or SAL every other for four days. After a one-week withdrawal period, all animals were administered a low challenge dose of AMPH (IP, 1.0 mg/kg). AMPH-injected rats displayed significantly more locomotor activity compared to controls across all testing days. CXCR4 antagonism significantly attenuated AMPH-induced locomotor activity. On challenge day, AMD3100 pre-treated animals exhibited diminutive AMPH-induced locomotor activity compared to SAL pre-treated animals. Postmortem analyses of brain tissue revealed elevated CXCR4 protein levels in the striatum of all experimental groups. Our results implicate CXCR4 signaling in the development of AMPH sensitization and may represent an important therapeutic target for future research in psychostimulant abuse.
Introduction
Psychostimulant drug abuse during adolescence potentiates the risk for progression to drug dependence in adulthood for human [1, 2] and rodent [3, 4] populations. Psychostimulant abuse remains a pervasive and significant issue for adolescents that may rise within the next decade [5, 6]. Preclinical and clinical models implicate adolescence, compared to adulthood, as a vulnerable developmental period for the development of psychostimulant-induced neurobehavioral alterations [1, 7, 8, as reviewed by 9]. Psychiatric and substance use disorders can be explored in validated animal models [as reviewed by 10] to investigate the biological substrates that underlie psychostimulant abuse vulnerability during adolescence to further aid the development of viable pharmacotherapies.
A novel line of scientific inquiry operationalizes psychostimulants as activators of neuroimmune signaling mechanisms that affect neuroplasticity and drug-seeking behavior in preclinical models [as reviewed by 11], including adolescent paradigms [as reviewed by 12, 13]. Several rodent in vivo and in vitro models have shown that drug classes such as alcohol [14, 15] and opioids [16–19] enhance neuroinflammatory signaling through increased activity of chemoattractant cytokines (“chemokines”). CXC Chemokine Receptor 4 (CXCR4) is a G-protein coupled receptor that induces and propagates significant proinflammatory signaling cascades following activation by its only ligand, CXC Chemokine Ligand 12 (CXCL12, stromal derived factor 1; SDF-1) [20, as reviewed by 21]. CXCR4 was first discovered in vitro as a co-receptor for human immunodeficiency virus infection, and, when antagonized, prevents viral replication [22, 23]. In rodent and human neural in vitro [24] and in vivo models [25], CXCR4 may determine cell fate by activating secondary messengers like extracellular signal-regulated kinases 1/2 and Jun N-terminal kinase to reduce cyclic adenosine monophosphate and increase intracellular calcium levels. These activities ultimately lower the threshold for action potentials, altering neuronal and glial signaling. CXCR4 has been associated with the onset of several diseases such as Alzheimer’s and Parkinson’s diseases, chronic pain, and the development of various cancers [25, as reviewed by 26–31]. CXCR4 antagonism reduces disease symptomatology [32–35]. Similarly, CXCR4 antagonism has been shown to reduce the characteristic neurobehavioral patterns of dysregulation associated with the reinforcing effects of stimulant drugs [36]. Kim and colleagues [37] showed that CXCR4 antagonism prior to an acute administration of cocaine prevented increased locomotor activity and disrupted the cocaine-induced conditioned place preference. Clinical evidence from abstinent lifetime cocaine users implicates CXCL12-CXCR4 and other proinflammatory substrates as predictors of cocaine symptom severity and suggests the use of these substrates as biomarkers for the development of intervention protocols for psychostimulant use disorders and psychiatric comorbidities [38]. Furthermore, clinical and rodent models demonstrate anti-inflammatory agents as effective therapies for psychiatric and substance use disorders [as reviewed by 39, 40].
Although stimulants affect multiple monoamine systems, actions on dopamine receptors in human and rodent populations are critical [as reviewed by 41]. Psychostimulants like cocaine can cause long-term damage to the human and rodent brain by inducing severe neurotoxicity to cells, altering the overall rate of enzymatic activity, and disrupting dopamine release in neural regions associated with reward [42–44]. Dopaminergic neurons originate in the ventral tegmental area (VTA) and substantia nigra (SN) and project to the nucleus accumbens (NAc), striatum (caudate putamen/CPu in animal models), frontal cortex, and regions that are collectively defined within the limbic system [as reviewed in 45] and use dopamine and its receptors to communicate throughout the circuit in the mammalian brain [46, 47].
Modulation of dopaminergic activity in the mesocorticolimbic system is requisite for general psychostimulant seeking behavior and the reinforcing effects of psychostimulants in mammals [48–50]. We build upon this preclinical and clinical evidence and hypothesize that CXCR4 may play a role in psychostimulant-addiction like behavior. CXCR4 is expressed on the plasma membrane surface of dopaminergic neurons, microglia, and astrocytes in the mammalian brain [51, 52]. Psychostimulant exposure affects dopaminergic and neuroimmune substrates in the mammalian striatum [as reviewed by 53, 54]. Furthermore, rodent models report evidence that implicates dopamine receptor activity as a regulator of nervous system immune activity in other psychiatric and physiological pathologies [55, as reviewed in 56]. The CXCL12-CXCR4 axis interacts with neurons to influence synaptic pruning and growth in rodent and in vitro models [57, as reviewed in 58], neurotrophic factors in rodent and clinical models [59, 60], and rodent hippocampal neurogenesis [61].The hippocampus is of interest as dopamine transmission affects hippocampal inputs to the striatum in vitro [62] and goal-directed behavior in a rat model [63]. During rodent nervous system development, CXCR4 may influence adolescent response to stimulants since CXCR4 binding to CXCL12 and subsequent signaling activity induces progenitor glial cell migration to layers of the early cortex and the hippocampus in the initial organization of the brain [64–66] and cultured human neural precursor cells [67]. While many facets of CXCR4’s developmental role are unexplored, it has been shown that amygdalar CXCR4 expression remains upregulated in adulthood, following an initial adolescent exposure to cocaine [68]. Collectively, these data suggest the adolescent brain may be differentially affected by psychostimulant-induced alterations in neuroimmune signaling and thus, confers a persisting vulnerability that increases the risk for progression to addiction in adulthood.
It is, therefore, probable that CXCL12-CXCR4 dysregulation may be implicated in the effects of repeated psychostimulant use and arguably influences adolescent vulnerability to addiction-like behaviors. The present study examined the role of CXCR4 antagonism on the development of AMPH sensitization in a cohort of adolescent male Long Evans rats. We hypothesized that pretreatment with a CXCR4 receptor antagonist, the bicyclam drug AMD3100 (Plerixafor; 1,19-[1,4-phenylenebis(methylene)]-bis1,4,8,11-azatetradecane), would affect the development of AMPH-induced locomotor sensitization. Additionally, we measured CXCR4 protein levels using immunohistochemistry to determine if differences in striatal CXCR4 expression mapped onto differential AMPH-induced locomotor behaviors.
Materials and methods
Ethics
All animal experiments and listed protocols were conducted in accordance with guidelines established by and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Massachusetts Boston. Procedures detailed here also followed the applicable portions of the Animal Welfare Act and the National Institute of Health’s ‘Guide for the Care and Use of Laboratory Animals’ (NIH Publications No. 80–23; Revised 1996).
Experimental subjects
A total of 40 adolescent (purchased on postnatal day [PND] 32–34, 200–350 g) male Long Evans rats were ordered from Charles River Breeding Laboratories (Wilmington, MA, United States). A power analysis to determine the number of animals required to detect significant differences between groups, was calculated using G*Power software [69] and was based on past analyses performed in our laboratory [70, 71]. Statistical significance was set to p ≤ 0.05, and β = 0.80. After arrival, animals were housed in groups of 2–3 in ventilated cages (229.90 × 82.50 × 81.06 cm) (Lab Products; Seaford, DE, United States) with 3.85–4.00 cm of contact bedding. Animals were randomly sorted into experimental groups by cage (SAL-SAL, SAL-AMPH, AMD-SAL, AMD3100 (Plerixafor)-AMPH, n = 9–10 per group) and were maintained in a temperature- and humidity-controlled environment with food and water accessible ad libitum. Animals were maintained on a 12:12 h light/dark cycle, and lights were on from 0700 h to 1900 h. Testing did not begin until after animals were habituated to the facility for 10 days (PND 42–44). All testing was performed during the light cycle between 1200–1600 h.
Drug pretreatment and four-day amphetamine sensitization regimen
Every other day for four days, animals were brought into the testing room and allowed to habituate for 30 minutes. Subjects were weighed and then placed into one of four locomotor activity chambers. Locomotor activity (LMA) testing was performed as previously described in our laboratory [72]. LMA chambers were directly connected to a computer running MedAssociates locomotion tracking software (MedAssociates, St. Albans, VT, United States). Each commercial LMA chamber (dimensions: 17 × 17 × 12 cm) was equipped with photoelectric beams to record distance traveled, stereotypies, and rears when photobeam transmissions were broken by an animal subject. Stimulants are known to induce persistent locomotor movements that are classically considered as consequences of increased dopamine activation to the striatum. Distance traveled was defined by each rat’s individual gait and their movement from one point to another. Stereotypies were defined as head bobbing, twitching, licking, or biting that were captured as rapid movements between photobeams. Rearing behavior was defined as the animal raising up above typical height and breaking the vertical photobeam transmission. Animals were placed in the LMA chamber for 15 minutes prior to pretreatment to obtain a baseline measurement of typical LMA across each of these three dependent variables. After baseline observations, LMA recordings were paused and pretreatment injections were intraperitoneally (IP) administered as appropriate to group: isotonic saline (0.9% sterile isotonic saline/SAL) or AMD3100 (4.0 mg/kg, dissolved in saline; Plerixafor/CXCR4 antagonist; MedChem Express, Monmouth Junction, NJ, United States). Animals were then returned to their respective chamber and the LMA tracking software was resumed. At the 30 min time point, recording software was paused again and animals were injected with either SAL or AMPH 4.0 mg/kg D-amphetamine sulfate; Sigma, St. Louis, MO, United States). Following drug injections, animals were returned for a final 60 min recording for a total of 90 min for the testing period. Between testing periods, LMA data were extracted and the LMA chambers were cleaned between each set of experimental subjects with mild soap and water. This was repeated every 48 h for four days after which all animals were given a 7-day withdrawal period. Finally, animals were challenged with a low dose of AMPH (1 mg/kg, IP), sacrificed, and brain tissue was harvested and stored at -80°C for later histological analyses.
Challenge dose
One week following the termination of the four-day AMPH sensitization regimen, all animals were retrieved from their cages, weighed, and brought to the LMA testing room where they were habituated to one of four LMA chambers for 30 min. Animals were injected with a challenge dose of AMPH (IP, 1.0 mg/kg) and tested for an additional 60 min, for a total of 90 min. Data were extracted from MedAssociatesTM tracking software after testing completion. Animals were sacrificed via live decapitation and brain tissue was immediately harvested and stored in -80°C for later immunohistochemical analyses. The comprehensive timeline for this experiment can be seen in Fig 1.

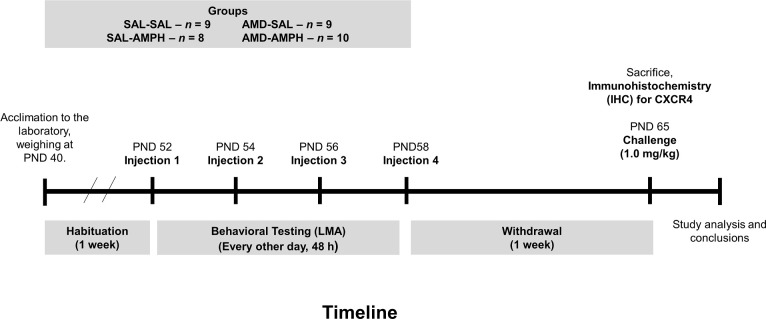
Timeline that depicts for the experimental design and procedures used in the current study.
Animals were habituated to the laboratory setting for one week prior to the start of testing. Following this, animals were placed in the LMA chambers for a 15 min habituation, and then given a pretreatment (SAL, 0.9% NaCl, IP) or (AMD,4.0 mg/kg, IP). This was followed by drug treatment, (SAL or AMPH 4.0 mg/kg, IP) at 30 min, every 48 h for four days. One week after the final day of testing, which was the withdrawal period, all animals were challenged with a low dose of AMPH (1.0 mg/kg, IP: timepoint 30 min) after a 30 min habituation in the LMA.
Euthanasia and brain extraction
Following the final AMPH challenge and subsequent LMA assessment, animals were removed from the LMA chamber and prepped for sacrifice. Animals were placed inside decapicones (Braintree Scientific, Braintree, MA; United States) and were promptly sacrificed via guillotine. Upon decapitation, brains were extracted and snap frozen on dry ice and stored at -80°C in a freezer until microsectioning was performed for later immunohistochemical analyses.
Immunohistochemistry for CXCR4
At the time of histology, brains were removed from the -80°C freezer, embedded in freezing O.C.T. compound (mounting medium), and adhered to a metal chuck for frozen (-20°C) microsectioning in a cryostat (Leica CM 3050S; Leica Biosystems, Welzlar, Germany). Brains were blocked and 30-μm coronal sections were taken to target the dorsal striatum. Next, sections were mounted on frosted glass sides (Fisherbrand Superfrost Plus; ThermoScientific, Waltham, MA, United States). Anatomical assessment and location of the caudate putamen was determined using a rat brain atlas [73]. The slides were thoroughly covered and post-fixed in 2 mL of 4% paraformaldehyde for 30 min. Slides were rinsed, and then cryoprotected by dousing in incremental sucrose-paraformaldehyde solutions (5% - 20%). Next, slides were gently agitated in 0.05M NaPBS repeatedly for 1 h, with the NaPBS solution changed every 20 min. Following this, slides were rinsed in H2O2. A mixture of 1.5% normal goat serum-NaPBS to block endogenous peroxidases, and after a 5 min NaPBS rinse, slides were incubated overnight at 4°C in primary anti-CXCR4 antibody (1:1000 in Triton-X and NaPBS; Abcam, Boston, MA; United States; Abcam Antibody Code ab2074).
On the next day, the slides were removed from the refrigerator and then rinsed in NaPBS and incubated in goat-anti-rabbit secondary antibody (1:600 in Triton-X and NaPBS; ABC Elite Kit, Vector Labs, Burlingame, CA, USA) at room temperature for 1 h. Following this, sections were rinsed again in NaPBS and then processed through an avidin-biotin complex (ABC) method (Vectastain ABC HRP kit; Denver, CO, United States) and processed in Trizma-based buffer (Sigma Aldrich; Natick, MA). Finally, the sections were stained with a 3,3’-diaminobenzidine (DAB) horseradish peroxidase (HRP) substrate kit (Vectastain ABC HRP kit; Denver, CO, United States) for 5 min, at which time the slides were again rinsed in Tris buffer to terminate the reaction. The slides were left to dry overnight under a ventilated fumehood with cover to protect from dust artifacts. The next day, the slides were rehydrated with increasing concentrations of ethanol and xylene, and then cover slipped with Permount for microscopy and subsequent image analysis. Negative controls were run following the exact protocol outlined above except for incubation in the primary antibody.
Image analysis
Digital images of the stained slides were taken using light microscopy (Olympus BX-40; Pennsylvania, United States) fitted with a monochrome Scion Image camera and software (4× and 10× magnification). Magnification at 4× was used to confirm impregnation of neuronal and glial cells for all animals relative to the negative controls. Higher magnification images were then used for counting positive CXCR4 immunoreactive cells. Counts were made using CellTargetTM with a threshold set to count the number of CXCR4 positive cells. When imaged at 4× magnification, experimenters blind to group representation were guided to count all the CXCR4 positive neurons visible in the dorsolateral, dorsomedial, and dorsoventral striatum. When imaged at 10× magnification, experimenters utilized the quadrant function of CellTargetTM [74] to maintain consistent cell counts from sample to sample.
Statistical analysis
All statistical analyses were completed using SPSS software (Windows and Mac, version 22.0). Data were first analyzed for normality using the Kolmogorov-Smirnov Test. To identify significant differences between groups, multivariate two-way analyses with repeated measures of variance (ANOVAs) were used. For behavioral data, three independent 2 (pretreatment) X 2 (drug treatment) repeated measures (Day and testing time block) mixed factor ANOVAs were employed to evaluate main and interaction effects across the independent variables for (1) distance traveled (2) rearing behavior and (3) stereotypies. To support these findings, we averaged total distance traveled (cm), stereotypies, or rears made by each subject across the four days and performed a univariate ANOVA on the average cumulative recorded movements for each behavioral measure for each subject. To identify AMPH-induced locomotor activation following a low-dose AMPH (IP, 1 mg/kg) challenge after a one-week withdrawal period, 2 (pretreatment) X 2 (drug treatment) mixed factors were utilized to identify main and interaction effects for each dependent measure. All behavioral data were reported as group means ± SEM. For neural data, a 2 (pretreatment) X 2 (drug treatment) mixed factor ANOVA was employed to evaluate main and interaction effects for CXCR4 positive cells in the dorsal striatum. Three experimenters who were blinded to experimental conditions and showed 0.90 inter-rater reliability quantified immunohistochemistry data. Immunohistochemistry data are reported as group means ± SEM. All significant findings were interpreted using Bonferroni correction and Tukey’s Honest Significant Difference (HSD) post-hoc tests.
Results
Three subjects were excluded from analyses due to low weight gain, data loss, and, in the case of one animal, escape from the LMA chamber (N = 37, n = 8–10 subjects per group). There were no significant differences in weight in the remaining subjects when measured (S1 Fig). We analyzed locomotor behavior (distance traveled, rears, and stereotypies) extracted from MedAssociates LMA software using a 2 (pretreatment) X 2 (drug treatment) mixed factors ANOVA f with repeated measures for Day and testing time period.
Data were analyzed for normality using the Kolmogorov-Smirnov test of normality. On the first [D(19) = 0.260; p = 0.001], second [D(19) = 0.237, p = 0.006], and fourth day of testing [D(19) = 0.289, p = 0.0001], the data violated normality assumptions. Data from animals in the antagonist group also violated the test for normality on the first and [D(19) = 0.302, p = 0.0001] and fourth [D(19) = 0.203, p = 0.039] days of testing. We elected to retain the data for analysis due to (1) retention of normality in the habituation period on all four days of sensitization testing, (2) positive skewness towards zero as a result of low distance traveled and related movements, and (3) no more than two outliers becoming apparent per group.
AMD3100 reduces AMPH-induced distance traveled, stereotypies, and rears
Distance traveled
In the analyses performed, we observed no significant differences for Day [F3,31 = 1.562, p = 0.218] or Time Period [F1,33 = 0.553, p = 0.462] alone on distance traveled. All animals moved around the locomotor activity chamber over the progression of testing, and this movement increased each day [Day × Time Period, F3,31 = 7.424, p = 0.001 < 0.05, ηp2 = 0.418]. There were no significant interaction effects for Day × Time Period × Pretreatment [F3,31 = 2.268, p = 0.100 > 0.05] or Day × Time Period × Pretreatment × Treatment [F3,31 = 1.229, p = 0.316 > 0.05]. Pretreatment with AMD3100 alone did not produce any change in rat behavior as indicated by non-significant results for Day × Pretreatment [F3,31 = 1.692, p > 0.05].
The two-way ANOVA did reveal significant interaction effects of Day × Treatment [F3, 31 = 4.917, p = 0.007 < 0.05, ηp2 = 0.322] and Day × Pretreatment × Treatment [F3,31 = 4.046, p = 0.015 < 0.05, ηp2 = 0.281]. Rats in the SAL-AMPH group traveled further than their drug-naïve counterparts in the SAL-SAL group. Additionally, AMD3100-AMPH animals traveled less than SAL-AMPH animals overall, especially on the first day of testing. Furthermore, AMD3100-AMPH animals exhibited significantly greater LMA activity compared to SAL-SAL and AMD-SAL animals (Fig 2). We supplemented our data analysis by calculating the percent change (distance traveled from 30 to 90 min minus distance traveled from 0–30 min) for each subject across the four main days of testing to account for general individual differences and treatment effects. We observed a significant effect of Treatment for Day 1 [F1,32 = 39.501, p < 0.001, ηp2 = 0.552], Day 2 [F1,32 = 43.030, p < 0.001, ηp2 = 0.574], Day 3 [F1,32 = 14.738, p < 0.001, ηp2 = 0.315], and Day 4 [F1,32 = 16.237, p < 0.001, ηp2 = 0.337]. These findings indicated that AMPH treatment caused a significant increase in the distance traveled for adolescent rats regardless of pretreatment (Fig 3).

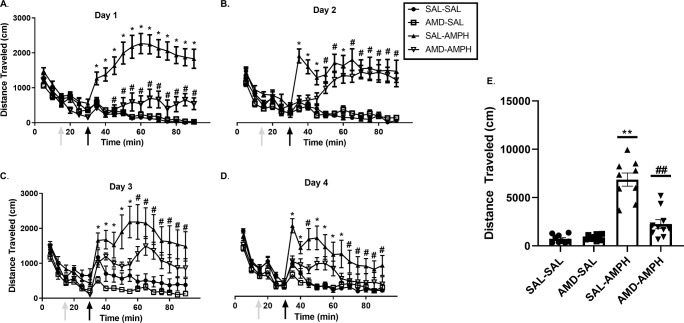
The locomotor effects of repeated pretreatment and treatment schedule on all experimental groups.

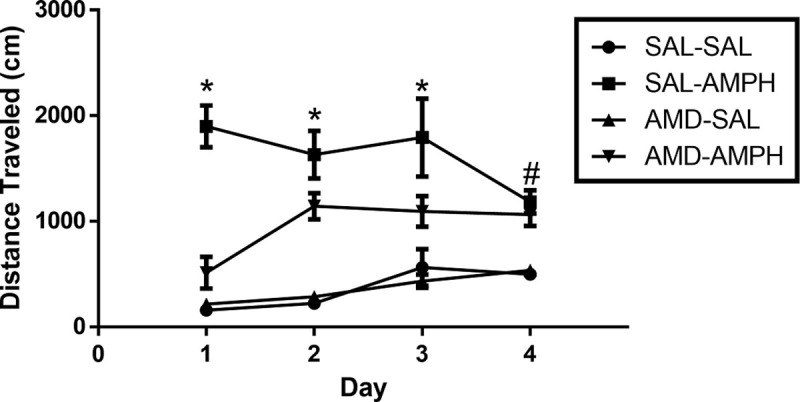
The effect of pretreatment and treatment conditions on average cumulative distance traveled (cm) across the four days of testing and on challenge.
Locomotor activity was measured for 90 min (data represented as Mean ± SEM). Arrows indicate an initial 0–15 min habituation period to the LMA chamber, followed by (1) the pretreatment injection period 15–30 min, (2) the post-injection 30–90 min period on (A) Day 1, (B) Day 2, (C) Day 3, and (D) Day 4. Single asterisks (*) represent a significant difference compared to all other groups (p < 0.05), and single hash marks (#) represent a significant differences compared to SAL-SAL and AMD-SAL groups (p < 0.05). (E) Data are presented as a scatterplot, with bars indicating average distance traveled (cm) in the Treatment testing period (30–90 minutes) ± SEM. Double asterisks (**) and double hash marks (##) represent significant differences between labeled groups and all other groups (p < 0.01).
Effect of pretreatment and drug treatment on the average distance traveled (cm) across each of the 4 days of testing (Mean ± SEM). Asterisks (*) represent significant differences (p < 0.05) as compared to other groups, and a hash mark (#) represents significant differences (p < 0.05) collapsed between AMD-AMPH and SAL-AMPH groups as compared to the following control groups, SAL-SAL and AMD-SAL.
Stereotypies
We report significant main and interaction effects of Day [F3,31 = 6.716, p = 0.001 < 0.05, ηp2 = 0.394], Day × Time Period [F3,31 = 14.688, p = 0.0001 < 0.05, ηp2 = 0.587] and Day × Time Period × Pretreatment [F3,31 = 3.151, p = 0.039 < 0.05, ηp2 = 0.234] for stereotypies. There was also a significant interaction effect of Time Period × Pretreatment × Treatment [F1,33 = 4.947, p = 0.033 < 0.05, ηp2 = 0.130], demonstrating the combined effect of repeated testing on overall stereotypies (Fig 4). Although total stereotypies made over the four individual days were not significantly different for Pretreatment × Treatment, [F1,132 = 1.608, p = 0.207 > 0.05, n.s.], there was a significant interaction effect of Day × Pretreatment × Treatment [F3,132 = 3.272, p = 0.023 < 0.05, ηp2 = 0.069]. SAL-AMPH treated rats engaged in significantly more stereotypies across the total 4 days as compared to all other groups, where AMD-AMPH animals exhibited diminished amounts of stereotypies compared to SAL-AMPH animals on Day 1.

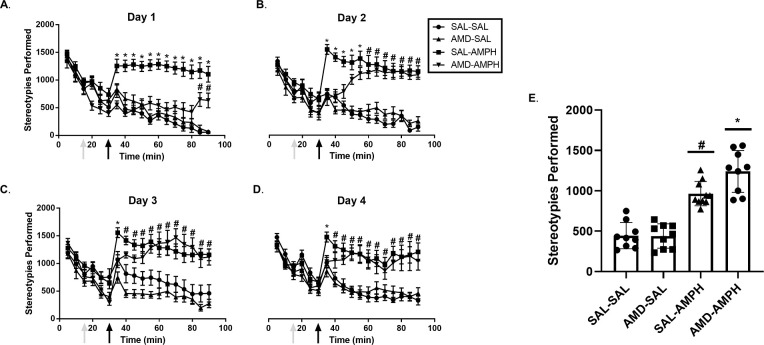
Average stereotypies performed following pretreatment and treatment conditions over the four-day period.
Average stereotypies over the 4-day period following Pretreatment (AMD or SAL, grey arrows at time point 15 min) and Treatment (SAL or AMPH, black arrows at time point 30 min) conditions on (A) Day 1, (B) Day 2, (C) Day 3, and (D) Day 4. After observing main and interaction effects, post-hoc analyses identified significant pairwise groups differences. Asterisks (*) represent significant differences between the SAL-AMPH relative to all other groups. Hash marks (#) represent significant differences between the AMD-AMPH and all other groups. (E) The data are represented as a scatterplot of the cumulative average of stereotypies completed by each group over the four days of testing (Mean ± SEM).
Rears
There were no significant group differences for rears that were identified in the analysis of the habituation period (p < 0.05) on each day of the 4-day sensitization regimen. For all other testing periods, data analysis revealed that SAL-AMPH animals reared significantly more than all other groups across all four days of testing, and this was confirmed through a Tukey HSD test with significance set at p < 0.05. Furthermore, AMD3100-AMPH animals were not significantly different from SAL-SAL or AMD3100-SAL groups in terms of cumulative rears on Day 1 of testing (p < 0.05). In the remaining experimental days, multivariate analysis revealed a significant effect of Day × Pretreatment [F3,31 = 9.808, p = 0.002 < 0.05, ηp2 = 0.371] as well as an interaction effect of Day × Pretreatment × Treatment [F3,31 = 7.624, p = 0.001 < 0.05, ηp2 = 0.425]. Tukey HSD post-hoc test indicated that SAL-AMPH animals reared the most as indicated by an interaction of Day × Treatment effect [F8,26 = 9.493, p = 0.0001 < 0.05, ηp2 = 0.723], and AMD3100-AMPH animals reared significantly more than SAL-SAL and AMD3100-SAL groups but significantly less than the SAL-AMPH group (p < 0.05) (Fig 5).

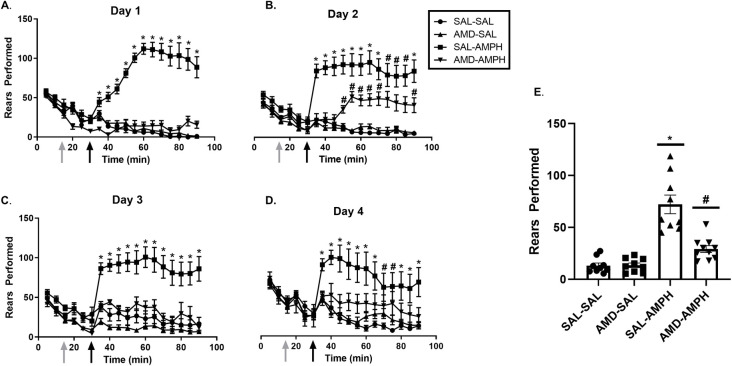
The effects of the treatment conditions on rears performed over the four days of testing.
Data are represented as Mean ± SEM. Cumulative average vertical counts (Mean ± SEM) performed over the four -day (A-D) testing period. (E) The mean of rears for each group over the four days of testing. Asterisks (*) represent significant differences between the SAL-AMPH group and all other groups (p < 0.05), and hash marks (#) indicate significant differences between the AMD-AMPH and SAL-SAL, AMD-SAL, and SAL-AMPH groups (p < 0.05).
AMD3100 pretreatment reduces AMPH sensitization following a one-week drug-free period
After a one week drug withdrawal period from behavioral experimentation, all animals were challenged with a low dose of AMPH (1.0 mg/kg, IP) to test the expression of AMPH sensitization. Two-way ANOVA analysis revealed significant main effects of pretreatment [F2,32 = 5.237, p = 0.01 < 0.05, ηp2 = 0.247] and treatment [F2,33 = 54.263, p = 0.001 < 0.05, ηp2 = 0.772] on total distance traveled. We also report a significant interaction effect of Pretreatment × Treatment [F2,32 = 4.133, p = 0.025 < 0.05, ηp2 = 0.205] (Fig 6A). A Tukey post-hoc HSD test (p < 0.05) revealed that animals pretreated with AMD3100 exhibited locomotor responses to a low dose AMPH challenge (IP, 1mg/kg) similarly to that of SAL pretreated animals. However, the AMD3100-AMPH group traveled significantly less in response to low dose AMPH challenge compared to SAL-AMPH animals. Past pretreatment with AMD3100 or SAL did not have an effect on overall stereotypies (Baseline: F[2,33 = 0.037, p = 0.848 > 0.05]; Treatment: F[2,33 = 4.077, p = 0.052 > 0.05]). We performed a Bonferroni correction on these data and again found that Pretreatment did not reach significance (p = 0.65 > 0.05). A significant main effect of Treatment [F2,33 = 143.452, p = 0.001 < 0.05, ηp2 = 0.813] and an interaction effect of Pretreatment × Treatment [F2,33 = 5.001, p = 0.032 < 0.05, ηp2 = 0.132] were found in our analyses. This indicated that AMD3100-AMPH and SAL-AMPH groups exhibited near-equivalent numbers of stereotypies in response to a low dose AMPH challenge (Fig 6B).

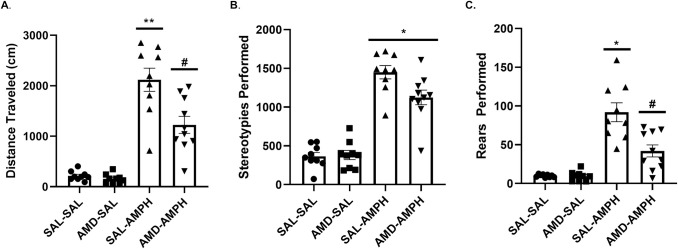
Distance traveled, stereotypies, and rears observed after a 1.0 mg/kg challenge dose of AMPH one week following the four-day AMPH sensitization regimen.
Data are represented as Mean (A) distance traveled (B) stereotypies, and (C) rears performed ± SEM. Asterisks (*) represent statistically significant differences set at p < 0.05 as compared to AMD-SAL and SAL-SAL groups, double asterisks represent statistically significant differences at p < 0.01, and hash marks (#) represent statistically significant differences between AMD-AMPH and all other groups at p < 0.05.
Behavioral rearing responses for the four groups were similar to the responses we observed for distance traveled. We found a significant main effect of Pretreatment [F2,33 = 5.730, p = 0.007 < 0.05, ηp2 = 0.205] as well as an interaction effect of Pretreatment × Treatment [F2,32 = 6.231, p = 0.005 < 0.05, ηp2 = 0.280]. We determined this to mean that the AMD-AMPH group showed significantly reduced rears relative to the SAL-AMPH group, but potentiated activity as compared to controls (SAL-SAL, AMD-SAL groups) that had never been treated with AMPH in the past. Animals in the control conditions (SAL-SAL, AMD-SAL) reared significantly less than AMD-AMPH and SAL-AMPH animals (p < 0.05), as identified through a Tukey HSD post-hoc test (Fig 6C).
CXCR4 protein expression is upregulated following repeated AMPH exposure
We investigated changes in dorsal striatal CXCR4 receptor protein levels following repeated SAL or AMPH exposure with or without AMD3100 pretreatment. Brain sections ranging from Bregma +0.20 mm to +0.70 mm were taken from n = 16 animals. Representative images of CXCR4 staining are shown in Fig 7A and 7B). Pearson’s product-moment correlation coefficient assessed inter-rater reliability at 4× magnification [r = 0.693, p = 0.03] and 10× magnification [r = 0.697, p = 0.001]. Counts at 20× magnification were also correlated between independent researchers [r = 0.679, p = 0.05].

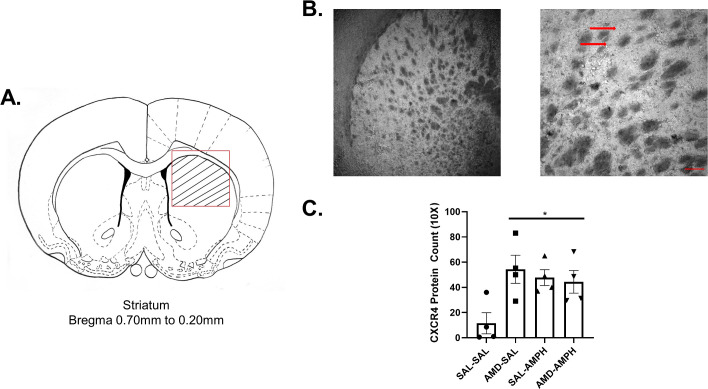
Repeated administration of the CXCR4 antagonist AMD3100 and D-amphetamine sulfate increased CXCR4 protein levels in the dorsomedial striatum.
Striatal neurons and glia were visualized with Nickel-DAB after immunostaining for CXCR4, viewed, and imaged on a light microscope (N = 16, n = 4 per group). (A) Cells were counted specifically within the striated targeted region outlined in red on the line drawing, delineating the dorsal striatum. Bregma +0.70 mm to + 0.20 mm ranges were selected based on rat brain atlas coordinates [73]. (B) Sample staining at 4× magnification and 10× magnification. The scale bar on the bottom right represents ~240 pixels (154.8 um). The red arrows identify deeply stained striatal puncta and cellular processes. (C) All groups expressed CXCR4 within the dorsal striatum. These numbers were elevated across experimental groups relative to the control (data represented as Mean ± SEM), asterisks (*) represent significant differences set at p<0.05.
CXCR4 positive cell counts between all independent researchers were averaged for all subject tissues. A 2 (pretreatment) X 2 (drug treatment) mixed factor ANOVA revealed a significant main effect of Pretreatment [F3,12 = 6.774, p = 0.023 < 0.05]. Repeated exposure to AMD3100 pretreatment upregulated the number of CXCR4 positive cells and clusters or puncta in the dorsal striatum of AMD3100-AMPH and AMD3100-SAL animals (Fig 7C).
Discussion
The current study evaluated the role of CXCR4 signaling in the development of AMPH-induced locomotor sensitization in adolescent male Long Evans rats. We antagonized CXCR4 receptor protein prior to repeated AMPH treatment to determine if it would interfere with the development of AMPH sensitization. Our findings indicate that pretreatment with the CXCR4 antagonist, AMD3100, interferes with the development of AMPH-induced locomotor sensitization and attenuates the sensitized AMPH response to a low dose challenge following a one-week drug withdrawal period. Accordingly, CXCR4 protein levels in the dorsomedial striatum were significantly elevated in response to repeated AMPH treatment for both levels of the pretreatment independent variable (AMD3100 and SAL), directly linking the effects of repeated AMPH exposure to dorsal striatal neuroimmune function in vivo.
We have previously shown that a low to moderate dose of amphetamine (3.0 mg/kg) is sufficient to induce sensitization and neuronal GABAergic alterations in a sexually dimorphic manner, where control and ovariectomized females were more locomotive and hyperactive than males [75]. We adapted this behavioral sensitization model for the current experiment and found that AMD3100 pretreatment significantly reduced total distance traveled and rears, but not stereotypies, in adolescent male AMPH-treated rats. All AMPH-treated rats displayed an upregulation of CXCR4-immunopositive cells in the dorsomedial striatum with no differences for animals pretreated with AMD3100. Taken together, the data implicate CXCL12-CXCR4 activity in the development of behavioral sensitization to repeated AMPH administration. The present experimental data does not allow for a causal link between neuroimmune data and behavioral outcomes. However, due to the timing of our AMPH sensitization model it is possible that we missed acute effects of AMD3100 pretreatment on the neuroimmune axis. Thus, at the time of measurement we were unable to distinguish any differences in CXCR4 expression between the pretreatment groups. Further work is required to determine if CXCR4 and CXCL12 mRNA and protein levels vary as a function of day(s) of AMPH- or AMD3100-treatment across neural regions in the dopaminergic mesocorticolimbic circuit.
Chemokines are emerging as critical feedback and regulatory system messengers in response to the use of psychostimulant drugs. As chemokines become better elucidated in the literature, there is an emerging body of evidence that highlights the role of chemokine signaling in neuronal communication and thus, could be necessary for the development and expression of responses to psychostimulant drugs. For example, human in vitro models [76] implicate CC Chemokine Receptor 5 (CCR5) mechanisms in the cellular response to methamphetamine, while silencing genes for and blocking CC Chemokine Receptor 2 (CCR2) and CCR5 elicit an attenuation in conditioned place preference and locomotor behavior to methamphetamine or cocaine in rodent in vivo models [77–79]. We have likewise demonstrated that systemic CXCR4 antagonism attenuates AMPH-induced hyperlocomotion in adolescent male Long Evans rats. Indeed, CXCR4 is evidenced to be expressed throughout the rodent adolescent [51] and adult brains [80, 81]. Furthermore, chronic amphetamine exposure amplifies the excitability of dopaminergic and glutamatergic neurons in the rodent prefrontal cortex [82, 83], which is one of the last areas of the mammalian brain to fully mature structurally and functionally [84–90]. As a critical developmental period, adolescence confers a lasting vulnerability to structural and functional neurobehavioral changes in response to environmental challenges [as reviewed by 91] in human adolescents as compared to adults [92]. Additionally, in a cohort of male and female rats, it was found that the timing of repeated amphetamine exposure centered around the onset of puberty determined later susceptibility to anxiety-like behavior and modulated dopamine D1 receptor activity levels in the ventral striatum during AMPH withdrawal periods [93]. Similarly, psychostimulant-induced striatal fos expression is dependent upon dopamine D1 and D2 receptor activation in the rat brain [94]. The current study reports that CXCR4 antagonism during the development of AMPH-sensitization had a significant effect on the expression of AMPH-induced hyperlocomotion and significantly upregulated the expression of striatal CXCR4 protein expression following a terminal AMPH challenge. CXCR4 protein expression was also significantly upregulated in all AMPH-treated adolescent animals as compared to SAL-SAL controls, providing in vivo evidence of AMPH-induced neuroimmune alterations in the adolescent rat striatum. While the current study did not examine dopamine receptor expression in conjunction with striatal CXCR4 expression, future work should aim to further delineate the role of CXCL12-CXCR4 signaling in relation to dopamine receptor activity following repeated adolescent AMPH exposure.
Future work should also address sex differences, since modifications in impulsivity [95], vigilance [96], cognitive flexibility [97], memory [98], and an enhanced sensitivity to the effects of psychostimulants in adulthood are reported [as reviewed by 99 and 100] highlighting risk factors for the progression to addiction. Biological females are considered more sensitive to the effects of psychostimulants than males, and often exhibit exaggerated behavioral responses to psychostimulants that are regulated by circulating female hormone levels [101, as reviewed by 102]. In our future research, we intend to incorporate female animals to examine the relation between adolescence and sex as a biological variables in the development and expression of AMPH sensitization.
The decreased effectiveness of AMD3100 in our AMD-AMPH group over the four days of testing could represent the characteristic neuromolecular plasticity associated with adolescence and synaptogenesis within the CXCL12-CXCR4 axis. Accordingly, it is not surprising that AMD3100 pretreatment had no effect on the development of sensitized AMPH induced stereotypies. In their 1997 review, Pierce and Kalivas argue that repeated administration to psychostimulants can induce differential sensitization of ambulatory and stereotyped behavior [103]. Furthermore, exposure to stress and subsequent glucocorticoid receptor activation alters rodent locomotor behavioral sensitization in response to repeated psychostimulant administration [104, 105]. Adolescence also modulates sensitized behavioral responses to psychostimulant exposure in rodents, as a single exposure to a high dose of cocaine differentially induced behavioral sensitization to a challenge dose [106]. Taken together, the discrepancy in sensitized locomotor responses between ambulatory and stereotyped behaviors may be attributed to (1) rodent experimental differences in sensitized locomotor responses to repeated psychostimulant administration, (2) natural differences in trait-anxiety phenotypes and subsequent activation of the hypothalamic-pituitary-adrenal (HPA) axis, and (3) the adolescent developmental period conferring differentiated responses to repeated amphetamine administration in rodents. Thus, it is possible that these mechanisms are unique to the adolescent brain. The development and reorganization of the adolescent brain require greater immune and nutrient availability for growth that could be shifted by psychostimulant experience. The refinement and modification of neuronal and glial systems in the prefrontal cortex and throughout the rest of the brain, is aided in part by chemokines [107, as reviewed by 108]. The CXCL12-CXCR4 axis has specific functions to aid progenitor cell migration, proliferation, and axonal pathfinding in the neonatal period [66, 109–111]. Although CXCR4 levels decrease two weeks following the neonatal period, when they have reached their temporary maximal peak [112], we report evidence that repeated exposure to a moderate dose of AMPH significantly increased adolescent CXCR4 expression in the dorsomedial striatum. We, therefore, hypothesize that CXCR4 is recruited in the presence of repeated AMPH exposure to increase signaling, synaptic plasticity, and neuronal activation throughout the adolescent brain.
We also observed that AMD3100-SAL treated rats exhibited a significant increase in CXCR4 protein levels comparable to that of AMD3100-AMPH and SAL-AMPH treated rats. CXCR4 is downregulated in cortical circuit neurons from the early postnatal period throughout adolescence and into adulthood in mammals [66, as reviewed by 113]. In the present study, the observed changes in CXCR4 protein expression may be a compensatory attempt by the adolescent neuroimmune microenvironment to correct cell positioning and function after the neurotoxic effects of repeated AMD3100 and AMPH exposure on cell activity. Furthermore, it is also likely that given AMD3100 affects other signaling mechanisms, their recruitment may serve to augment CXCR4 protein levels. Another possibility is that any suppressive effects of AMD3100 on striatal CXCR4 expression may be acute and, therefore, our one-week withdrawal period is too long to detect transient changes that may have occurred during the four-day AMPH sensitization regimen. Future experiments should be designed to delineate these theoretical possibilities. Moreover, differential CXCR4 protein expression is evidenced in other psychiatric disorders [114] and physiological system dysregulation [115, 116] as well. Our findings demonstrate that striatal CXCR4 receptors are upregulated in response to repeated AMPH exposure and CXCR4 antagonism and these changes are linked to CXCL12-CXCR4 axis alterations observed in other diseased/disordered states.
Although we did not extend our findings to additional dopaminergic mesocorticolimbic regions that regulate psychostimulant addiction-like behaviors, there is an emerging body of literature that implicates other dopaminergic region alterations in chemokine activity and relates this to psychostimulant exposure related rodent behaviors [37, 77, 117–120]. For example, exogenous CXCL12 administration prior to cocaine injection into the nucleus accumbens shell has inhibitory effects on rat activity [120]. Furthermore, CXCL12 signaling mediates the migratory process and maintenance of in vitro and in vivo rodent dopaminergic neurons within the VTA [121] and CXCL12 protein levels are required for executive function and inhibitory gating within the mPFC [122, 123]. Thus, chemokine activity is implicated in psychostimulant-related behaviors throughout the dopaminergic mesocorticolimbic pathway. Future research should center on further elucidating the role of various chemokines systems in the mesocorticolimbic pathway on the rewarding and psychomotor-activating effects of psychostimulants.
One potential direction that could clarify the depth of CXCR4 activity on the development and maintenance of AMPH sensitization would be to better understand the genetic and epigenetic properties of CXCR4. In the present study, increased expression of striatal CXCR4 protein could be indicative of compensatory mechanisms for intracellular regulation, such as heterologous dimerization with CXCR7 or the production of upregulated proinflammatory signaling factors like nuclear factor kappa beta (NF-κB), toll-like receptor 4 (TLR4), and Protein kinase B that follow G-protein coupled receptor activation [96, 124, 125]. A future direction for our animal model would be to assess and map genetic and epigenetic substrates within these proinflammatory pathways to psychostimulant-induced behavioral outcomes. For example, repeated methamphetamine exposure upregulates mouse striatal CCR2 mRNA and increases expression of the epigenetic marker histone H3 lysine 4 (H3K4) trimethylation at the CCR2 promoter region [79]. Additionally, in a mouse model, paternal sire cocaine use affected filial 1 offspring’s drug preference along with significant chemokine (and other systems associated with psychostimulant exposure and neurodevelopment) and gene expression changes in the ventral striatum [126]. Drug-induced behavioral and epigenetic alterations to chemokine systems within the rodent mesocorticolimbic pathway extends to other drugs of abuse as well, such as morphine [127]. Taken together, genetic and epigenetic markers map onto psychostimulant exposure and chemokine activity in the rodent brain and this relation should be further explored in future work.
The present study demonstrates that CXCR4 antagonism with AMD3100 is sufficient to modify the development of amphetamine sensitization in a male adolescent rat model. We show here that dorsomedial striatal CXCR4 protein levels are enhanced by repeated AMPH exposure and CXCR4 antagonism. These findings further implicate the CXCL12-CXCR4 axis in the development of amphetamine sensitization and encourages additional experimental research to examine adolescent psychostimulant vulnerabilities and/or therapeutic development involving this neuroimmune axis.
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
 CXCR4 inhibition with AMD3100 attenuates amphetamine induced locomotor activity in adolescent Long Evans male rats
CXCR4 inhibition with AMD3100 attenuates amphetamine induced locomotor activity in adolescent Long Evans male rats
