
Competing Interests: The authors have declared that no competing interests exist.
Cognitive dysfunction after surgery under general anesthesia is a well-recognized clinical phenomenon in the elderly. Physiological effects of various anesthetic agents have been studied at length. Very little is known about potential effects of anesthesia on brain structure. In this study we used Diffusion Tensor Imaging to compare the white matter microstructure of healthy control subjects under sevoflurane anesthesia with their awake state. Fractional Anisotropy, a white mater integrity index, transiently decreases throughout the brain during sevoflurane anesthesia and then returns back to baseline. Other DTI metrics such as mean diffusivity, axial diffusivity and radial diffusivity were increased under sevoflurane anesthesia. Although DTI metrics are age dependent, the transient changes due to sevoflurane were independent of age and sex. Volumetric analysis shows various white matter volumes decreased whereas some gray matter volumes increased during sevoflurane anesthesia. These results suggest that sevoflurane anesthesia has a significant, but transient, effect on white matter microstructure. In spite of the transient effects of sevoflurane anesthesia there were no measurable effects on brain white matter as determined by the DTI metrics at 2 days and 7 days following anesthesia. The role of white matter in the loss of consciousness under anesthesia will need to be studied and MRI studies with subjects under anesthesia will need to take these results into account.
Cognitive dysfunction in elderly patients after they undergo surgery under general anesthesia is a well-known phenomenon, but it is unclear whether this is caused by the surgical procedures or the anesthesia. Little is known about how general anesthesia causes a reduction in nerve transmission and subsequent loss of consciousness and the effects of general anesthesia on brain structure has not been studied before. Some neuroimaging studies necessitate the use of anesthesia. Examples are patients that cannot stay still long enough to complete an imaging protocol, certain pediatric populations, patients on ventilators, patients with claustrophobia or other complicating factors [1–3]. Another situation where patients are scanned under anesthesia is in intraoperative MRI procedures [4]. We collected these data in the context of the TORIE (Trajectory of Recovery in the Elderly) study [5] which was designed to investigate the recovery of cognitive function after general anesthesia without surgery in healthy adults 40–80 years old, spanning the age range of individuals that are at elevated risk of postoperative neurocognitive disorders including Postoperative Delirium (PD) and Postoperative Cognitive Dysfunction (POCD) [6, 7]. In this study we sought to analyze the effects of general anesthesia on brain white matter.
This analysis is part of a larger study on the effects of anesthesia on cognitive function in the elderly in the absence of surgery (Trajectory of Recovery in the Elderly [TORIE] (Trajectory of Recovery in the Elderly), NIH 1R01AG046634, clinicaltrials.gov registration NCT 2275026). The full protocol for this study is published [5]. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (New York, NY, USA; IRB@mssm.edu, 212-824-8200). Participants were recruited through local contacts and IRB-approved advertisements in local media and online. Potential participants were pre-screened by telephone by both research staff and a study anesthesiologist. Informed written consent was obtained by participants at the first in-person visit. Specific inclusion criteria were adults aged 40–80, American Society of Anesthesiologists (ASA) Physical Status 1 (no medical comorbidities) or 2 (one or more medical comorbidities which do not impact the patient’s function), and no underlying cognitive dysfunction as determined from baseline cognitive testing before general anesthesia. Exclusion criteria included contraindication to MRI scanning (implanted metal, presence of tattoos, claustrophobia), current smoking, use of illicit drugs, excessive use of alcohol, or other diseases that could affect response to anesthesia or alter brain physiology. Participants were excluded after recruitment and consent if the scan prior to anesthesia revealed any of the following: cerebral microvascular disease, any mass, evidence of old infarct (even without clinical signs), atrophy and/or ventriculomegaly greater than expected for age in the neuroradiologist’s judgment. Age-appropriate changes, such as mild cortical atrophy, were not grounds for exclusion. Participants were also excluded if baseline neuropsychological testing suggested poor or abnormal baseline cognitive function in the judgment of the study neuropsychologist. Sixty-eight healthy participants were analyzed in this study with an average age of 58.6 years (30F/38M). Although not used in the current analysis, participants received a battery of cognitive tests including the Postoperative Quality of Recovery Scale (PQRS) and NIH Toolbox Cognitive Battery to assess executive function, attention, episodic memory, working memory and processing speed.
In the TORIE imaging protocol scans were acquired at 5 different time points: before induction of anesthesia (PRE), twice (at 40 mins (A1) and 100 mins (A2) post induction) during a 2 hour general anesthesia at a depth of one age-adjusted MAC (minimum alveolar concentration) of sevoflurane, 1 day post anesthesia (D1) and 7 days post anesthesia (D7). All scans were performed on a Siemens 3T Skyra using a 32 channel head coil. Sequences included Dual Echo TSE (PD-T2), GE-EPI BOLD N-Back, GE-EPI ME-BOLD Resting State, Diffusion Tensor Imaging (DTI), T1 3D MP-RAGE, T2/FLAIR, pulsed arterial spin labeling (PASL), and Susceptibility Weighted Imaging (SWI). For this study only the DTI and anatomical T1’s were analyzed. T1 MP-RAGE was performed with the following protocol: T1-weighted anatomical images will be acquired with an MPRAGE sequence (FOV 256 × 256 × 176 mm, 0.8 mm isotropic resolution, TR/TE/TI = 2400/3.2/1000ms, bandwidth 280 Hz/Pixel, echo spacing 7.6 ms, in-plane acceleration factor 2, and total acquisition time ~ 7 min). DTI: diffusion MRI data were acquired with a MB accelerated single shot spin echo EPI sequence (FOV 208 × 176 mm, matrix 114 × 96, slice thickness 1.8 mm, 72 slices for whole brain coverage, TR/TE = 3650/85 ms, Stejskal-Tanner (i.e., monopolar) diffusion encoding with diffusion Gmax ~ 43mT/m, phase partial Fourier 6/8, MB factor 3, blipped CAIPIRINHA phase-encoding shift = FOV/3, bandwidth ~ 1700 Hz/Pixel, echo spacing ~0.7 ms, diffusion encoding directions 64 with 4 non-diffusion weighted (i.e., b0) images, b value 1250 s/mm2, total acquisition time ~ 12 min, with two phase-encoding direction reversed averages to correct eddy current distortion and improve signal-to-noise ratio (SNR).
The first day of imaging included the pre-anesthesia scan and the scans during anesthesia. Anesthesia staff performed a preanesthesia evaluation and confirmed the participant’s eligibility. The imaging suite has a complete anesthesia setup, including an MRI compatible anesthesia machine and a set of vital sign monitors (blood pressure, ECG, oximetry, end-tidal CO2 and gas analysis). Following the pre-anesthesia scans, the MRI bed was moved out of the scanner and the anesthesia induced using propofol (approximately 2 mg/kg) followed by the insertion of a laryngeal mask airway (LMA). Anesthesia was maintained using sevoflurane. Anesthetic depth during the transition between propofol and sevoflurane was adjusted to a bispectral index between 40 and 60 using a Bispectral Index Monitor (Covidien, MA, USA) which was removed for subsequent scanning. Participants resumed spontaneous respiration under anesthesia. This induction procedure lasted about 15 minutes. The participant was then moved back into the scanner and imaged under anesthesia for about 2 hours. After the last scan, the subject emerged from anesthesia and the LMA was removed. After it was determined that they were sufficiently conscious to perform a functional task, which took about 15 minutes on average, the scanner bed was moved back into the scanner bore for the post anesthesia scans. Participants were reassessed using the PQRS battery and transported to a postanesthesia care unit (PACU). Follow up scans without anesthesia was performed on the next day and again 7 days later.
In this analysis we focused on diffusion tensor metrics. Diffusion Tensor Images are eddy-current-corrected and fractional anisotropy (FA), Radial Diffusivity (RD), Axial Diffusivity (AD) as well as mean diffusivity maps (MD) were calculated using FSL (www.fmrib.ox.ac.uk/fsl). Exploratory whole brain group comparisons of the diffusion parameters are performed. FA (and other DTI metrics) images were spatially normalized to the ICBM template using Tract Based Spatial Statistics (TBSS) [8]. The procedure involves a skeletonization of the FA images to obtain centers of white matter tracts. Voxel-wise statistics are performed only on the white matter skeleton in order to reduce the chance of type I errors due to imperfections in normalization. The parameters used to warp the FA images to ICBM template to the white matter skeleton were applied to the MD, RD and AD images for statistical comparisons. Randomise is an FSL routine for permutation based inference testing that is used for voxel-wise general linear modeling to test for differences between the conditions. Clusters are identified using the TFCE (Threshold-Free Cluster Enhancement) that is optimized for permutation (n = 5000) based inference testing of skeletonized images [9]. Age and sex were used as covariates. We separated into 5 conditions per subject: 1 before anesthesia induction, 2 during anesthesia (A1 (at approximately 40 mins), A2 (at approximately 100 mins)), 1 at 1 day follow up and 1 at 7 day follow up. Whole brain as well as individual tracts’ DTI metrics were computed using the Johns Hopkins University ICBM-DTI-81 white matter atlas: mean values were calculated based on the average voxel intensities averaged over all the regions of interests of the atlas. Statistical analysis of individual tracts was performed using Statistica V13 (Statsoft Inc., Tulsa, OK).
T1-MPRAGE images were processed through Freesurfer. Cortical reconstruction and volumetric segmentation was performed using the standard recon-all pipeline of the Freesurfer image analysis software (version 5.3.0), which is documented and freely available online (http://surfer.nmr.mgh.harvard.edu). Regions of interest (ROIs) were labeled using an automatic labeling system [10]. Processing included motion correction, removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical regions and deep gray matter structures intensity normalization, tessellation of the gray matter-white matter boundary, automated topology correction, and surface deformation following intensity gradients. Gray matter volume, white matter volume, and cortical thickness measures were computed.
All voxel by voxel wise statistics were performed using FSL as described above. ROI and whole brain based analysis were performed using Statistica V13. All time point comparisons were performed using a paired t-test and corrected for age and sex. Pearson correlation analyses were performed between whole brain DTI metrics and age. Multiple linear regression was used to test for effect of age and sex on the change in DTI metrics.
Whole brain voxel-based analyses using TBSS showed that Fractional Anisotropy (FA) was decreased throughout the brain during anesthesia when compared to the awake state before anesthesia. On the other hand, MD, RD and AD were all increased during anesthesia (Fig 1). Although AD had slightly fewer significant voxels than RD. Statistical analysis on whole brain white matter DTI metrics showed that FA was decreased while MD, AD and RD were increased during anesthesia when compared to time points before or after the administration of anesthesia. In addition, FA was lower and MD, AD and RD were higher at 100 min than at 40 min post administration of anesthesia. There were no differences between pre-anesthesia and day 1 or day 7 post anesthesia (Fig 2). These significances were corrected for age and sex. Table 1 shows the magnitude of the white matter mean values and the percent changes between the time points.

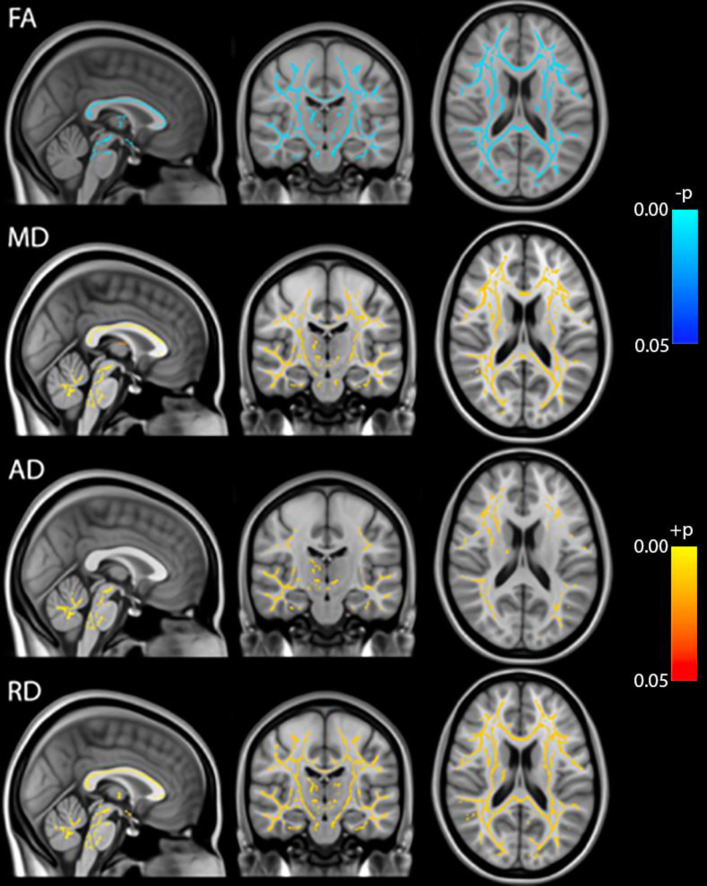
TBSS analysis of DTI metrics (FA, MD, AD, RD).
Blue: Pre-anesthesia > during anesthesia, Red: Pre-anesthesia < during anesthesia, p<0.05 FWE-corrected.

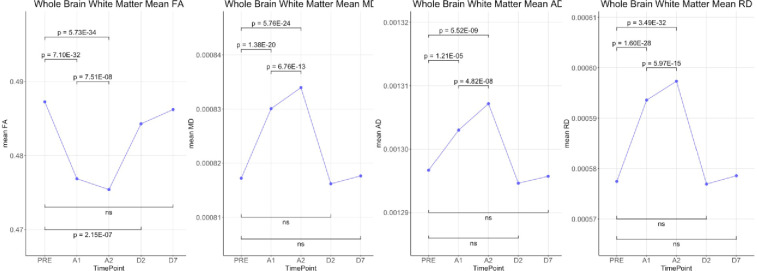
Statistical comparisons of whole brain means of DTI metrics (FA, MD, AD and RD) over the 5 time-points.

| Time point | FA | MD (mm2/s) | AD (mm2/s) | RD (mm2/s) |
|---|---|---|---|---|
| PRE | 0.487 | 0.000817 | 0.001297 | 0.000577 |
| A1 | 0.477 | 0.000830 | 0.001303 | 0.000594 |
| A2 | 0.475 | 0.000834 | 0.001307 | 0.000597 |
| D2 | 0.484 | 0.000816 | 0.001295 | 0.000577 |
| D7 | 0.486 | 0.000818 | 0.001296 | 0.000579 |
| % change (A1-Pre) | -2.13 * | 1.57 * | 0.49 * | 2.79 * |
| % change (A2-Pre) | -2.43 * | 2.05 * | 0.81 * | 3.44 * |
| % change (A2-A1) | -0.31 * | 0.47 * | 0.32 * | 0.63 * |
| % change (D2-Pre) | -0.61 * | -0.13 | -0.16 | -0.09 |
| % change (D7-pre) | -0.21 | 0.05 | -0.07 | 0.19 |
At baseline, linear correlation analysis showed that whole brain white matter FA had a significant negative correlation with age (p = 1.09e-5, R-sqr = 0.256) while MD (p = 4.07e-9, R-sqr = 0.410), AD (p = 5.781e-8, R-sqr = 0.362) and RD (p = 6.269e-9, R-sqr = 0.403) were positively correlated (Fig 3A). To visualize age differences we split up the data into 4 age groups [40–49 (n = 19), 50–59 (n = 18), 60–69 (n = 13), and 70–80 (n = 18)] as per the main protocol [5]. Group means of the DTI metrics are shown in Fig 3B. Multiple linear regression was used to investigate the relationship between change in FA, calculated as the value at 40 minutes after drug administration minus the baseline value, with age and sex. Baseline FA was included as a covariate in the model. Adjusting for sex and baseline FA, there was no significant association between change in FA and age (β = 0.000003; 95% CI: -.000098 to 0.000104; p = 0.95). Similarly, there was no significant association between change in FA and sex (β = -0.000178; 95% CI: -.002184 to 0.001827; p = 0.86) after controlling for age and baseline FA. No significance associations were found between changes in MD, AD and RD and age or sex (Tables 2 and 3).

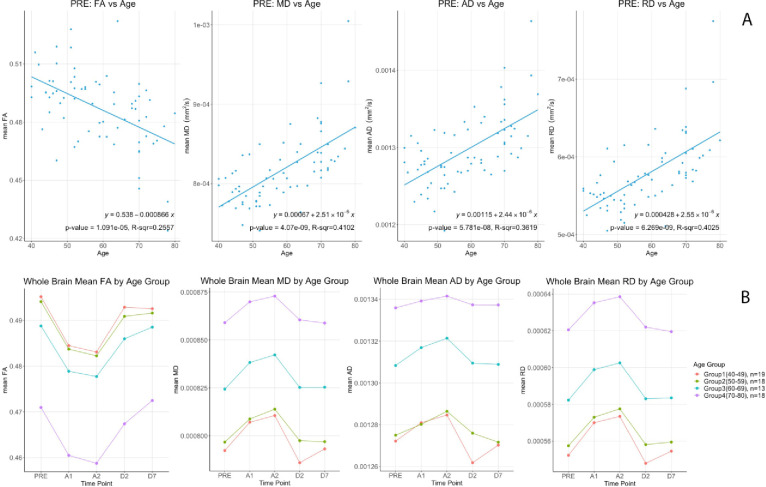
Top (A) DTI metrics (FA, MD, AD & RD) correlations versus age at baseline (PRE). Bottom (B) Differential changes of (FA, MD, AD & RD) by age groups for the 5 time points.

| Sex | N | Mean | Std Dev | Median | 25th Pctl | 75th Pctl | |
|---|---|---|---|---|---|---|---|
| FA | F | 30 | -0.011 | 0.0044 | -0.0108 | -0.0133 | -0.0083 |
| M | 38 | -0.010 | 0.0037 | -0.0098 | -0.0133 | -0.0072 | |
| MD | F | 30 | 0.000012 | 0.000008 | 0.000011 | 0.000007 | 0.000016 |
| M | 38 | 0.000014 | 0.000008 | 0.000012 | 0.000009 | 0.000018 | |
| AD | F | 30 | 0.000004 | 0.000011 | 0.000005 | -0.000003 | 0.000011 |
| M | 38 | 0.000008 | 0.000011 | 0.000006 | 0.000002 | 0.000013 | |
| RD | F | 30 | 0.000015 | 0.000007 | 0.000015 | 0.000012 | 0.000019 |
| M | 38 | 0.000017 | 0.000007 | 0.000015 | 0.000012 | 0.000021 |

| Parameter | Estimated β | Lower 95% CI | Upper 95% CI | P-Value | |
|---|---|---|---|---|---|
| FA | Age | 3.00E-06 | -9.80E-05 | 1.04E-04 | 0.950 |
| Sex F | -1.78E-04 | -2.18E-03 | 1.83E-03 | 0.860 | |
| PRE | -1.868E-02 | -7.87E-02 | 4.13E-02 | 0.536 | |
| MD | Age | -6.23E-08 | -2.87E-07 | 1.62E-07 | 0.582 |
| Sex F | -2.56E-06 | -6.56E-06 | 1.43E-06 | 0.205 | |
| PRE | -1.79E-02 | -7.65E-02 | 4.07E-02 | 0.544 | |
| AD | Age | 2.50E-08 | -2.66E-07 | 3.16E-07 | 0.864 |
| Sex F | -4.90E-06 | -1.02E-05 | 4.42E-07 | 0.072 | |
| PRE | -6.88E-02 | -1.42E-01 | 4.27E-03 | 0.065 | |
| RD | Age | -6.23E-08 | -2.87E-07 | 1.62E-07 | 0.582 |
| Sex F | -2.56E-06 | -6.56E-06 | 1.43E-06 | 0.205 | |
| PRE | -1.79E-02 | -7.65E-02 | 4.07E-02 | 0.544 |
There were no significant differences with sex or age.
The 48 ROIs from the Johns Hopkins white matter atlas showed the same significances and directions except for two regions near the inferior cerebellar peduncles (not significant). The other 46 white matter tracts all had significances between p<10−5 and p<10−31 after correction for age and sex (Table 4).

| Pre-A1 | Pre-A2 | Pre-D2 | Pre-D7 | A1-A2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region of Interest | tstat | p-value | diff | tstat | p-value | diff | tstat | p-value | diff | tstat | p-value | diff | tstat | p-value | diff |
| Middle.cerebellar.peduncle | -9.1262 | 2.2756E-13 | -0.0254 | -8.6354 | 1.7256E-12 | -0.0238 | -1.9269 | 5.8233E-02 | -0.0052 | 4.5911 | 1.9956E-05 | 0.0146 | 3.5393 | 7.3457E-04 | 0.0016 |
| Pontine.crossing.tract..a.part.of.MCP. | 12.6599 | 1.9486E-19 | 0.0343 | 10.4572 | 1.0161E-15 | 0.0308 | -8.1996 | 1.0507E-11 | -0.0259 | 1.9632 | 5.3778E-02 | 0.0069 | -3.2719 | 1.6905E-03 | -0.0035 |
| Genu.of.corpus.callosum | 17.7870 | 4.4639E-27 | 0.0104 | 21.3089 | 1.4919E-31 | 0.0123 | 0.0940 | 9.2542E-01 | 0.0001 | 0.6360 | 5.2694E-01 | 0.0007 | 5.4411 | 8.0685E-07 | 0.0018 |
| Body.of.corpus.callosum | 12.7469 | 1.4063E-19 | 0.0110 | 15.8670 | 2.2095E-24 | 0.0137 | 1.0212 | 3.1082E-01 | 0.0009 | 0.8763 | 3.8399E-01 | 0.0011 | 7.0589 | 1.1884E-09 | 0.0027 |
| Splenium.of.corpus.callosum | 17.0967 | 3.9487E-26 | 0.0126 | 19.4728 | 2.7299E-29 | 0.0150 | -0.5829 | 5.6191E-01 | -0.0005 | 0.5594 | 5.7773E-01 | 0.0009 | 5.8760 | 1.4556E-07 | 0.0024 |
| Fornix..column.and.body.of.fornix. | 7.8372 | 4.7315E-11 | 0.0116 | 9.2470 | 1.3848E-13 | 0.0133 | 2.5501 | 1.3064E-02 | 0.0034 | 2.3444 | 2.2030E-02 | 0.0040 | 1.7258 | 8.8995E-02 | 0.0016 |
| Corticospinal.tract.R | 13.8332 | 2.5963E-21 | 0.0426 | 16.3608 | 4.2941E-25 | 0.0478 | -8.6303 | 1.7626E-12 | -0.0334 | -3.7032 | 4.3277E-04 | -0.0166 | 2.5474 | 1.3155E-02 | 0.0051 |
| Corticospinal.tract.L | 15.9815 | 1.5071E-24 | 0.0440 | 18.9881 | 1.1446E-28 | 0.0515 | -9.1014 | 2.5198E-13 | -0.0322 | -1.3301 | 1.8798E-01 | -0.0078 | 4.6550 | 1.5799E-05 | 0.0074 |
| Medial.lemniscus.R | 19.0499 | 9.5194E-29 | 0.0683 | 17.6236 | 7.4421E-27 | 0.0659 | -5.2352 | 1.7879E-06 | -0.0155 | -1.6530 | 1.0301E-01 | -0.0124 | -2.2362 | 2.8670E-02 | -0.0024 |
| Medial.lemniscus.L | 20.9969 | 3.5303E-31 | 0.0718 | 20.1507 | 3.8325E-30 | 0.0699 | -5.1350 | 2.6237E-06 | -0.0191 | -2.5014 | 1.4821E-02 | -0.0212 | -1.6779 | 9.8024E-02 | -0.0019 |
| Inferior.cerebellar.peduncle.R | -3.7803 | 3.3590E-04 | -0.0133 | -1.8634 | 6.6796E-02 | -0.0065 | -6.0035 | 8.7465E-08 | -0.0214 | 2.6971 | 8.8395E-03 | 0.0121 | 5.0176 | 4.0964E-06 | 0.0068 |
| Inferior.cerebellar.peduncle.L | -0.7856 | 4.3488E-01 | -0.0030 | 0.2453 | 8.0696E-01 | 0.0010 | 1.2767 | 2.0611E-01 | 0.0032 | 18.0103 | 2.2311E-27 | 0.0773 | 2.5496 | 1.3080E-02 | 0.0040 |
| Superior.cerebellar.peduncle.R | 11.5231 | 1.5057E-17 | 0.0256 | 12.0462 | 1.9976E-18 | 0.0280 | 7.7841 | 5.8978E-11 | 0.0143 | -0.1618 | 8.7194E-01 | -0.0009 | 2.8598 | 5.6466E-03 | 0.0024 |
| Superior.cerebellar.peduncle.L | 13.6635 | 4.7967E-21 | 0.0300 | 14.6504 | 1.4238E-22 | 0.0322 | 14.6385 | 1.4840E-22 | 0.0232 | -4.5292 | 2.4985E-05 | -0.0225 | 2.5270 | 1.3871E-02 | 0.0022 |
| Cerebral.peduncle.R | 11.0236 | 1.0672E-16 | 0.0121 | 11.7606 | 5.9940E-18 | 0.0130 | 0.1044 | 9.1713E-01 | 0.0001 | 1.2102 | 2.3044E-01 | 0.0033 | 1.5221 | 1.3268E-01 | 0.0009 |
| Cerebral.peduncle.L | 10.8710 | 1.9523E-16 | 0.0111 | 11.6737 | 8.3912E-18 | 0.0117 | 0.7856 | 4.3488E-01 | 0.0009 | 0.7420 | 4.6068E-01 | 0.0028 | 0.8793 | 3.8236E-01 | 0.0005 |
| Anterior.limb.of.internal.capsule.R | 12.9157 | 7.4885E-20 | 0.0108 | 15.2070 | 2.0683E-23 | 0.0129 | 0.0139 | 9.8899E-01 | 0.0000 | 0.6467 | 5.2002E-01 | 0.0008 | 5.2958 | 1.4162E-06 | 0.0020 |
| Anterior.limb.of.internal.capsule.L | 14.3838 | 3.6361E-22 | 0.0106 | 14.9851 | 4.4420E-23 | 0.0117 | 1.1154 | 2.6867E-01 | 0.0009 | 1.1441 | 2.5664E-01 | 0.0013 | 2.7409 | 7.8484E-03 | 0.0011 |
| Posterior.limb.of.internal.capsule.R | 16.6291 | 1.7851E-25 | 0.0166 | 17.6387 | 7.0962E-27 | 0.0183 | 0.6917 | 4.9154E-01 | 0.0007 | 1.1328 | 2.6134E-01 | 0.0018 | 4.1879 | 8.4044E-05 | 0.0016 |
| Posterior.limb.of.internal.capsule.L | 16.1854 | 7.6569E-25 | 0.0123 | 17.7376 | 5.2084E-27 | 0.0140 | 0.0262 | 9.7919E-01 | 0.0000 | 0.3456 | 7.3075E-01 | 0.0005 | 4.1415 | 9.8774E-05 | 0.0017 |
| Retrolenticular.part.of.internal.capsule.R | 16.3333 | 4.7003E-25 | 0.0179 | 16.7208 | 1.3255E-25 | 0.0193 | 1.3283 | 1.8859E-01 | 0.0018 | 0.1624 | 8.7151E-01 | 0.0003 | 2.9207 | 4.7542E-03 | 0.0014 |
| Retrolenticular.part.of.internal.capsule.L | 7.7812 | 5.9700E-11 | 0.0121 | 8.6751 | 1.4643E-12 | 0.0130 | -0.3730 | 7.1029E-01 | -0.0004 | 0.2945 | 7.6932E-01 | 0.0007 | 1.5365 | 1.2911E-01 | 0.0009 |
| Anterior.corona.radiata.R | 15.2749 | 1.6392E-23 | 0.0090 | 15.0101 | 4.0749E-23 | 0.0100 | 1.0346 | 3.0460E-01 | 0.0008 | 0.9016 | 3.7048E-01 | 0.0009 | 3.1669 | 2.3199E-03 | 0.0011 |
| Anterior.corona.radiata.L | 11.9238 | 3.1954E-18 | 0.0071 | 11.9041 | 3.4473E-18 | 0.0079 | 0.6193 | 5.3781E-01 | 0.0004 | 0.9847 | 3.2829E-01 | 0.0011 | 2.6944 | 8.9046E-03 | 0.0008 |
| Superior.corona.radiata.R | 16.8404 | 8.9987E-26 | 0.0114 | 18.0527 | 1.9572E-27 | 0.0124 | 0.3992 | 6.9102E-01 | 0.0003 | 0.7596 | 4.5014E-01 | 0.0008 | 3.1914 | 2.1558E-03 | 0.0011 |
| Superior.corona.radiata.L | 17.6042 | 7.9083E-27 | 0.0108 | 18.9524 | 1.2733E-28 | 0.0124 | 0.6374 | 5.2602E-01 | 0.0004 | 0.5880 | 5.5849E-01 | 0.0006 | 5.1001 | 2.9963E-06 | 0.0015 |
| Posterior.corona.radiata.R | 16.5913 | 2.0189E-25 | 0.0117 | 19.5151 | 2.4115E-29 | 0.0133 | 1.2844 | 2.0342E-01 | 0.0010 | 0.9371 | 3.5205E-01 | 0.0012 | 4.2280 | 7.3048E-05 | 0.0016 |
| Posterior.corona.radiata.L | 17.6404 | 7.0593E-27 | 0.0102 | 18.3170 | 8.6882E-28 | 0.0114 | 0.2206 | 8.2609E-01 | 0.0001 | 0.7944 | 4.2977E-01 | 0.0010 | 3.6439 | 5.2492E-04 | 0.0012 |
| Posterior.thalamic.radiation.R | 15.7533 | 3.2359E-24 | 0.0122 | 18.0177 | 2.1811E-27 | 0.0140 | 1.5005 | 1.3819E-01 | 0.0014 | 0.2541 | 8.0017E-01 | 0.0004 | 5.0556 | 3.5486E-06 | 0.0017 |
| Posterior.thalamic.radiation.L | 11.8964 | 3.5500E-18 | 0.0099 | 15.2176 | 1.9948E-23 | 0.0121 | -0.0493 | 9.6079E-01 | 0.0000 | 1.1020 | 2.7442E-01 | 0.0013 | 6.4184 | 1.6396E-08 | 0.0021 |
| Sagittal.stratum.R | 11.2313 | 4.7109E-17 | 0.0119 | 12.5739 | 2.6923E-19 | 0.0139 | 1.6721 | 9.9176E-02 | 0.0018 | 0.7756 | 4.4069E-01 | 0.0016 | 3.2109 | 2.0333E-03 | 0.0020 |
| Sagittal.stratum.L | 10.2353 | 2.4787E-15 | 0.0081 | 11.0947 | 8.0635E-17 | 0.0089 | 1.2304 | 2.2285E-01 | 0.0012 | 1.5945 | 1.1554E-01 | 0.0032 | 1.4310 | 1.5707E-01 | 0.0007 |
| External.capsule.R | 14.4599 | 2.7800E-22 | 0.0092 | 12.9036 | 7.8343E-20 | 0.0096 | 0.9711 | 3.3498E-01 | 0.0009 | 1.2101 | 2.3048E-01 | 0.0013 | 1.3466 | 1.8265E-01 | 0.0004 |
| External.capsule.L | 11.7365 | 6.5787E-18 | 0.0080 | 12.5250 | 3.2373E-19 | 0.0079 | 1.0025 | 3.1971E-01 | 0.0006 | 0.9206 | 3.6058E-01 | 0.0013 | -0.3574 | 7.2195E-01 | -0.0001 |
| Cingulum..cingulate.gyrus..R | 9.9238 | 8.7333E-15 | 0.0091 | 10.8180 | 2.4090E-16 | 0.0110 | 1.9750 | 5.2395E-02 | 0.0018 | 0.7865 | 4.3434E-01 | 0.0010 | 3.0003 | 3.7849E-03 | 0.0019 |
| Cingulum..cingulate.gyrus..L | 10.1565 | 3.4056E-15 | 0.0081 | 11.5322 | 1.4532E-17 | 0.0095 | -0.8958 | 3.7356E-01 | -0.0007 | 0.3275 | 7.4430E-01 | 0.0005 | 2.7051 | 8.6496E-03 | 0.0013 |
| Cingulum..hippocampus..R | 8.0776 | 1.7435E-11 | 0.0093 | 8.1270 | 1.4202E-11 | 0.0108 | 2.3744 | 2.0447E-02 | 0.0030 | 1.9577 | 5.4438E-02 | 0.0045 | 1.6322 | 1.0734E-01 | 0.0014 |
| Cingulum..hippocampus..L | 4.5659 | 2.1869E-05 | 0.0060 | 6.9327 | 1.9985E-09 | 0.0084 | 0.5085 | 6.1277E-01 | 0.0007 | 0.9029 | 3.6983E-01 | 0.0024 | 3.7963 | 3.1858E-04 | 0.0024 |
| Fornix..cres. . . .Stria.terminalis.R | 8.7587 | 1.0362E-12 | 0.0106 | 9.9927 | 6.6056E-15 | 0.0129 | 1.4098 | 1.6322E-01 | 0.0018 | 1.7206 | 8.9935E-02 | 0.0032 | 3.1427 | 2.4934E-03 | 0.0023 |
| Fornix..cres. . . .Stria.terminalis.L | 6.0362 | 7.6740E-08 | 0.0088 | 6.6659 | 5.9734E-09 | 0.0099 | 1.3253 | 1.8957E-01 | 0.0023 | 0.9384 | 3.5142E-01 | 0.0023 | 1.4670 | 1.4704E-01 | 0.0011 |
| Superior.longitudinal.fasciculus.R | 18.3319 | 8.3032E-28 | 0.0115 | 19.0502 | 9.5112E-29 | 0.0122 | 1.6419 | 1.0529E-01 | 0.0011 | 0.8852 | 3.7920E-01 | 0.0010 | 1.9507 | 5.5277E-02 | 0.0007 |
| Superior.longitudinal.fasciculus.L | 12.2758 | 8.3158E-19 | 0.0087 | 16.2023 | 7.2406E-25 | 0.0103 | -0.7031 | 4.8442E-01 | -0.0004 | 0.2406 | 8.1060E-01 | 0.0003 | 4.2880 | 5.9167E-05 | 0.0016 |
| Superior.fronto.occipital.fasciculus.R | 8.1819 | 1.1310E-11 | 0.0082 | 9.4445 | 6.1617E-14 | 0.0100 | -1.3281 | 1.8865E-01 | -0.0013 | -1.0899 | 2.7964E-01 | -0.0013 | 2.4779 | 1.5741E-02 | 0.0018 |
| Superior.fronto.occipital.fasciculus.L | 4.7591 | 1.0770E-05 | 0.0060 | 6.0560 | 7.0888E-08 | 0.0079 | -0.5052 | 6.1506E-01 | -0.0006 | -0.1136 | 9.0990E-01 | -0.0002 | 2.0901 | 4.0405E-02 | 0.0019 |
| Uncinate.fasciculus.R | 5.7290 | 2.6077E-07 | 0.0081 | 6.2181 | 3.6914E-08 | 0.0085 | -0.2483 | 8.0469E-01 | -0.0003 | 0.8655 | 3.8987E-01 | 0.0023 | 0.4105 | 6.8276E-01 | 0.0004 |
| Uncinate.fasciculus.L | 5.6344 | 3.7876E-07 | 0.0072 | 6.0927 | 6.1155E-08 | 0.0077 | 0.2388 | 8.1196E-01 | 0.0003 | 0.8173 | 4.1667E-01 | 0.0021 | 0.5464 | 5.8660E-01 | 0.0005 |
| Tapetum.R | 11.3724 | 2.7106E-17 | 0.0103 | 12.3854 | 5.4867E-19 | 0.0108 | 1.1157 | 2.6854E-01 | 0.0008 | 1.1200 | 2.6673E-01 | 0.0016 | 0.8858 | 3.7889E-01 | 0.0005 |
| Tapetum.L | 6.4521 | 1.4297E-08 | 0.0060 | 9.4271 | 6.6155E-14 | 0.0084 | -0.0606 | 9.5183E-01 | -0.0001 | 1.4514 | 1.5134E-01 | 0.0013 | 2.9667 | 4.1699E-03 | 0.0025 |
Pre: Before anesthesia, A1: 40 mins after administration of anesthesia, A2: 100 mins after administration of anesthesia, D2: 1 day after anesthesia, D7: 7 days after anesthesia. Significant differences highlighted in bold.
Analysis of the structural MRI (T1-MPRage) using Freesurfer showed that both left and right hemispheric white matter volumes were smaller during anesthesia (p<0.018 and p<0.008 respectively). Out of the 68 white matter regions extracted, 21 were smaller and 2 (left and right entorhinal cortex) were larger during anesthesia. Out of the 68 gray matter regions, 42 regions were larger under anesthesia while 2 (left and right frontal poles) were smaller (Table 5).

| White Matter Regions | tstat | p-value | diff | %change | Gray Matter Regions | tstat | p-value | diff | %change |
|---|---|---|---|---|---|---|---|---|---|
| wm.lh.bankssts | 0.081 | 9.354E-01 | 2.162 | -0.073 | bankssts | 5.353 | 1.140E-06 | 80.088 | -3.285 |
| wm.lh.caudalanteriorcingulate | -0.297 | 7.676E-01 | -8.876 | 0.345 | caudalanteriorcingulate | 0.222 | 8.247E-01 | 3.706 | -0.228 |
| wm.lh.caudalmiddlefrontal | -3.229 | 1.926E-03 | -205.525 | 3.204 | caudalmiddlefrontal | 0.235 | 8.149E-01 | 17.868 | -0.302 |
| wm.lh.cuneus | -3.091 | 2.909E-03 | -68.284 | 2.620 | cuneus | 1.447 | 1.525E-01 | 30.941 | -0.975 |
| wm.lh.entorhinal | 2.168 | 3.375E-02 | 30.219 | -3.479 | entorhinal | 2.609 | 1.120E-02 | 74.794 | -3.742 |
| wm.lh.fusiform | 0.302 | 7.635E-01 | 12.413 | -0.198 | fusiform | 5.594 | 4.430E-07 | 333.029 | -3.422 |
| wm.lh.inferiorparietal | -1.668 | 9.998E-02 | -123.734 | 1.266 | inferiorparietal | 6.209 | 3.830E-08 | 388.603 | -3.160 |
| wm.lh.inferiortemporal | 0.017 | 9.867E-01 | 0.634 | -0.010 | inferiortemporal | 5.264 | 1.600E-06 | 387.750 | -3.759 |
| wm.lh.isthmuscingulate | 0.007 | 9.948E-01 | 0.176 | -0.005 | isthmuscingulate | 1.233 | 2.218E-01 | 22.279 | -0.864 |
| wm.lh.lateraloccipital | -3.379 | 1.217E-03 | -376.244 | 3.707 | lateraloccipital | 3.935 | 2.000E-04 | 246.853 | -2.014 |
| wm.lh.lateralorbitofrontal | 0.689 | 4.935E-01 | 31.135 | -0.468 | lateralorbitofrontal | 6.832 | 3.020E-09 | 248.868 | -3.289 |
| wm.lh.lingual | -1.544 | 1.273E-01 | -59.632 | 1.117 | lingual | 6.553 | 9.470E-09 | 193.559 | -2.875 |
| wm.lh.medialorbitofrontal | -0.439 | 6.618E-01 | -18.801 | 0.497 | medialorbitofrontal | 1.497 | 1.390E-01 | 55.824 | -1.084 |
| wm.lh.middletemporal | 1.433 | 1.564E-01 | 54.701 | -1.006 | middletemporal | 6.053 | 7.170E-08 | 502.132 | -4.765 |
| wm.lh.parahippocampal | 1.162 | 2.494E-01 | 11.321 | -0.757 | parahippocampal | 4.734 | 1.180E-05 | 81.603 | -4.056 |
| wm.lh.paracentral | -1.810 | 7.480E-02 | -65.051 | 1.645 | paracentral | 0.847 | 3.999E-01 | 26.147 | -0.739 |
| wm.lh.parsopercularis | -2.525 | 1.394E-02 | -63.713 | 1.857 | parsopercularis | 2.346 | 2.196E-02 | 52.529 | -1.156 |
| wm.lh.parsorbitalis | -1.481 | 1.433E-01 | -17.015 | 1.626 | parsorbitalis | 3.944 | 1.941E-04 | 77.912 | -3.363 |
| wm.lh.parstriangularis | -3.147 | 2.465E-03 | -60.168 | 2.008 | parstriangularis | 3.694 | 4.458E-04 | 70.279 | -1.989 |
| wm.lh.pericalcarine | -3.140 | 2.516E-03 | -76.857 | 2.507 | pericalcarine | 0.617 | 5.391E-01 | 12.647 | -0.590 |
| wm.lh.postcentral | -1.932 | 5.763E-02 | -111.412 | 1.452 | postcentral | 4.601 | 1.920E-05 | 233.324 | -2.428 |
| wm.lh.posteriorcingulate | -0.907 | 3.674E-01 | -29.943 | 0.678 | posteriorcingulate | 0.324 | 7.473E-01 | 6.809 | -0.227 |
| wm.lh.precentral | -4.929 | 5.710E-06 | -567.278 | 4.094 | precentral | 4.610 | 1.860E-05 | 513.647 | -3.716 |
| wm.lh.precuneus | -1.333 | 1.871E-01 | -91.800 | 1.039 | precuneus | 3.868 | 2.508E-04 | 214.221 | -2.216 |
| wm.lh.rostralanteriorcingulate | 1.392 | 1.684E-01 | 37.394 | -1.545 | rostralanteriorcingulate | 1.140 | 2.585E-01 | 35.735 | -1.442 |
| wm.lh.rostralmiddlefrontal | -4.133 | 1.016E-04 | -420.491 | 3.423 | rostralmiddlefrontal | 1.633 | 1.072E-01 | 116.338 | -0.803 |
| wm.lh.superiorfrontal | -3.255 | 1.779E-03 | -537.750 | 3.103 | superiorfrontal | -0.158 | 8.751E-01 | -22.956 | 0.108 |
| wm.lh.superiorparietal | -2.265 | 2.672E-02 | -273.254 | 2.233 | superiorparietal | 3.886 | 2.359E-04 | 350.632 | -2.641 |
| wm.lh.superiortemporal | -1.089 | 2.799E-01 | -65.050 | 0.783 | superiortemporal | 6.719 | 4.800E-09 | 443.118 | -3.467 |
| wm.lh.supramarginal | -1.082 | 2.832E-01 | -88.826 | 0.980 | supramarginal | 4.124 | 1.051E-04 | 303.779 | -2.710 |
| wm.lh.frontalpole | -2.010 | 4.843E-02 | -8.896 | 3.347 | frontalpole | -2.789 | 6.882E-03 | -40.941 | 4.227 |
| wm.lh.temporalpole | -1.542 | 1.278E-01 | -15.319 | 2.313 | temporalpole | 1.850 | 6.868E-02 | 83.088 | -3.455 |
| wm.lh.transversetemporal | -0.549 | 5.847E-01 | -8.178 | 0.951 | transversetemporal | 4.309 | 5.480E-05 | 44.706 | -3.843 |
| wm.lh.insula | -0.752 | 4.549E-01 | -37.959 | 0.389 | insula | 2.243 | 2.819E-02 | 75.294 | -1.072 |
| wm.rh.bankssts | 0.595 | 5.537E-01 | 12.163 | -0.435 | bankssts | 4.625 | 1.760E-05 | 89.559 | -4.034 |
| wm.rh.caudalanteriorcingulate | 0.343 | 7.324E-01 | 9.994 | -0.390 | caudalanteriorcingulate | -0.790 | 4.324E-01 | -12.779 | 0.712 |
| wm.rh.caudalmiddlefrontal | -1.490 | 1.409E-01 | -99.388 | 1.681 | caudalmiddlefrontal | 2.202 | 3.113E-02 | 158.529 | -2.656 |
| wm.rh.cuneus | -4.176 | 8.770E-05 | -79.881 | 2.931 | cuneus | -0.080 | 9.363E-01 | -2.059 | 0.059 |
| wm.rh.entorhinal | 2.358 | 2.132E-02 | 23.166 | -2.904 | entorhinal | 2.408 | 1.881E-02 | 70.691 | -3.646 |
| wm.rh.fusiform | 0.549 | 5.849E-01 | 22.657 | -0.367 | fusiform | 6.037 | 7.640E-08 | 356.103 | -3.735 |
| wm.rh.inferiorparietal | -3.354 | 1.316E-03 | -304.735 | 2.656 | inferiorparietal | 7.046 | 1.250E-09 | 515.868 | -3.409 |
| wm.rh.inferiortemporal | -0.215 | 8.307E-01 | -7.449 | 0.126 | inferiortemporal | 5.182 | 2.190E-06 | 298.985 | -2.946 |
| wm.rh.isthmuscingulate | -1.842 | 6.988E-02 | -50.916 | 1.496 | isthmuscingulate | 0.386 | 7.009E-01 | 6.926 | -0.287 |
| wm.rh.lateraloccipital | -4.164 | 9.120E-05 | -329.579 | 3.228 | lateraloccipital | 4.216 | 7.630E-05 | 295.338 | -2.379 |
| wm.rh.lateralorbitofrontal | 1.139 | 2.588E-01 | 95.641 | -1.362 | lateralorbitofrontal | 5.302 | 1.380E-06 | 256.074 | -3.413 |
| wm.rh.lingual | -2.246 | 2.804E-02 | -87.431 | 1.531 | lingual | 2.640 | 1.030E-02 | 91.015 | -1.259 |
| wm.rh.medialorbitofrontal | 1.042 | 3.009E-01 | 36.841 | -0.988 | medialorbitofrontal | 1.402 | 1.656E-01 | 52.471 | -0.972 |
| wm.rh.middletemporal | 0.076 | 9.400E-01 | 3.079 | -0.049 | middletemporal | 7.015 | 1.430E-09 | 413.824 | -3.591 |
| wm.rh.parahippocampal | 1.762 | 8.261E-02 | 21.209 | -1.379 | parahippocampal | 4.931 | 5.670E-06 | 80.309 | -4.123 |
| wm.rh.paracentral | -1.852 | 6.839E-02 | -111.200 | 2.367 | paracentral | 1.798 | 7.667E-02 | 47.471 | -1.205 |
| wm.rh.parsopercularis | -1.137 | 2.597E-01 | -42.666 | 1.307 | parsopercularis | 2.917 | 4.803E-03 | 101.647 | -2.582 |
| wm.rh.parsorbitalis | -1.211 | 2.302E-01 | -21.096 | 1.648 | parsorbitalis | 5.471 | 7.190E-07 | 94.162 | -3.487 |
| wm.rh.parstriangularis | -4.285 | 5.990E-05 | -90.421 | 2.656 | parstriangularis | 2.822 | 6.277E-03 | 60.132 | -1.420 |
| wm.rh.pericalcarine | -2.016 | 4.783E-02 | -60.822 | 1.822 | pericalcarine | 2.142 | 3.580E-02 | 45.574 | -1.833 |
| wm.rh.postcentral | -2.236 | 2.869E-02 | -144.635 | 1.917 | postcentral | 4.355 | 4.670E-05 | 190.074 | -2.052 |
| wm.rh.posteriorcingulate | -1.959 | 5.425E-02 | -57.569 | 1.389 | posteriorcingulate | 0.214 | 8.311E-01 | 4.985 | -0.163 |
| wm.rh.precentral | -4.330 | 5.110E-05 | -537.878 | 3.858 | precentral | 4.649 | 1.620E-05 | 494.897 | -3.681 |
| wm.rh.precuneus | -4.148 | 9.650E-05 | -249.516 | 2.693 | precuneus | 1.902 | 6.142E-02 | 139.382 | -1.387 |
| wm.rh.rostralanteriorcingulate | 0.599 | 5.514E-01 | 9.610 | -0.517 | rostralanteriorcingulate | 1.222 | 2.260E-01 | 25.868 | -1.432 |
| wm.rh.rostralmiddlefrontal | -1.814 | 7.420E-02 | -265.241 | 2.032 | rostralmiddlefrontal | 1.696 | 9.458E-02 | 205.824 | -1.370 |
| wm.rh.superiorfrontal | -1.288 | 2.022E-01 | -207.425 | 1.220 | superiorfrontal | 1.614 | 1.112E-01 | 233.765 | -1.137 |
| wm.rh.superiorparietal | -4.960 | 5.100E-06 | -470.591 | 3.948 | superiorparietal | 2.396 | 1.935E-02 | 227.412 | -1.728 |
| wm.rh.superiortemporal | -1.682 | 9.724E-02 | -105.721 | 1.515 | superiortemporal | 7.441 | 2.450E-10 | 316.191 | -2.704 |
| wm.rh.supramarginal | -2.688 | 9.070E-03 | -199.659 | 2.299 | supramarginal | 4.199 | 8.070E-05 | 339.559 | -3.307 |
| wm.rh.frontalpole | -3.039 | 3.385E-03 | -18.993 | 5.704 | frontalpole | -2.823 | 6.260E-03 | -48.382 | 4.188 |
| wm.rh.temporalpole | 0.971 | 3.352E-01 | 9.363 | -1.364 | temporalpole | 1.611 | 1.119E-01 | 57.456 | -2.262 |
| wm.rh.transversetemporal | 0.288 | 7.740E-01 | 2.841 | -0.433 | transversetemporal | 3.647 | 5.200E-04 | 33.235 | -3.576 |
| wm.rh.insula | -0.743 | 4.602E-01 | -63.557 | 0.661 | insula | 1.285 | 2.032E-01 | 78.059 | -1.110 |
| Left.UnsegmentedWhiteMatter | -1.546 | 1.269E-01 | -269.732 | 0.976 | |||||
| Right.UnsegmentedWhiteMatter | -1.023 | 3.099E-01 | -179.632 | 0.640 | |||||
| Left.hemisphere.cerebral.white.matter.Vol | -2.420 | 1.825E-02 | -2982.322 | 1.349 | |||||
| Right.hemisphere.cerebral.white.matter.Vol | -2.736 | 7.950E-03 | -3230.282 | 1.454 | |||||
| Estimated.Total.Intracranial.Vol | 1.933 | 5.746E-02 | 10491.750 | -0.639 |
Regions of interests were computed using Freesurfer and a paired t-test was computed between the two time-points. Differences represent anesthesia (A2)-Day 7.
Analysis of ventricular volume showed that overall CSF volume was increased during anesthesia. Individual ventricles showed that the 3rd ventricle was increased while the 4th ventricle was decreased. Lateral ventricle volumes were also increased but were not significant (Table 6).

| Regions | tstat | p-value | diff | %change |
|---|---|---|---|---|
| Left.Lateral.Ventricle | 0.720 | 0.474 | 44.768 | -0.304 |
| Left.Inf.Lat.Vent | 1.352 | 0.181 | 14.551 | -2.843 |
| 3rd.Ventricle | 2.237 | 0.029 | 18.806 | -1.310 |
| 4th.Ventricle | -3.746 | 0.000 | -58.512 | 3.000 |
| CSF | 3.338 | 0.001 | 60.884 | -4.893 |
| Right.Lateral.Ventricle | 1.137 | 0.260 | 61.112 | -0.458 |
| Right.Inf.Lat.Vent | 1.624 | 0.109 | 9.740 | -2.234 |
| 5th.Ventricle | -0.141 | 0.888 | -0.013 | 18.367 |
Differences are Anesthesia (A2)—Day 7. Significant differences highlighted in bold.
The source of Diffusion Weighted signal based on the acquisition sequences used in this study is believed to be most sensitive to extracellular water [11, 12]. FA measures the coherence of diffusion of water molecules between myelinated axonal bundles. RD measures the diffusion perpendicular to the axons whereas AD measures the diffusion parallel to the axons. MD is an overall measure of the diffusion properties independent of the directions. The combination of the differences in FA (decrease) and MD (increase) is normally observed when there are increases in the extracellular space between the axons due to axonal degeneration or edema [13, 14]. Changes in AD and RD usually reflect specific directional changes in diffusivity parallel or perpendicular to the axons respectively. Our data showed a significant transient decrease in FA and increases in MD, AD and RD during sevoflurane anesthesia. These results suggest a widening of the inter-axonal space (Fig 4). These transient changes were highly significant and were detected diffusely throughout the brain white matter regions using both a voxel-wise technique (TBSS) (Fig 1) as well as regions of interest approaches (the Johns Hopkins Atlas ROIs) (Fig 2 and Tables 1 and 4). Our data also showed the well-known finding of declining FA and increases of MD with age after the 4th decade of life (Fig 3A) [13, 15–17]. Interestingly, the magnitude of the changes in FA, MD, AD and RD during anesthesia were the same for all age groups (Fig 3B, Tables 2 and 3), there were no age or sex associations with the effects of the anesthesia. Volumetric analysis suggests an overall shrinkage of white matter volumes and increases of gray matter volume (Table 5) while CSF volume showed a significant increase (Table 6) during anesthesia. This is the first time that these changes in white matter microstructure have been reported during general anesthesia and the underlying physiology is not well-understood.

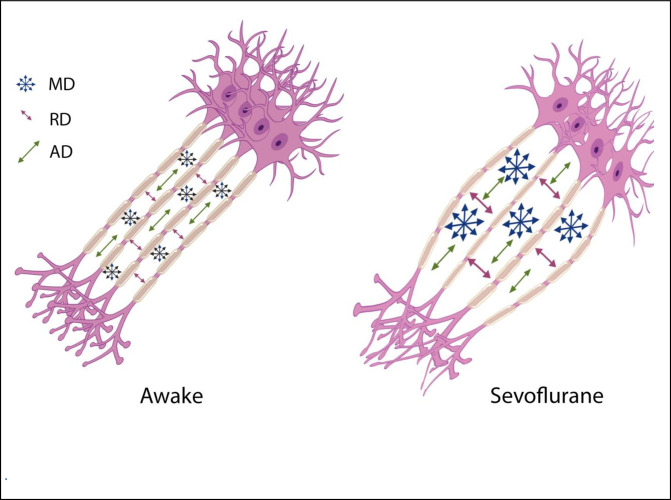
Illustration of one possible geometric implication of the DTI metrics.
Increases in the interaxonal space will result in increased MD and RD, but to a lesser extend the AD.
We review here several possible mechanisms consistent with the changes in the DTI metrics that we have detected:
Previous studies exploring the sensitivity of DTI to functional activation have shown significant correlations between FA and task based activity (functional DTI or fDTI) in thalamocortical and optic tracts for tactile and visual stimuli respectively [18, 19]. Changes in glial cell morphology during activation may contribute to the physical changes of the extracellular space [20]. Unlike the BOLD effect in functional MRI [21] which is sensitive to blood oxygenation changes upon brain activation, DTI metrics such as FA, MD, RA and AD measure physical and geometrical attributes of the underlying tissue. The positive correlation of FA with brain activity [18] was believed to be due to glial cell swelling which changes the extra cellular space geometry. Shrinkage of the extracellular space upon neuronal firing has been studied for many years [22]. In particular, astrocytes, being the most abundant of glia cells in the brain, swell upon hyperactivity due to imbalances in Na+ and K+ concentrations inside and outside of the cells [23, 24]. In another study using diffusion weighted imaging on a rat optic nerve it was shown that levels of potassium concentrations in the extracellular space could control the diffusivity measures [25]. They also observed that the changes in diffusivity in the radial direction (similar to our RD measure) were larger than in the parallel direction (similar to our AD measure). In our study the changes in AD, although significant, the % change was much less than MD or RD (Table 1). Increases in extracellular potassium concentrations with neuronal activity have been well described before [26, 27]. The earlier fDTI study showed an increase in FA upon neuronal activation; our study showed decreased FA under sevoflurane anesthesia. One possibility is that the reduced brain activity in the anesthesia state causes potassium concentration levels to drop below baseline and thereby causes glial cells to shrink. Indeed, as we will discuss in the next section on the glymphatic system, glial cells shrink during sleep to make room for clearance of waste material in the brain [28]. This shrinkage would lead increased extracellular space, and MD, RD and AD would increase and FA would decrease. The volumetric results (Table 6) that showed increased CSF volume and an overall trend in increased ventricular size may corroborate this interpretation. In addition, we showed that the volumes of several white matter regions were reduced during anesthesia. This reduction might be related to the shrinkage of the glial cells. It should be noted that DTI metrics such as FA that measure the microstructure of white matter does not have a direct relationship with white matter volumetric measures; the increase in extracellular space does not necessarily contradict the reduction of overall white matter volume [29, 30].
The glymphatic system is involved with clearance of waste matter in the central nervous system [31–33]. It is part of the perivascular system that drives CSF into brain parenchyma and drains waste solutes from the interstitial space fluid (ISF) into the perivascular space around the veins. Astrocytic endfeet cover almost the entire brain vasculature regulating endothelial tight junctions that form the blood brain barrier as well vascular tone through vasoactive agents [34]. Astrocytic endfeet also express water channels called aquaporin-4 (AQP4); these channels facilitate the movement of interstitial fluids into a perivascular space around draining veins [35]. AQP4 channels are mainly engaged during sleep and under anesthesia [28]. In addition, interstitial space was dilated during sleep and anesthesia states when compared to awake states [28]. Sevoflurane upregulates AQP4 expression [36]. It should be noted that different types of anesthesia seem to have different effects on the glymphatic transport system; dexmedetomidine (an alpha-2 adrenergic agonist) had 32% more enhanced glymphatic transport when compared with isoflurane [37]. In this study it was speculated that the lowered adrenergic tone with dexmedetomidine increased the interstitial fluid volume fraction which then facilitated glymphatic transport [38]. Using two-photon microscopy and fluorescent tracers, it was shown that the interstitial space was increased by 60% during sleep as well as under anesthesia using ketamine/xylazine in a mouse model [28]. We speculate that this increase in the ISF is the cause of the changes in the DTI metrics such as FA, MD, AD and RD. Increases in ISF would push axonal bundles apart and thereby reduce FA (a measure of bundle coherence), increase MD (increase of isotropic free water movement), and increase RA (the space perpendicular to the axons) [Fig 4]. Although AD was decreased but its change was only 0.4% vs 2.4% for RD. From a geometric point of view, increases in ISF has less effect on AD then RD [Fig 4]. In another study of normal pressure hydrocephalus (NPH), a lower FA was associated with better glymphatic clearance [39]. In summary the increases of ISF is tightly coupled with the glial cell shrinkage suggested in the previous paragraph as it is believed that it is the response of astrocytes to a reduced wakefulness that is changing the ISF space [40].
Microtubules are the major components of the cytoskeleton of most eukaryotic cells and in particular axonal structures [41, 42]. Several studies have shown the effects of diverse anesthetics on microtubule structural stability [43]. Among the mechanisms that have been proposed are Tau hyperphosphorylation [44–47]. Temporary impairment of Tau function by hyperphosphorylation at several positions can be induced by the administration of anesthetics. In one study, 2.5% sevoflurane in 5–6 month-old C57B16/J mice increased Tau phosphorylation level on Ser 396/404 at 1 h following anesthesia. This short-term increase in Tau phosphorylation was reversible as no significant increase was detected 1 day after anesthesia [44]. Isoflurane anesthesia in mice with tauopathy elevated phospho-tau for at least 1 week after the anesthesia [48]. A study on the effects of sevoflurane on young mice comparing wild type and Tau-KO showed that sevoflurane increased activation of glycogen synthase kinase 3β (the kinase that is related to Tau phosphorylation) in young WT resulting in cognitive impairment but not in the Tau-KO [46]. Another study showed the same anesthesia induced microtubule instability through the same GSK3β pathway using sevoflurane, urethane and ketamine [43]. The instability of microtubules is rather complex and it would be difficult to propose a geometric explanation for the changes that we have found in the different DTI metrics, but anesthesia induced microtubule instability could lead to deformation of axonal formations and affect DTI metrics that depend on structural coherence of axonal bundles.
Another potential source of these changes might be vasodilatory effects of sevoflurane that give rise to microscopy vasogenic swelling and subsequent increase in water content of the extra-axonal space. Halothane, fentanyl, and thiopental can all cause an increase in brain water and electrolyte distribution [49, 50]. Sevoflurane is one of the more common inhaled anesthetics and like most other volatile anesthetics is known to cause vasodilation [51, 52]. Changes in intracranial pressure (ICP) following sevoflurane have also been reported but the results have been inconclusive [53–55]. Increases in ICP might be due to a combination of vasodilation and accumulation of CSF due to reduced reuptake [56]. It has been previously reported that the apparent diffusion coefficient (ADC) in deep white matter increases in idiopathic intracranial hypertension [57]. Diffusion imaging in hydrocephalus has shown increased diffusion coefficients in periventricular white matter [58, 59]. Increases in ADC reflect increases in extracellular water or increased fraction of mobile water in intracellular space. Increased ICP can cause increases in CSF in the extracellular space of white matter. This transepedymal pathway of CSF has been studied recently [60]. Although increased CSF in the extracellular space could explain some of our findings, targeted research in the relationship between ICP and FA have not provided any consistent results, some have shown a decrease [61, 62], increased [63] and some no change [64], so we tend to discount this explanation of our findings.
Although these results show that the changes in the white matter microstructure are transient, it is important to consider these results in research studies that necessitate the use of anesthesia, given that general anesthesia can produce significant changes in diffusion measures of white matter integrity as well as in volume of specific brain regions. We have suggested several possible mechanisms for the observed changes in white matter microstructure as detected using standard in-vivo DTI techniques. To better unravel the underlying physiological changes more research need to be performed in vitro using imaging technologies such as electron microscopy (EM). In addition, investigations into whether these changes in white matter microstructure are related to loss of consciousness are warranted.
We thank Helen Chang, M.S., Senior Statistician, Icahn School of Medicine at Mount Sinai for her advice on some of the statistical analysis.
| AD | Axial Diffusivity |
| ADC | Apparent Diffusion Coefficient |
| AQP4 | Aquaporin-4 |
| BOLD | Blood Oxygenation Level Dependent |
| CSF | Cerebral Spinal Fluid |
| DTI | Diffusion Tensor Imaging |
| FA | Fractional Anisotropy |
| fDTI | functional Diffusion Tensor Imaging |
| ICP | Intracranial Pressure |
| ISF | Interstitial Space Fluid |
| MD | Mean diffusivity |
| RD | Radial Diffusivity |
| ROI | region of Interest |
| TBSS | Tract Based Spatial Statistics |
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64