
Competing Interests: The authors have declared that no competing interests exist.
- Altmetric
Ever since the outbreak of the COVID-19 epidemic, various public health control strategies have been proposed and tested against the coronavirus SARS-CoV-2. We study three specific COVID-19 epidemic control models: the susceptible, exposed, infectious, recovered (SEIR) model with vaccination control; the SEIR model with shield immunity control; and the susceptible, un-quarantined infected, quarantined infected, confirmed infected (SUQC) model with quarantine control. We express the control requirement in metric temporal logic (MTL) formulas (a type of formal specification languages) which can specify the expected control outcomes such as “the deaths from the infection should never exceed one thousand per day within the next three months” or “the population immune from the disease should eventually exceed 200 thousand within the next 100 to 120 days”. We then develop methods for synthesizing control strategies with MTL specifications. To the best of our knowledge, this is the first paper to systematically synthesize control strategies based on the COVID-19 epidemic models with formal specifications. We provide simulation results in three different case studies: vaccination control for the COVID-19 epidemic with model parameters estimated from data in Lombardy, Italy; shield immunity control for the COVID-19 epidemic with model parameters estimated from data in Lombardy, Italy; and quarantine control for the COVID-19 epidemic with model parameters estimated from data in Wuhan, China. The results show that the proposed synthesis approach can generate control inputs such that the time-varying numbers of individuals in each category (e.g., infectious, immune) satisfy the MTL specifications. The results also show that early intervention is essential in mitigating the spread of COVID-19, and more control effort is needed for more stringent MTL specifications. For example, based on the model in Lombardy, Italy, achieving less than 100 deaths per day and 10000 total deaths within 100 days requires 441.7% more vaccination control effort than achieving less than 1000 deaths per day and 50000 total deaths within 100 days.
1 Introduction
The COVID-19 pandemic [1] has caused over 28 million confirmed cases and over 0.91 million deaths globally as of September 12, 2020. Ever since the outbreak of COVID-19, various public health control strategies have been proposed and tested against the coronavirus SARS-CoV-2 [2–4].
Currently, over 90 vaccines are being developed against SARS-CoV-2 by research teams across the world [5]. Besides vaccination, other strategies have also been proposed to control the spread of SARS-CoV-2. In [6], the authors proposed shield immunity to protect the susceptible people from getting infected with SARS-CoV-2. Specifically, shield immunity works by first identifying and deploying recovered individuals who have protective antibodies to SARS-CoV-2, and then increasing the proportion of interactions with recovered individuals as opposed to other individuals. In [7], the authors analyzed how quarantine has mitigated the spread of SARS-CoV-2 based on a model that differentiates quarantined infected individuals and un-quarantined infected individuals.
Despite the fact that various promising control strategies have been proposed against SARS-CoV-2, such control strategies still suffer from several limitations. (a) The control strategies against SARS-CoV-2 often treat the control inputs (e.g., the shield strength in shield immunity and the quarantine rate in quarantine control) as parameters that stay constant during one stage of time, while in reality such parameters may change on a daily basis with more fine-tuned control. (b) The control inputs in the literature are often tuned manually through trial-and-error instead of being synthesized systematically. (c) There is a lack of specific and formal specifications for the expected effects and outcomes of the control strategies.
To address these limitations, we propose a systematic control synthesis approach for three control strategies against SARS-CoV-2. We use metric temporal logic (MTL) formulas to specify the expected control outcomes such as “the deaths from the infection should never exceed one thousand per day within the next three months” or “the population immune from the disease should eventually exceed 200 thousand within the next 100 to 120 days”. Such temporal logic formulas have been used as high-level knowledge or specifications in many applications in artificial intelligence [8], robotic control [9], power systems [10], etc.
The proposed control synthesis approach is based on three specific COVID-19 epidemic mitigation models: the susceptible, exposed, infectious, recovered (SEIR) model with vaccination control; the SEIR model with shield immunity control; and the susceptible, un-quarantined infected, quarantined infected, confirmed infected (SUQC) model with quarantine control. We develop methods for synthesizing control strategies based on the three specific COVID-19 epidemic models with MTL specifications. Specifically, we convert the synthesis problem into mixed-integer bi-linear programming or mixed-integer fractional constrained programming problems, and solve the optimization problems using highly efficient solvers [11].
We provide simulation results in three different case studies: vaccination control for COVID-19 epidemic with model parameters estimated from data in Lombardy, Italy; shield immunity control for COVID-19 epidemic with model parameters estimated from data in Lombardy, Italy; and quarantine control for COVID-19 epidemic with model parameters estimated from data in Wuhan, China. The proposed synthesis approach can generate control inputs such that the time-varying numbers of individuals in each category (e.g., infectious, immune) satisfy the MTL specifications.
Based on the simulation results, we observe that early control is essential in mitigating the spread of COVID-19, and more control effort is needed for more stringent MTL specifications. For example, based on the model in Lombardy, Italy, achieving less than 100 deaths per day and 10000 total deaths within 100 days requires 441.7% more vaccination control effort than achieving less than 1000 deaths per day and 50000 total deaths within 100 days. As the control inputs are generated on a daily basis, the proposed approach can be used to assist and provide quantitative guidelines in public health control strategies to achieve specific specifications for mitigating the spread of COVID-19.
2 Related work
COVID-19 epidemic modeling and control strategies. Ever since the outbreak of COVID-19, there has been numerous research focusing on the modeling of COVID-19 epidemic based on data collected from both the epicenters and other places [12–17]. Among the various models, compartmental models such as SEIR and SUQC models have been used frequently for the analysis of COVID-19. There has also been work in analyzing or predicting the spread of COVID-19 using artificial intelligence models [17, 18], stochastic intensity models [13], etc. The models we use in this paper are based on the SEIR (both the standard and with shield immunity) and SUQC models, but we have replaced some essential parameters (e.g., the shield strength in shield immunity, the quarantine rate in quarantine control) with control inputs which can be synthesized to vary on a daily basis.
Optimal control of epidemic models. There exist works in designing vaccination control for the SEIR or SIR models of epidemics [19, 20]. However, such methods have not been applied in the setting of COVID-19. Besides, there has been no work in optimal control of epidemic models with formal specifications (e.g., expressed in temporal logic formulas).
Control synthesis with temporal logic specifications. There are three main categories of approaches to designing controllers that meet temporal logic specifications [21–31]. The first category of approaches abstract the system as a transition system and transform the control syntheses problem into a series of constrained reachability problems [32–34]. The second category of approaches mainly focus on linear dynamical systems and they convert the control synthesis problem into a mixed-integer linear programming (MILP) problem [35–40] which can be solved efficiently by MILP solvers. The third category of approaches substitute the temporal logic constraint into the objective function of the optimization problem and apply a functional gradient descent algorithm on the resulting unconstrained problem [10, 41–43]. The control synthesis approach in this paper is based on the second category of approaches, but we have extended the method to non-linear dynamical systems to fit the epidemic models for COVID-19.
3 Methodology
In this section, we provide the descriptions of methodology for synthesizing control strategies based on the three specific COVID-19 epidemic models with metric temporal logic (MTL) specifications. We first review MTL in Section 3.1, then introduce three control models for COVID-19 epidemic (vaccination control, shield immunity control and quarantine control) in Section 3.2, and finally present the control synthesis methods for the three COVID-19 control models in Section 3.3.
3.1 Metric Temporal Logic (MTL)
In this subsection, we briefly review metric temporal logic (MTL) [44] interpreted over discrete-time trajectories. The state x belongs to the domain . The time set is
The syntax of MTL is defined recursively as follows:
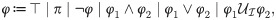
We define the set of states that satisfy the atomic proposition π as
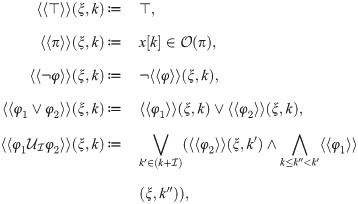
3.2 COVID-19 models with control strategies
In this subsection, we study three models for COVID-19 epidemic [6, 7, 14] and introduce the corresponding models with vaccination control, shield immunity control and quarantine control.
COVID-19 SEIR model with vaccination control. The susceptible, exposed, infectious, recovered (SEIR) model has been frequently used in epidemic analyses. As shown in Fig 1, the total population is divided into five subgroups:
The susceptible population S: everyone is susceptible to the disease by birth since immunity is not hereditary;
The exposed population E: the individuals who have been exposed to the disease, but are still not infectious;
The infectious population I: the individuals who are infectious;
The immune (recovered) population R: the individuals who are vaccinated or recovered from the disease, i.e., the population who are immune to the disease;
The dead population D: the dead individuals from the disease.

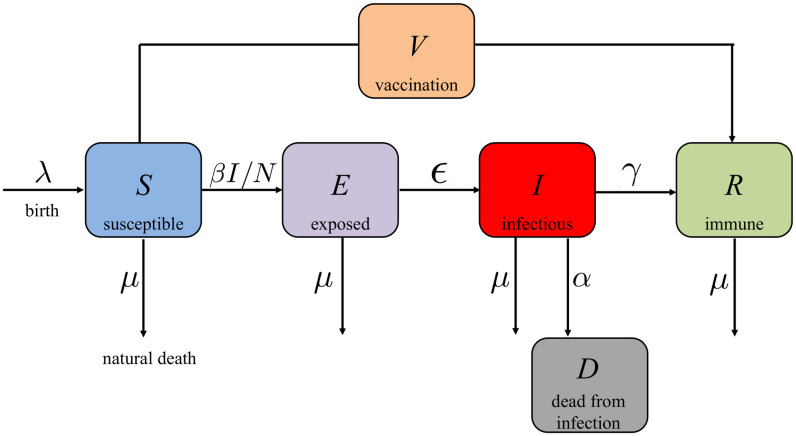
Block diagram of the COVID-19 SEIR model with vaccination control.
We consider a COVID-19 SEIR model [14, 15] with vaccination control [19] as follows.
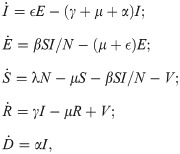
Remark 1. Note that one difference between this model and the vaccination control model in [19] is that we control V as the number of vaccinated individuals per day (constrained to be less than the susceptible population S), while in [19] the control input is the ratio of the vaccinated individuals per day to the average born population per day. We found it more convenient this way for computational convenience in the control synthesis in Section 3.3.
COVID-19 SEIR model with shield immunity control. Shield immunity is a strategy recently proposed in [6] to limit the transmission of SARS-CoV-2. The basic idea of this strategy is to increase the proportion of interactions with recovered individuals as opposed to the other individuals in the population. The effectiveness of this strategy is based on the assumption that recovered individuals (virus-negative and antibody-positive) can safely interact with both susceptible and infectious individuals without getting infected with the disease.
As the model used in [6] is modified from an SIR model, we consider a corresponding SEIR model with shield immunity control as follows (see Fig 2 as an illustration).
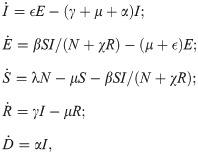

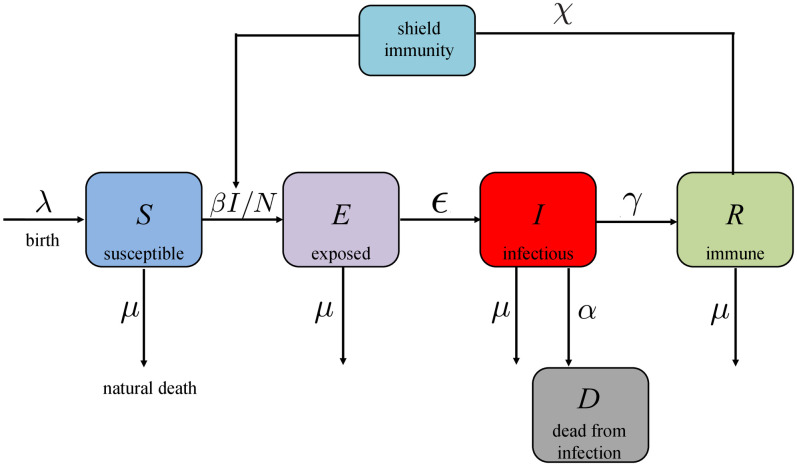
Block diagram of the COVID-19 SEIR model with shield immunity control.
COVID-19 SUQC model with quarantine control. The susceptible, un-quarantined infected, quarantined infected, confirmed infected (SUQC) model was recently proposed in [7] based on the COVID-19 data in Wuhan, China. As shown in Fig 3, we consider four subgroups in the population:
The susceptible population S: everyone is susceptible to the disease by birth since immunity is not hereditary;
The un-quarantined infected population U: the individuals who are infected and un-quarantined, and they can be either asymptomatic or symptomatic;
The quarantined infected population Q: the individuals who are infectious and quarantined (the un-quarantined infected become quarantined infected by isolation or hospitalization, and the quarantined infected lose the ability of infecting the susceptible individuals);
The confirmed infected population C: the individuals who are confirmed to be infected with the disease (i.e., the positive cases).

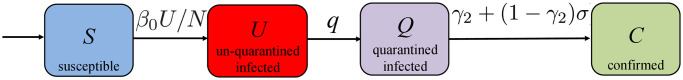
Block diagram of the COVID-19 SUQC model with quarantine control.
We consider the SUQC model with quarantine control as follows.
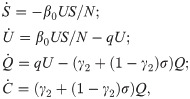
3.3 Control synthesis of COVID-19 epidemic with metric temporal logic specifications
In this subsection, we present the control synthesis methods for the three COVID-19 epidemic models in Section 3.2 with vaccination control, shield immunity control and quarantine control, respectively.
Vaccination control. For the COVID-19 SEIR model with vaccination control, we discretize the model in (3) as follows.
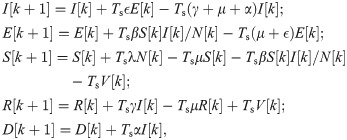
Following the notations in Section 3.1, we use xV = [I, E, S, R, D] to denote the state of (6) and
Problem 1 (Vaccination control). Given the SEIR model with vaccination control in
(6)
and an MTL specification
φV, compute the control input V[⋅] that minimizes the vaccination control efforts ‖V[⋅]‖ while satisfying
The vaccination control synthesis problem can be formulated as a constrained optimization problem as follows.

The above optimization problem is generally a mixed-integer non-linear programming problem. We refer the readers to [38] for a detailed description of how the constraint
Shield immunity control. For the COVID-19 SEIR model with shield immunity control, we discretize the model in (4) as follows.
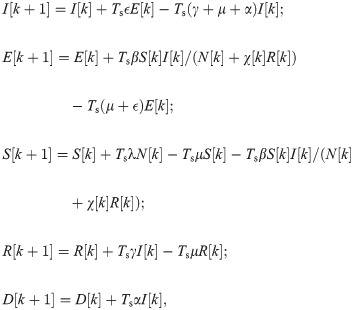
Following the notations in Section 3.1, we use xS = [I, E, S, R, D] to denote the state of (8) and
Problem 2 (Shield immunity control). Given the SEIR model with shield immunity control in
(8)
and an MTL specification φS, compute the control input χ[⋅] that minimizes the shield immunity control efforts ‖χ[⋅]‖ while satisfying
The shield immunity control synthesis problem can be formulated as a constrained optimization problem as follows.
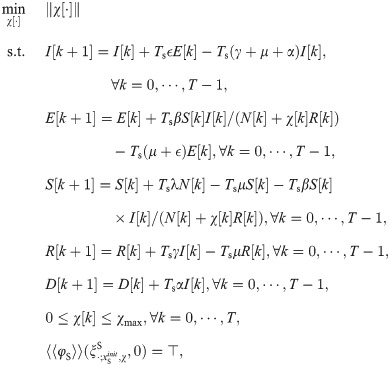
The above optimization problem is generally a mixed-integer fractional constrained programming problem. If the MTL specification φ consists of only conjunctions (∧) and the always operator (□), the integers in the optimization problem can be eliminated [38] and the problem becomes a fractional constrained programming problem.
Quarantine control. For the COVID-19 SUQC model with quarantine control, we discretize the model in (5) as follows.
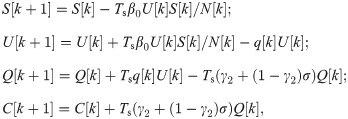
Following the notations in Section 3.1, we use xQ = [S, U, Q, C] to denote the state of (10) and
Problem 3 (Quarantine control). Given the SUQC model with quarantine control in
(10)
and an MTL specification φQ, compute the control input q[⋅] that minimizes the quarantine control efforts ‖q[⋅]‖ while satisfying
The quarantine control synthesis problem can be formulated as a constrained optimization problem as follows.
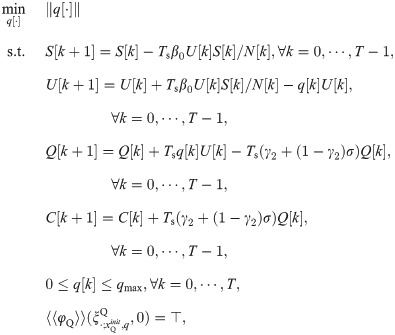
The above optimization problem is generally a mixed-integer non-linear programming problem. As the change of total population is relatively small compared to the multiplication of the susceptible population and the un-quarantined infectious population, we approximate the term Ts
β0
U[k]S[k]/N[k] with
4 Results
In this section, we implement the proposed control synthesis methods in the COVID-19 models estimated from data in Lombardy, Italy and Wuhan, China.
4.1 COVID-19 SEIR model with vaccination control
The parameters of the COVID-19 SEIR model are shown in Table 1. They were estimated using the data from February 23 to March 16, 2020 in Lombardy, Italy with no isolation measures (see Section 4.1 in [14] for details). Specifically, in Table 1, the values of λ and μ are from “μ−1 ≈ 83 years” (as 83 years = 30295 days) in Section 4 in [14] and our assumption that λ = μ, the values of α, β, ϵ and γ are from the initial values listed in Table 1 in [14], and the value of N0 is from Lombardy’s population listed in Wikipedia (https://en.wikipedia.org/wiki/Lombardy). The start time for the simulations in this subsection is February 23, 2020. We consider three MTL specifications as shown in Table 2. For example,

Simulation results for COVID-19 SEIR model estimated from data from Lombardy, Italy with no isolation measures.

| parameter | value | parameter | value |
|---|---|---|---|
| λ | 1/30295 | ϵ | 0.2/day |
| μ | 1/30295 | γ | 0.2/day |
| α | 0.006/day | N0 | 10 million |
| β | 0.75/day | Ts | 1 day |

| MTL specification | control effort | computation time |
|---|---|---|
 | 1.28 | 1.365 s |
 | 1.927 | 2.276 s |
 | 6.934 | 3.289 s |
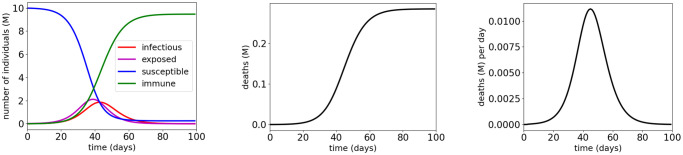
We use the solver GEKKO [11] to solve the optimization problems formulated in Section 3.3. Fig 5 and Table 2 show the simulation results for vaccination control of COVID-19 SEIR model with MTL specifications

Simulation results for the COVID-19 SEIR model with vaccination control and MTL specifications
The red dotted lines indicate the thresholds in the atomic propositions of the MTL specifications
4.2 COVID-19 SEIR model with shield immunity control
We use the same parameters of the COVID-19 SEIR model as shown in Table 1 (in the same setting as in Section 4.1 for Lombardy, Italy, with no isolation measures). Following Section 4.1 in [14], we choose the initial values of the states as I[0] = 1000 (people), E[0] = 0.02 million, S[0] = 9.979 million, R[0] = 0 and D[0] = 0, with S[0] + E[0] + I[0] + R[0] + D[0] = N0 = 10 million. We set χmax = 100. The start time for the simulations in this subsection is February 23, 2020. We set the three MTL specifications

| MTL specification | control effort | computation time |
|---|---|---|
 | 16879.53 | 2.112 s |
 | 45595.10 | 2.881 s |
 | 67786.88 | 5.323 s |
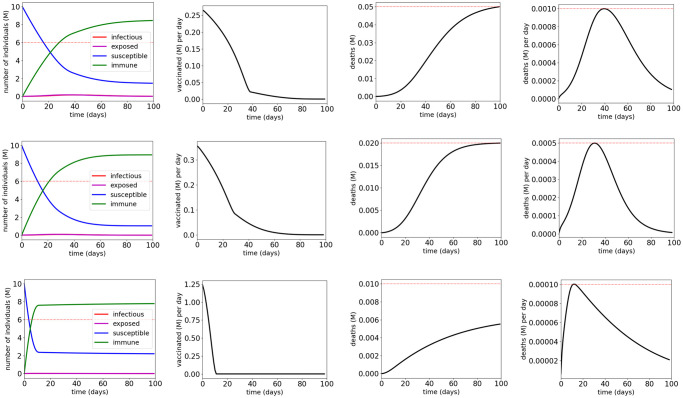
Fig 6 and Table 3 show the simulation results for shield immunity control of the COVID-19 SEIR model with MTL specifications

Simulation results for the COVID-19 SEIR model with shield immunity control and MTL specifications
The red dotted lines indicate the thresholds in the atomic propositions of the MTL specifications
4.3 COVID-19 SUQC model with quarantine control
The parameters of the COVID-19 SUQC model are shown in Table 4. In Table 4, the value of the infection rate β0 is from “Methods/Parameter inference” in [7] (the authors in [7] used α to denote the infection rate), the value of

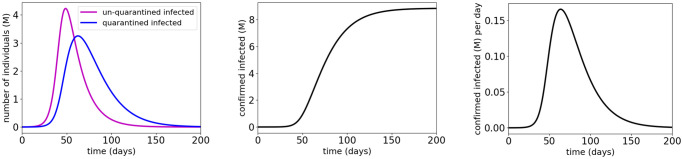
Simulation results for the COVID-19 SUQC model estimated from data in Stage I of Wuhan, China.

| parameter | value | parameter | value |
|---|---|---|---|
| β0 | 0.2967 | γ2 | 0.05 |
 | 8.9 million | σ | 0 |
| Ts | 1 day |

| MTL specification | control effort | computation time |
|---|---|---|
 | 15.146 | 2.296 s |
 | 15.638 | 2.598 s |
 | 15.894 | 4.578 s |
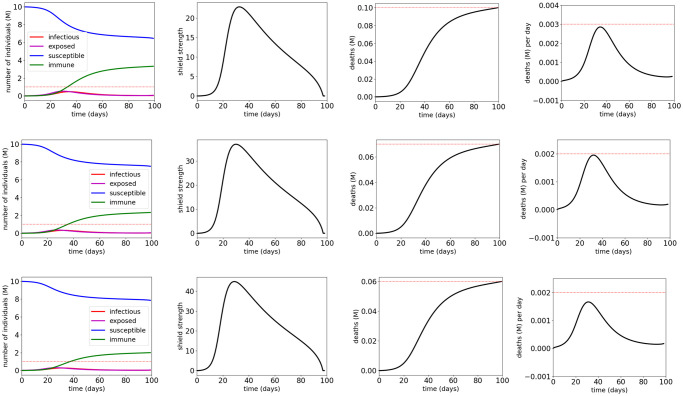
Fig 8 and Table 5 show the simulation results for quarantine control of the COVID-19 SUQC model with MTL specifications

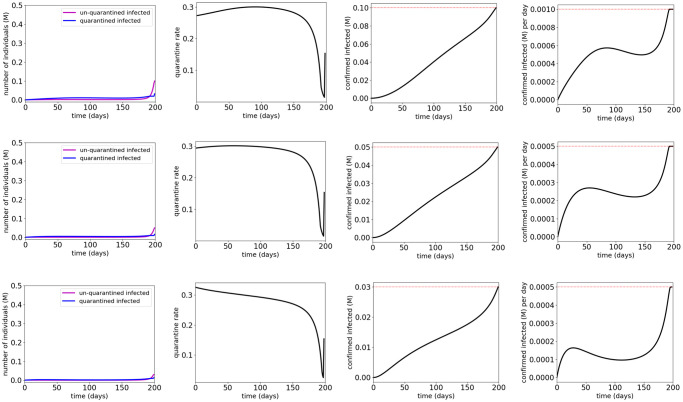
Simulation results for the COVID-19 SUQC model with quarantine control and MTL specifications
The red dotted lines indicate the thresholds in the atomic propositions of the MTL specifications
5 Discussion
In this paper, we proposed a systematic control synthesis approach for mitigating the COVID-19 epidemic based on three control models with vaccination, shield immunity and quarantine, respectively. We used metric temporal logic (MTL) formulas to formally specify the required performance of the control strategies. The proposed approach can synthesize control inputs that lead to satisfaction of the MTL specifications. The methodology and results in this paper contribute to several useful suggestions on limiting the spread of COVID-19.
Firstly, from the results with three control strategies (vaccination, shield immunity and quarantine) we observe the unanimous trend that the computed optimal control values are larger in the earlier days after the outbreak. This indicates that early intervention is essential and the most efficient (in comparison with late intervention) in controlling the spread of COVID-19.
Secondly, while it is obvious that more control efforts are needed to achieve more stringent control specifications, we observe that the required control efforts are not linear with respect to the “stringency” of the control specifications. For example, based on the model in Lombardy, Italy, achieving less than 20000 total deaths and 500 deaths per day within 100 days requires 50.55% more vaccination control effort than achieving less than 50000 total deaths and 1000 deaths per day within 100 days. However, achieving less than 10000 total deaths and 100 deaths per day within 100 days requires 259.83% more vaccination control effort than achieving less than 20000 total deaths and 500 deaths per day within 100 days. This “diminishing return” kind of property indicates that the same amount of additional (vaccination) control efforts will generally achieve less improvement for the control performance when the control specifications are increasingly more stringent.
The work in this paper opens the door to the formal synthesis of control strategies based on epidemic models. We believe that the methodology we developed in this paper can be readily used in controlling COVID-19 (and potentially other epidemic diseases) in various places where the control outcome needs to be specified in precise manners. In the following, we list several future directions that may readily derive from the work in this paper.
Firstly, the validity of the results depends on the accuracy of the model parameters (e.g., the recovery rate γ of infectious individuals). However, as the computation time is relatively short (within 6 seconds for all the scenarios in this paper), the user (or decision maker) can always change the model parameters to the latest estimated parameters and compute the optimal controls in a short time. The work in this paper can be readily extended to online control synthesis so that control inputs can be generated in real-time based on the latest information (e.g., using online parameter identification and receding horizon control).
Secondly, as we investigated the three control strategies separately in this paper, we will study the benefits and costs of joint control of different control strategies (vaccination, shield immunity and quarantine) so that the specifications can be satisfied with coordinated efforts. For example, to study the coordinated control of vaccination and shield immunity, we can use an integrated SEIR model with both V and N + χR. By selecting a cost function being the weighted sum of ‖V[⋅]‖ and ‖χ[⋅], we can achieve coordinated control of vaccination and shield immunity where the weights for ‖V[⋅]‖ and ‖χ[⋅] represent the relative costs of vaccination and shield immunity. To further include quarantine control, we can resort to more detailed models that differentiate between quarantined and un-quarantined population (e.g., as described in [49]).
Thirdly, the results in this paper focus on the control of COVID-19 in one specific region (i.e., Lombardy, Italy and Wuhan, China). Due to different geographic and demographic characteristics, the parameters in the COVID-19 models in different regions may be different, and the specifications in different regions may also be different (more stringent specifications for regions where the policy focuses more on mitigating the spread of COVID-19 than other factors such as the impact on the economy). The methodology in this paper can be readily applied to synthesizing coordinated regional control strategies for multiple different yet somewhat connected regions (e.g., as described in [49]).
References
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
 Control strategies for COVID-19 epidemic with vaccination, shield immunity and quarantine: A metric temporal logic approach
Control strategies for COVID-19 epidemic with vaccination, shield immunity and quarantine: A metric temporal logic approach
