
Competing Interests: The authors have declared that no competing interests exist.
We assessed the association of suPAR (soluble urokinase plasminogen activator receptor) plasma levels with fibrotic and vascular manifestations in patients with systemic sclerosis (SSc).
suPAR plasma levels were measured in 121 consecutive patients with SSc and correlated to pulmonary and vascular features of SSc, including interstitial lung disease as characterized by percentage of predicted CO diffusing capacity (DLco) and forced vital capacity (FVC), pulmonary fibrosis by computed tomography, and pulmonary arterial hypertension, telangiectasias, and digital ulcers.
Overall, 121 SSc patients (84% females; mean age, 57 ± 12 [range: 22–79] years) were enrolled; 35% had diffuse cutaneous SSc. suPAR plasma levels ranged from 1.3–10.2 [median: 2.9 (p25–p75: 2.3–3.9)] ng/mL. Log(suPAR) levels correlated with DLco (r = -0.41, p <0.0001) and FVC (r = -0.26, p = 0.004), also when adjusted for age, sex, and pulmonary hypertension. A suPAR cut-off level of >2.5 ng/mL showed a sensitivity of 91% for identifying patients with either DLco <50% or FVC < 60% of the predicted values. Similarly, 19 (90%) had a suPAR >2.5 ng/mL among those diagnosed with pulmonary fibrosis vs. 59 (60%) among those who did not (p = 0.008). suPAR values were not associated with vascular manifestations.
suPAR levels strongly correlated with pulmonary involvement in SSc. Future studies should test if suPAR estimation can be used for surveillance of severe pulmonary involvement in SSc.
Systemic sclerosis (SSc, scleroderma) is a rare autoimmune disease affecting nearly all organ-systems with substantially increased morbidity and mortality [1, 2]. Microvascular impairment, progressive fibrosis, and innate and adaptive immune dysregulation are hallmark pathophysiological features of SSc. The disease presents as several highly variable phenotypes [3, 4]. Therefore, there is an unmet demand for biomarkers that can reflect disease activity and severity in affected patients with severe fibrotic and vascular complications (such as interstitial lung disease and pulmonary hypertension). In this context, a number of hematological biomarkers indicative of interstitial lung disease, including surfactant protein, monocyte chemoattractant protein-1, interleukin-34, CCL-18, CXCL, and Kerbs von Lungren (KL)-6, have been identified [5–7]. Nevertheless, the clinical role of these biomarkers in the assessment of fibrotic manifestations (e.g., interstitial lung disease) or vasculopathy in SSc remains unclear. Furthermore, these tests are generally unavailable for routine clinical use.
C-reactive protein (CRP) is used as a general inflammatory marker in patients with autoimmune diseases. Prior studies have suggested that the pro-inflammatory interleukin-6 exerts transcriptional control on CRP release, and consequently, its blood levels. An elevated CRP level may therefore be associated with certain inflammatory SSc phenotypes, e.g. those involving progressive skin and lung involvement [8–12]. However, the diagnostic and prognostic sensitivity and specificity of a non-specific acute phase reactant, such as CRP may be limited, as it is not directly involved in SSc pathophysiology. Conversely, mechanistic molecular biomarkers that reflect biological dysregulation in pathogenic pathways specific to the disease promise to have more predictive potential for clinical and therapeutic management [3].
The urokinase plasminogen activator receptor (uPAR) is considered an important factor in the regulation of proteolysis, degradation of extracellular matrix, angiogenesis, and inflammation. An experimental deficiency of uPAR has been demonstrated to decrease angiogenesis and alter endothelial cell morphology [13]. Abnormal protein cleavage resulting in dysfunctional uPAR-pathways affects several immune mechanisms adversely, e.g. for differentiation of fibroblasts into myofibroblasts, an important step causing excessive production and deposition of extracellular matrix in SSc [14]. Systemic levels of soluble uPAR (suPAR) have been shown to correlate positively with immune system activation, with observational studies showing a positive correlation of this protein with both prognosis and mortality in a wide range of diseases [15]. When compared to many other inflammatory biomarkers, suPAR is biologically and chemically more stable both in vivo [16] and in vitro [17] and remains unaffected by the circadian rhythm or analytical preparations [17, 18]. A recent study demonstrated a possible association of suPAR levels with interstitial pulmonary and vascular involvement (e.g. development of pulmonary hypertension) in SSc patients as compared to those of healthy controls [19]. These data suggest that despite the fact that there is a possibility of an association between suPAR and systemic sclerosis, few studies have assessed this association. Therefore, in the present study, we measured suPAR levels and determined their clinical correlations with fibrotic and vascular complications occurring in patients with SSc.
This cross-sectional and analytical study included 121 consecutive SSc patients fulfilling the American College of Rheumatology criteria for classification of the disease [20]. All data were collected in 2009 (January until February) to investigate the correlation of selected biomarkers with subclinical organ damage, as detailed in previously published studies [21, 22]. The patients were recruited at the outpatient clinics at the Department of Dermatology, Bispebjerg Hospital, and at the Department of Rheumatology, Rigshospitalet, both part of Copenhagen University Hospitals. All but one patient was of Western European descendants. Clinical examinations were performed at the respective departments. The biochemical analysis of suPAR were performed at Amager and Hvidovre University Hospital, Copenhagen, Denmark. The patients were informed on the project by mail or during the planed clinical consultation. Patients were subscribed only after having fully understood the benefits and risks of the project and after having been given time for consideration.
Patients who had not received biological medications within the last 6 months and were free of cancer, pregnancy, or systemic infections at the time of screening were included in the study. The population of SSc patients is considered representative of a larger cohort of patients with this disease. Demographical data found in this population is in accordance with demographical data from other SSc populations [23].
The study was approved by the local ethics committee (“De Videnskabsetiske Komiteer for Region Hovedstaden”, approval number H-B-2008-131) and carried out in accordance with the principles of the Declaration of Helsinki. All patients provided written, informed consent for study participation.
All patients were screened and investigated by a single investigator, thereby eliminating the possibility of inter-individual variations in observations and evaluations. All patients were screened for the presence of abnormal skin thickness in 17 integumentary regions at the time of enrolment, and the results of this assessment were compiled and expressed in the form of the modified Rodnan skin score [24]. The relevant medical history of all patients was elicited by screening their medical records and by interviewing and recording reported comorbidities. The duration of disease was calculated as the time period from the onset of first non-Raynaud’s manifestation. Classification of SSc as diffuse or limited was performed in accordance with the definitions provided by LeRoy et al. [25].
Pulmonary function tests had been performed as a part of the usual clinical evaluation and the recorded results of all patients were ≤1-year-old at baseline. Data of forced vital capacity (FVC) and diffusing lung capacity of carbon monoxide (DLco) were also collected for the present study and are expressed as percentages (%) of predicted values. Diffusion capacity was estimated using a single-breath carbon monoxide and helium-carbon monoxide dilution principle and was standardized according to the patient’s hemoglobin concentration at the time. Blood pressures were measured at rest using standard electronic blood pressure machines. Hypertension was defined as a blood pressure >140/90 mm Hg.
We measured suPAR and high sensitivity CRP (hsCRP) levels in thawed plasma that had been collected in 2009. Whole blood (drawn from venipuncture of the cubital fossa) was centrifuged immediately after extraction and subsequently plasma samples were stored at −80°C until the analysis of suPAR levels. Levels of suPAR were measured using the commercially available suPARnostic® kit (ViroGates, Copenhagen, Denmark). Intra- and inter-assay variations in these estimations were reportedly low (<10%). The suPAR levels have been shown to be stable in spite of freezing and thawing of plasma samples [26]. The hsCRP levels were estimated using a standard clinical platform, as in previous studies [27].
Baseline characteristics are expressed as means and standard deviations (continuous variables) or as percentages (discrete variables). Due to the skewed distribution of concentrations of suPAR and hsCRP, the estimated variables were log-transformed (base 10) before analysis. Associations between suPAR and continuous variables were determined by means of Pearsons correlation analysis. Given a type 1 error rate of 0.05, a type 2 error rate of 0.20 and the study sample size, allowed us to be conclusive on correlation coefficients down to +/- 0.25. Linear regression analysis was used to analyze sex- and age-adjusted associations. To evaluate the existence and level of associations of suPAR and hsCRP concentrations with those of other variables, generalized linear models were applied. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed using SAS studio® or the SAS version 9.4 (Cary, North Carolina, USA) software.
A total of 121 patients (including 120 white) were enrolled. Of these, 84% were females. The mean age with standard deviation (± SD) of the study group was 57 ± 12 (range: 22–79) years. The average disease duration of the group was 12 ± 9 (range: 0–53) years, and 35% and 65% of the study patients had diffuse cutaneous and limited cutaneous SSc, respectively. The concentrations range of suPAR was 1.3–10.2 ng/ml (median: 2.9, p25–p75: 2.3–3.9 ng/ml) and demonstrated a skewed distribution (Fig 1).

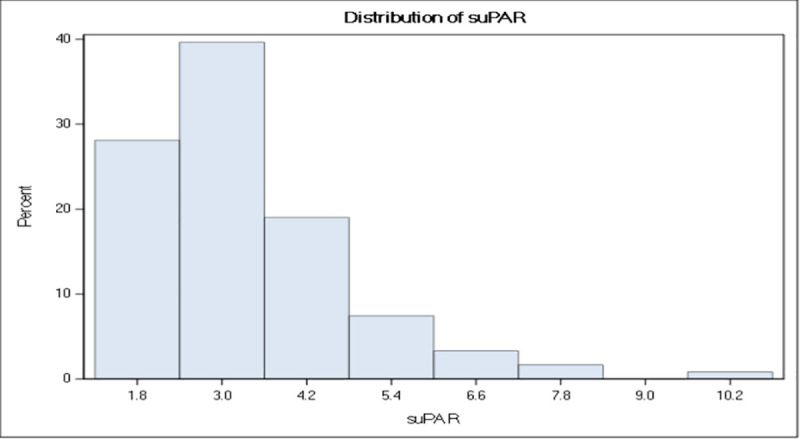
Concentration range of suPAR.
Histogram showing the distribution of suPAR concentrations (ng/mL) in the study population.
Baseline characteristics are presented in Table 1. Log suPAR was significantly associated with age, smoking and hsCRP (log10base). Furthermore, log suPAR was significantly associated with fibrotic features of SSc (modified Rodnan skin score, diffuse cutaneous SSc, radiographically confirmed lung fibrosis, FVC, DLco).

| Characteristics | N | r (p) | beta (p) |
|---|---|---|---|
| Sex, female (%) | 102 (84%) | -0.08 (0.37) | -0.08 (0.37) |
| Age, years, mean (SD) | 57 (12) | 0.22 (0.01) | 6.39 (0.01) |
| Disease duration, years, mean (SD) | 11.7 (8.9) | 0.00 (0.99) | -1.09 (0.59) |
| Body mass index, mean (SD) | 23.9 (3.8) | -0.06 (0.51) | -0.50 (0.57) |
| Current smoker, n (%) | 27 (25%) | 0.33 (<0.001) | 0.37 (<0.001) |
| hsCRP, mg/l, mean (SD) | 1.26 (1.21) | 0.33 (<0.001) | 1.10 (<0.001) |
| Anti-SCL70-antibodies, n (%) | 15 (13%) | 0.06 (0.54) | 0.05 (0.48) |
| Centromere-antibodies, n (%) | 50 (42%) | 0.11 (0.23) | 0.06 (0.56) |
| RNA-polymerase-III-antibodies, n (%) | 13 (11%) | 0.00 (0.98) | 0.05 (0.49) |
| Modified Rodnan Skin, Score20, n (%), | 11 (9) | 0.12 (0.20) | 4.29 (0.02) |
| Type of SSc, diffuse cutaneous, n (%) | 42 (35%) | 0.12 (0.18) | 0.22 (0.04) |
| Past or present cutaneous ulcers, n (%) | 45 (38%) | 0.00 (0.96) | -0.01 (0.95) |
| FVC, % of predicted value, mean (SD) | 96.7 (21.1) | -0.27 (0.004) | -15.67 (0.001) |
| DLco, % of predicted value, mean (SD) | 64.6 (19.4) | -0.41 (<0.0001) | -18.75 (<0.0001) |
| Radiographically confirmed lung fibrosis, n (%) | 21 (18%) | 0.25 (0.007) | 0.23 (0.01) |
| Evidence of pulmonal hypertension on echocardiography, n (%) | 19 (17%) | 0.16 (0.09) | 0.13 (0.14) |
| Telangiectasia, n (%) | 40 (34%) | -0.16 (0.08) | -0.09 (0.43) |
| Hypertension, n (%) | 30 (25%) | -0.06 (0.48) | -0.02 (0.82) |
| Systolic blood pressure, mm Hg, mean (SD) | 138 (23) | -0.05 (0.62) | -4.74 (0.36) |
| Diastolic blood pressure, mm Hg, mean (SD) | 77 (12) | -0.10 (0.28) | -3.01 (0.28) |
| History of thrombosis, n (%) | 19 (17%) | -0.22 (0.02) | -0.18 (0.05) |
| History of coronary artery disease, n (%) | 8 (7%) | -0.04 (0.74) | -0,02 (0.79) |
| History of intermittent claudication, n (%) | 3 (2.5) | -0.07 (0.50) | -0.02 (0.68) |
Demographic and clinical characteristics as well as association to logarithm of suPAR plasma levels by unadjusted and adjusted (sex and age) analyses.
Percentages are calculated based on the number of individuals without missing values. The maximal number of individuals with missing values was 32 (for medical history of coronary artery disease). For the other (non-medical history) variables, most patients had complete data: three individuals had missing values of pulmonary function tests, six individuals had missing data for pulmonary hypertension on echocardiography, and seven individuals had missing data on RNA-polymerase-III-antibodies. (SSc: systemic sclerosis; suPAR: soluble urokinase plasminogen activator receptor; hsCRP: high sensitivity C-reactive protein; FVC: forced vital capacity; DLco: lung diffusing capacity of carbon monoxide; mRSS: modified Rodnan skin score).
Vascular involvement was not associated with suPAR levels. There were no significant differences in logarithmic suPAR levels between individuals presenting with and without pulmonary hypertension (mean: 1.26 vs. 1.1, p = 0.09), telangiectasias (mean: 1.1 vs. 1.0, p = 0.08), or past or present digital ulcers (mean: 1.1 vs. 1.1, p = 0.96). Furthermore, the logarithmic hsCRP levels were also found to be comparable between patients with and without pulmonary hypertension (p = 0.60), telangiectasias (p = 0.40) and digital ulcers (p = 0.39).
To determine whether suPAR could be used as a screening biomarker for more severe pulmonary involvement in SSc, we estimated the concentrations of suPAR in patients with and without either diffusion capacity of <50% or forced vital capacity of <60% of predicted values. In total, 32 (27%) patients were found to have severe pulmonary involvement according to this definition. Median (p25–p75) concentrations of suPAR were estimated at 3.8 (3.0–4.6) ng/mL and 2.6 (2.1–3.4) ng/mL for patients with and without severe pulmonary involvement, respectively. A cut-off value >2.5 ng/mL encompassed 29 of the 32 patients, corresponding to a sensitivity of 91% and a negative predictive value of 96%. In total, 41 (34%) patients included in the study had suPAR values ≤2.5 ng/mL. Of those without severe pulmonary involvement (n = 80, 73%), 38 were correctly classified as not having severe pulmonary disease according to their suPAR concentrations, corresponding to a specificity of 43%. Similarly, 19 of 21 (90%) patients diagnosed with pulmonary fibrosis on X-ray and high-resolution computed tomography (CT), had suPAR values >2.5 ng/mL as compared to 59 of 98 (60%) of those who did not show any radiological signs of the condition (p for difference = 0.008). Further, among those with fibrosis on imaging (X-ray), of which only 7 underwent high-resolution computed tomography, unadjusted and adjusted linear regression models showed no significant association between suPAR and FVC/DLco.
Markers of pulmonary involvement (radiographically confirmed involvement, FVC, DLco) were associated with suPAR. Log(suPAR) values were found to demonstrate a high inverse correlation with both carbon monoxide diffusion capacity (r = -0.41, r2 = 0.17, p <0.0001) and forced vital capacity (r = -0.26, r2 = 0.07, p = 0.004) values, Figs 2 and 3. As compared to suPAR, log(hsCRP) was observed to be more closely related to forced vital capacity (r = -0.40, r2 = 0.16, p <0.0001) than to diffusion capacity (r = -0.26, r2 = 0.07, p = 0.006) estimates. After adjustments for age, sex and presence of pulmonary hypertension, the log(suPAR) level was found to be associated with percentage values of predicted diffusion capacity and forced vital capacity [β: -16.8% (p <0.0001) and -12.7 (p = 0.0005) per unit increase in log(suPAR), respectively]. After performing similar adjustments, hsCRP values were also observed to be associated with percentage values of both predicted diffusion capacity (beta estimate: -3.8%, p = 0.01) and forced vital capacity (beta estimate: -6.4%, p <0.0001) for every unit increase in the log(hsCRP) estimate.

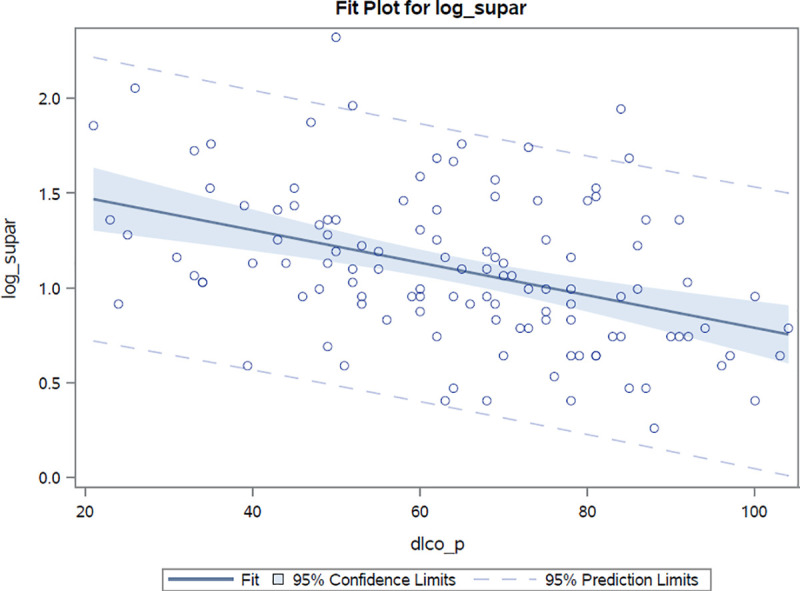
Correlation between log(suPAR) values and percentage of predicted diffusion capacity estimates.
Correlation coefficient r = -0.41 p<0.0001.

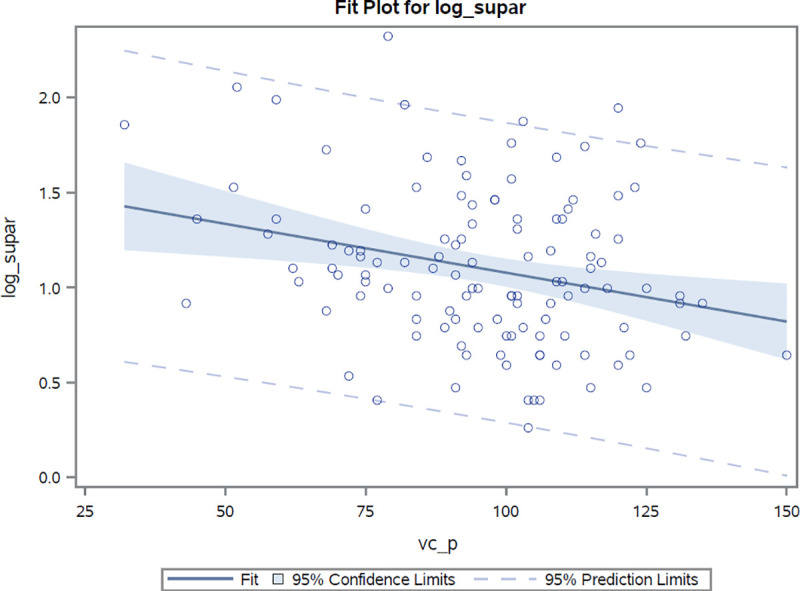
Correlation between log(suPAR) values and percentage of predicted total capacity estimates.
Correlation coefficient r = -0.27 p<0.004.
We investigated the association between suPAR concentrations and organ-level involvement in patients with SSc. We found a strong correlation between suPAR levels and surrogate markers of interstitial lung disease in SSc patients. Conversely, we did not observe a correlation of this biomarker with the extent of dermal fibrosis (defined as per the modified Rodnan skin score), serological markers (anti-Scl-, centromere-, and RNA-polymerase-III-antibodies), or vascular manifestations (pulmonary hypertension, telangiectasias, systolic blood pressure, past or present cutaneous ulcers). The detailed relationship between fibrotic and vascular dysfunction in SSc remains unclear and the pathophysiological overlap is incompletely understood. Because of the heterogeneity and spectrum of organ involvement in SSc, there is currently no single biomarker that can serve as an indicator of disease activity or severity for all phenotypes of the condition. Earlier studies have suggested suPAR as a valuable marker of organ damage or disease activity in various conditions including systemic lupus erythematosus [28–30], rheumatoid arthritis [31, 32], liver [33], and kidney [15] disease. It has been shown to correlate well with mortality and prognosis in a wide range of patient populations [34–37]. The few studies that have been conducted show that levels of suPAR have also been found to be elevated in SSc patients as compared to those of healthy controls [19, 38]. Abnormalities in the uPA or uPAR systems in endothelial cells have been implicated in vascular complications related to SSc [39–41], and suPAR has been described as a marker of cardiovascular risk and mortality [26, 42], We did not find a difference in log(suPAR) levels in SSc patients with and without vascular manifestations (pulmonary hypertension, telangiectasias, past or present digital ulcers). Although we did not have a healthy comparison cohort, our results are in contrast to those of a previous study [19], wherein suPAR levels were found to be higher in SSc patients with pulmonary arterial hypertension (determined using right heart catheterization) and digital ulcers than in patients without these manifestations. Pulmonary hypertension in our cohort was determined on echocardiography, limiting diagnostic accuracy as compared to that achieved with right heart catheterization [43], which can be used to differentiate pulmonary arterial hypertension from other inciting conditions such as pulmonary obstructive disorders, interstitial lung disease and chronic thromboembolism.
Experimental studies suggest that uPAR may be causally involved in dermal, renal and lung fibrosis in mice (as evidenced by gene deficiency or inactivation studies) [13, 44, 45]. A recent human study, however, found no significant difference in suPAR concentrations among SSc patients with and without fibrotic gastrointestinal involvement, or according to their extent of dermal involvement (evaluated using the modified Rodnan skin score) [19]. However, the same study found significantly higher suPAR levels in patients with interstitial lung disease (determined using high-resolution CT scans), as compared to that in individuals without, suggesting that this biomarker may be more closely related to the pulmonary manifestations of SSc than with the involvement of other organs; high suPAR levels, in particular, correlated with severe reductions in the DLco by <40% [median suPAR value: 6.14; interquartile range (IQR): 4.68–6.45 ng/ml] and with moderate reductions in FVC (50% <FVC <69%) (median suPAR value: 5.18; IQR: 3.91–6.42 ng/ml). Our study corroborates these earlier findings, underscoring the implication that suPAR levels appear to have a close correlation with the severity of interstitial lung involvement. Although further studies are warranted to investigate if estimation of suPAR concentration can be used as a screening tool to detect pulmonary involvement in patients, our exploratory analyses indicates that a suPAR concentration ≤2.5 ng/mL could be used to rule out clinically relevant pulmonary involvement in SSc.
It is important to establish biomarker validity and utility [46]. Estimation of suPAR has been demonstrated to have analytical validity (accuracy, reproducibility and reliability) in a number of studies [15, 37]. Our Western European cohort of SSc patients was clinically well-characterized and sampled under identical and controlled conditions, strengthening the reliability of our data. Nevertheless, the present study has certain limitations. First, our study findings can only lead us to hypothesize as to the clinical validity of using suPAR estimations in stratifying the degree of interstitial lung involvement in SSc. Although suPAR is a relatively stable biomarker and SSc a chronic disease, pulmonary function testing, echocardiography and high-resolution computed tomography did not take place at the same time, which represents a limitation on the exact correlation with suPAR. Therefore, whether or not suPAR estimation has clinical utility (implying that biomarker usage can result in better or similar outcomes at lower cost) as a diagnostic and prognostic biomarker in SSc cannot be extrapolated from the findings of our study. Second, the number of patients in our study cohort was low, which decreased the power of discrimination between degrees of disease severity. Finally, we did not have a healthy control cohort for comparison, which would have helped us demonstrate the screening and diagnostic capability of the biomarker for detection of SSc-related organ manifestations.
In conclusion, our study shows that the plasma suPAR-concentrations may reflect interstitial lung disease severity and extent of organ damage in SSc patients. Our findings also indicate the possibility of utilization of suPAR levels to detect the extent of lung involvement in SSc, which in turn can correlate with future prognosis of the affected patient. Our findings may facilitate the development of novel biomarkers for non-invasive disease staging, though large-scale, prospective studies are warranted to investigate the possibility of utilizing suPAR as a marker for screening, monitoring and predicting prognosis in SSc patients.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46