
Competing Interests: The authors have declared that no competing interests exist.
Arcobacter butzleri is an emerging zoonotic food-borne and water-borne pathogen that can cause diarrhea in humans. The global prevalence of A. butzleri infection is underestimated, and little is known about their phenotypic and genotypic characterization. The aim of this study was to determine antimicrobial susceptibility (AST) profiles, detect related virulence genes, and classify sequence type (ST) of A. butzleri isolates obtained from human stool and food samples. A total of 84 A. butzleri isolates were obtained from human diarrheal (n = 25), non-diarrheal (n = 24) stool, and food (n = 35) samples in Thailand. They were evaluated for phenotypic identification by conventional microbiological procedures and AST by Kirby-Bauer disc diffusion method as well as virulence genes detection. Representative isolates from each origin were selected based on the presence of virulence genes and AST profiles to analyze genetic diversity by multilocus sequence typing (MLST). All isolates showed resistance to nalidixic acid 40.5% (34/84), ciprofloxacin 11.9% (10/84), azithromycin 8.3% (7/84), and erythromycin 3.6% (3/84). Regarding the ten virulence genes detected, cj1349, mviN and pldA had the highest prevalence 100% (84/84), followed by tlyA 98.8% (83/84), cadF 97.6% (82/84), ciaB 71.4% (60/84), hecA and hecB 22.6% (19/84), iroE 15.5% (13/84) and irgA 10.7% (9/84), respectively. Three virulence genes were present among A. butzleri isolates of human diarrheal stool and food samples, with a significant difference observed among isolates; hecB [36% (9/25) and 8.6% (3/35)], hecA [36% (9/25) and 5.7% (2/35)], and irgA [24% (6/25) and 2.9% (1/35)] (p < 0.05), respectively. The hecA and hecB virulence genes functions are related to the mechanism of hemolysis, while irgA supports a bacterial nutritional requirement. MLST analysis of 26 A. butzleri isolates revealed that 16 novel STs exhibited high genetic diversity. The results of this study is useful for understanding potentially pathogenic and antimicrobial-resistant A. butzleri in Thailand. The pathogenic virulence markers hecB, hecA, and irgA have the potential to be developed for rapid diagnostic detection in human diarrheal stool. No significant relationships among STs and sources of origin were observed. Little is known about A. butzleri, the mechanism of action of these virulence genes, is a topic that needs further investigation.
Bacteria in the genus Arcobacter are emerging food-borne zoonotic pathogens. Recently, Arcobacter butzleri and Arcobacter cryaerophilus have been classified as microbial hazards to human health by the International Commission on Microbiological Specifications for Foods (ICMSF) [1–3]. Arcobacter spp. are slightly curved shape Gram-negative bacteria possessing one polar flagellum or bipolar flagella. The genus Arcobacter was first identified by Vandamme et al. [4] and currently includes 27 species [5]. Of the 27, A. butzleri, A. skirrowii, and A. cryaerophilus were reported to be associated with human foodborne diseases and isolated from human clinical stool specimens and blood cultures [1, 6–10]. Contaminated undercooked or raw meat i.e. chicken, pork, beef, shellfish, and water have been identified as major sources of infection [11–16]. Recently, a study in Thailand reported that 13% (9/70) of meals served in some restaurants in Bangkok were contaminated with A. butzleri [17]. Furthermore, A. butzleri was detected in 74% (54/73) of raw meat and poultry samples at the local market in Kanchanaburi province located in the western region of Thailand [18].
Antimicrobial susceptibility tests of Arcobacter using Etest, agar dilution, and disc diffusion have been reported [17, 19–21]. Macrolides (erythromycin and azithromycin) or fluoroquinolones (ciprofloxacin) are the recommended drugs of choice for treatment of Arcobacter infections [17, 20, 22]. Tetracyclines and aminoglycosides are alternative treatments for this infection in veterinary and human medicine to overcome resistance [20, 21, 23]. However, few studies investigating antimicrobial susceptibility of Arcobacter strains have been performed in Thailand [17, 18].
The genomic analysis of A. butzleri American Type Culture Collection (ATCC) 49616 revealed ten putative virulence genes: cadF, cj1349, ciaB, hecA, hecB, mviN, pldA, tlyA, irgA, and iroE [24]. Presence of these ten putative virulence genes in Arcobacter spp. isolates from human and food were determined by the PCR-based method [25–27]. The functions of each proposed virulence gene have previously been described in various pathogens. Genes cadF and cj1349 encode for fibronectin-binding proteins that promote the binding of bacteria to intestinal cells [28]. The invasive genes ciaB and Campylobacter invasive antigen B contributes to host cell invasion through a secretion system [29]. HecA is a member of the filamentous hemagglutinin family and was reported to be involved in the attachment, aggregation, and epidermal cell killing of Erwinia chrysanthemi [30]. HecB encodes a hemolysin activation protein [24]. MviN can produce an essential protein required for peptidoglycan biosynthesis in Escherichia coli [31]. The phospholipase gene pldA encoding the outer membrane phospholipase A is associated with lysis of erythrocytes [32]. The hemolysin gene tlyA is also present in Mycobacterium tuberculosis and Serpulina hyodysenteriae [33]. The irgA and iroE genes are part of the functional components for iron acquisition and therefore is required for establishing and maintaining infections [34]. Virulence genes harboring in A. butzleri are mainly cadF, cj1349, ciaB, mviN, pldA, and tlyA with 100% detection in clinical (n = 84), food (n = 218) and environmental (n = 45) samples [25–26, 35] whereas hecA, hecB and irgA genes were identified at 21% (16/78), 68% (53/78), and 35% (27/78) from human specimens, respectively [25]. The virulence mechanisms and pathogenicity of Arcobacter spp. have rarely been demonstrated and is poorly understood. In Thailand, no evidence of Arcobacter virulence genes has been reported in human diarrheal, non-diarrheal stool, and food samples.
The genotypic diversity of Arcobacter spp. is often discriminated by molecular typing methods. Pulsed-field Gel Electrophoresis (PFGE), Amplified Fragment Length Polymorphism (AFLP), Enterobacterial Repetitive Intergenic Consensus (ERIC)-PCR, Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight (MALDI-TOF) Mass Spectrometry (MS) and Multilocus Sequence Typing (MLST) methods have been used for Arcobacter typing from different strains isolated from different sources [36–40]. Miller et al. [39] proposed a MLST scheme for Arcobacter typing using seven housekeeping loci (aspA, atpA, glnA, gltA, glyA, pgm, and tkt). A total of 366 human-related Arcobacter isolates, from four continents and various sources were typed by MLST and found no association among STs and sources of origin or locations [25]. At present, this method currently has been identified as a valuable technique for genotyping and assessing the diversity of Arcobacter spp. in humans [36, 39]. This study aims to determine antimicrobial susceptibility patterns and virulence genes profiles of archived Arcobacter isolated from human stool and food samples. Subsequently, the genetic diversity of selected Arcobacter isolates was analyzed using MLST.
A total of 84 A. butzleri isolates from the Department of Bacterial and Parasitic Diseases, AFRIMS, Bangkok, Thailand were used in this study. Arcobacter spp. were previously isolated from human diarrheal (n = 25) and non-diarrheal (n = 24) stool samples, raw chicken (n = 15), raw beef (n = 11), raw pork (n = 8), and a chicken egg (n = 1) from 2001 to 2016 by the laboratory at AFRIMS. Arcobacter were identified by conventional phenotypic tests as described in Bodhidatta et al. [17]. All archived Arcobacter spp. isolates were grown on a blood agar plate (BAP; 5% sheep blood in Brucella agar, Becton, Dickinson and Company, Sparks, MD, USA) and incubated at 37°C in microaerobic condition for 24–48 h.
Antimicrobial susceptibility of Arcobacter isolates were performed by the Kirby-Bauer disc diffusion method [17, 23, 41]. Staphylococcus aureus ATCC 25923, E. coli ATCC 25922 were used as the reference strains. Eight antimicrobial discs (Becton, Dickinson and Company, Sparks, MD, USA) used in this study were azithromycin (AZM; 15 μg), ciprofloxacin (CIP; 5 μg), erythromycin (ERY; 15 μg), gentamicin (GM; 10 μg), kanamycin (KAN; 30 μg), nalidixic acid (NA; 30 μg), streptomycin (STR; 10 μg), and tetracycline (TE; 30 μg). The zone diameter of each Arcobacter isolate was interpreted by comparing with the zone diameter interpretive standards for Enterobacteriaceae and S. aureus according to the Clinical Laboratory Standards Institute (CLSI) [41]. Multidrug resistance was defined as acquired resistance to at least one antimicrobial agent in three or more antimicrobial drug classes [42].
Primers for cadF, cj1349, ciaB, hecA, hecB, mviN, pldA, tlyA, irgA and iroE genes amplification were obtained from previous studies [25, 26]. A. butzleri ATCC 49616 was used as a positive control. Briefly, the PCR mixture was prepared in a final volume of 25 μl per reaction on a PCR Thermocycler (Veriti 96 well Thermal Cycler, Applied Biosystems, Austin, TX, USA). The reaction mixture consisted of 1X PCR buffer, 0.2 mM dNTP, 50 μM specific primer set, 1.25 U Ampli-Taq Gold polymerase (Applied Biosystems, Austin, TX, USA), and 1 μl genomic DNA. The PCR parameters included initial denaturation at 94°C for 3 min, 32 cycles of 94°C for 45 s, 53°C for ciaB, cj1349, mviN, pldA, and tlyA genes; 55°C for cadF, hecB, and iroE genes; 56°C for hecA, and irgA genes for 45 s, 72°C for 45 s, and a final extension of 72°C for 3 min. Electrophoresis of PCR products in 1.0% agarose gel was performed and stained with ethidium bromide to visualize PCR fragment by using a transilluminator (Alpha Innotech, San Leandro, CA, USA).
MLST scheme and primer sets of seven housekeeping gene loci are available at the Arcobacter MLST website (http://pubmlst.org/arcobacter). A. butzleri ATCC 49616 were used as positive controls of the PCR assays. The PCR mixture was prepared with 50-μls per reaction on a PCR Thermocycler (Veriti 96 well Thermal Cycler, Applied Biosystems, Austin, TX, USA). The reaction mixture consisted of 1X PCR buffer, 0.2 mM dNTP, 50 μM each primer set, 1 U Taq DNA polymerase (Qiagen Inc., Germantown, MD, USA), and 2 μl genomic DNA. The optimal PCR conditions were initial denaturation at 95°C for 2 min, then 35 cycles of 95°C for 45 s, 55°C for pgm; 57°C for aspA, atpA, glnA, gltA, and tkt; 59°C for glyA locus for 45 s, and 72°C for 30 s, and a final extension of 72°C for 10 min. Gel electrophoresis and visualization were performed as described above. The amplicons were purified by using Wizard® SV Gel and PCR clean-up system (Promega, Madison, WI, USA) and were sequenced (1st BASE, The Gemini, Singapore Science Park II, Singapore). Sequences were submitted to the Bacterial Isolate Genome Sequence Database (BIGSDB) [43] at the Arcobacter MLST website (http://pubmlst.org/arcobacter/).
Association of virulence genes and antimicrobial susceptibility of Arcobacter spp. was analyzed by the Chi-Square test in the IBM SPSS Statistics 24 program (IBM, New York, NY, USA). The nucleotide sequences for MLST were aligned and checked for quality by using the Sequencher software version 5.4 (Gene Codes Corporation, Ann Arbor, MI, USA). The phylogenetic analysis and the Minimum Spanning Tree (MST) of Arcobacter isolates in Thailand were studied by using goeBURST implemented in PHYLOViZ [44] online at https://online.phyloviz.net.
A total of 84 A. butzleri isolates originating from human diarrheal (n = 25) and non-diarrheal stool (n = 24), and food samples (n = 35) were tested. The majority of isolates were resistant to NA at 40.5% (34/84) followed by CIP at 11.9% (10/84), AZM at 8.3% (7/84) and ERY at 3.6% (3/84). The resistance rate of A. butzleri isolates from human diarrheal and non-diarrheal stool, and food samples, the majority of the resistance was also to NA at 52% (13/25), 54.2% (13/24) and 22.9% (8/35), respectively. No resistance to aminoglycosides i.g. GM, KAN, and STR, and TE were detected (Table 1). No multidrug resistance was determined in all Arcobacter isolates.

| Antimicrobial agents | Disc content (μg) | No. (%) of isolates resistant to antimicrobial agents | |||
|---|---|---|---|---|---|
| Human diarrheal (n = 25) | Human non-diarrheal (n = 24) | Food (n = 35) | Total (N = 84) | ||
| Macrolide | |||||
| Azithromycin | 15 | 3 (12) | 4 (16.7) | 0 | 7 (8.3) |
| Erythromycin | 15 | 2 (8) | 1 (4.2) | 0 | 3 (3.6) |
| Quinolone | |||||
| Ciprofloxacin | 5 | 2 (8) | 4 (16.7) | 4 (11.4) | 10 (11.9) |
| Nalidixic Acid | 30 | 13 (52)a | 13 (54.2)a | 8 (22.9)a | 34 (40.5) |
| Aminoglycoside | |||||
| Gentamicin | 10 | 0 | 0 | 0 | 0 |
| Kanamycin | 30 | 0 | 0 | 0 | 0 |
| Streptomycin | 10 | 0 | 0 | 0 | 0 |
| Tetracyclines | |||||
| Tetracycline | 30 | 0 | 0 | 0 | 0 |
a Significantly different (Chi-square test; p < 0.05)
The percent resistant to NA in A. butzleri isolates in stool samples from human diarrheal (52%, 13/25) and non-diarrheal (54.2%, 13/24), were significantly higher than those isolates from food samples [(22.9%, 8/35), (p < 0.05)].
Among 84 A. butzleri isolates, the predominant virulence genes were cj1349, mviN, and pldA detected at 100% (84/84), followed by tlyA at 98.8% (83/84), cadF at 97.6% (82/84), ciaB at 71.4% (60/84), hecA and hecB at 22.6% (19/84), iroE at 15.5% (13/84), and irgA at 10.7% (9/84), respectively (Table 2).

| Source | n | No. (%) of isolates generating specific gene amplicon | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhesins | O-Antigen | Invasins | Pore-forming toxins/ haemolysin | Iron uptake systems | |||||||
| cadF | cj1349 | mviN | ciaB | pldA | hecA | hecB | tlyA | irgA | iroE | ||
| Human diarrheal stool | 25 | 25 (100) | 25 (100) | 25 (100) | 20 (80) | 25 (100) | 9a (36) | 9a (36) | 24 (96) | 6a (24) | 6 (24) |
| Human non-diarrheal stool | 24 | 24 (100) | 24 (100) | 24 (100) | 16 (66.7) | 24 (100) | 8a (33.3) | 7 (29.2) | 24 (100) | 2 (8.3) | 4 (16.7) |
| Food | 35 | 33 (94.3) | 35 (100) | 35 (100) | 24 (68.6) | 35 (100) | 2a (5.7) | 3a (8.6) | 35 (100) | 1a (2.9) | 3 (8.6) |
| Chicken eggs | 1 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 0 | 0 | 1 (100) | 0 | 0 |
| Fresh beef | 11 | 10 (90.9) | 11 (100) | 11 (100) | 5 (45.5) | 11 (100) | 1 (9.1) | 1 (9.1) | 11 (100) | 1 (9.1) | 1 (9.1) |
| Fresh chicken meat | 15 | 15 (100) | 15 (100) | 15 (100) | 10 (66.7) | 15 (100) | 0 | 0 | 15 (100) | 0 | 0 |
| Fresh pork | 8 | 7 (87.5) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 1 (12.5) | 2 (25) | 8 (100) | 0 | 2 (25) |
| Total | 84 | 82 (97.6) | 84 (100) | 84 (100) | 60 (71.4) | 84 (100) | 19 (22.6) | 19 (22.6) | 83 (98.8) | 9 (10.7) | 13 (15.5) |
a Significantly different (Chi-square test; p < 0.05)
The prevalence of hecA, hecB, and irgA in A. butzleri isolates from human diarrheal stool samples [hecA 36% (9/25), hecB 36% (9/25), and irgA 24% (6/25), respectively] were significantly higher than those isolates from food samples [5.7% (2/35), 8.6% (3/35), and 2.9% (1/35), respectively] (p < 0.05). Furthermore, hecA in A. butzleri isolates from human non-diarrheal stool samples (33.3%, 8/24) was significantly higher than those isolates from food samples [(5.7%, 2/35) (p < 0.05)].
Among 84 isolates of A. butzleri, the most common virulence genes profiled was cadF-cj1349-ciaB-mviN-pldA-tlyA, which were detected in 48.6% (17/35) from food samples, 41.7% (10/24) from human non-diarrheal stools, and 28% (7/25) from human diarrheal stools. The common virulence genes detected in all A. butzleri were cj1349, mviN, and pldA. Only 14.3% (5/35) of A. butzleri isolates from food samples possessed at least 7 virulence genes whereas 56% (14/25) of A. butzleri isolates from human diarrheal stools possessed those genes. Only one A. butzleri isolate from raw beef harbored all ten virulence genes. Regardless of the sources, all A. butzleri isolates possessed potential virulence genes that can cause diarrheal diseases in humans (Table 3).

| Profile of virulence genes | No. (%) of isolates | ||
|---|---|---|---|
| Human diarrheal stool (n = 25) | Human non-diarrheal stool (n = 24) | Food (n = 35) | |
| Quintuple | 4 (16) | 2 (8.3) | 13 (37.1) |
| cadF-cj1349-mviN-pldA-tlyA | 3 (12) | 2 (8.3) | 11 (31.4) |
| cadF-cj1349-ciaB-mviN-pldA | 1 (4) | 0 | 0 |
| ciaB-cj1349-mviN-pldA-tlyA | 0 | 0 | 2 (5.7) |
| Sextuple | 7 (28) | 12 (50.1) | 17 (48.6) |
| cadF-cj1349-hecA-mviN-pldA-tlyA | 0 | 1 (4.2) | 0 |
| cadF-cj1349-hecB-mviN-pldA-tlyA | 0 | 1 (4.2) | 0 |
| cadF-cj1349-ciaB-mviN-pldA-tlyA | 7 (28) | 10 (41.7) | 17 (48.6) |
| At least septuple | 14 (56) | 10 (41.8) | 5 (14.3) |
| Septuple | 4 (16) | 6 (25.1) | 3 (8.5) |
| cadF-cj1349-ciaB-hecB-mviN-pldA-tlyA | 2 (8) | 1 (4.2) | 1 (2.9) |
| cadF-cj1349-ciaB-hecA-mviN-pldA-tlyA | 0 | 1 (4.2) | 0 |
| cadF-cj1349-hecA-hecB-mviN-pldA-tlyA | 2 (8) | 4 (16.7) | 0 |
| cadF-cj1349-ciaB-mviN-pldA-tlyA-iroE | 0 | 0 | 2 (5.7) |
| Octuple | 6 (24) | 3 (12.5) | 1 (2.9) |
| cadF-cj1349-ciaB-hecA-hecB-mviN-pldA-tlyA | 3 (12) | 0 | 1 (2.9) |
| cadF-cj1349-ciaB-hecA-mviN-pldA-tlyA-iroE | 0 | 1 (4.2) | 0 |
| cadF-cj1349-ciaB-irgA-mviN-pldA-tlyA-iroE | 3 (12) | 2 (8.3) | 0 |
| Nonuple | 4 (16) | 1 (4.2) | 0 (0) |
| cadF-cj1349-ciaB-hecA-mviN-pldA-tlyA-iroE-irgA | 2 (8) | 0 | 0 |
| cadF-cj1349-ciaB-hecA-hecB-mviN-pldA-tlyA-irgA | 1 (4) | 0 | 0 |
| cadF-cj1349-ciaB-hecA-hecB-mviN-pldA-tlyA-iroE | 1 (4) | 1 (4.2) | 0 |
| Decuple | 0 (0) | 0 (0) | 1 (2.9) |
| cadF-cj1349-ciaB-hecA-hecB-mviN-pldA-tlyA- irgA-iroE | 0 | 0 | 1 (2.9) |
Nucleotide sequences of seven housekeeping genes (aspA, atpA, glnA, gltA, glyA, pgm, and tkt) of 26 Arcobacter isolates were analyzed (Table 4). A total of 26 representative isolates of Arcobacter spp. were selected based on the presence of virulence genes, antimicrobial susceptibility patterns, sources of sample, location, and year of isolation for the MLST assay. Among the 26 Arcobacter isolates, 140 alleles and 23 STs were identified across all seven loci. A total of 32 new allele numbers and 16 new STs (ST576, ST582, ST583, ST585, ST591, ST592, ST612-ST621) were identified in the present study. The predominant new alleles was pgm (45.5%; 10/22), followed by glyA (34.8%; 8/23), tkt (27.8%; 5/18), aspA (18.2%; 4/22), atpA (15.8%; 3/19), glnA (10.5%; 2/19), and gltA (5.9%; 1/17).

| No. | Code of isolate | ST | Allele ID of housekeeping genes | Source of sample | Location | Year of isolation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aspA | atpA | glnA | gltA | glyA | pgm | tkt | ||||||
| 1 | AF-ARCO-FC-77 | 30 | 5 | 12 | 7 | 9 | 33 | 7 | 24 | Food (raw chicken) | Bangkok | 2003 |
| 2 | AF-ARCO-FC-78 | 31 | 5 | 12 | 11 | 26 | 36 | 30 | 24 | Food (raw chicken) | Bangkok | 2003 |
| 3 | AF-ARCO-FC-79 | 94 | 21 | 22 | 21 | 24 | 48 | 27 | 25 | Food (raw chicken) | Bangkok | 2003 |
| 4 | AF-ARCO-FC-75 | 74 | 19 | 17 | 17 | 20 | 26 | 22 | 19 | Food (raw chicken) | Bangkok | 2003 |
| 5 | AF-ARCO-FP-80 | 3 | 2 | 2 | 24 | 27 | 112 | 35 | 20 | Food (raw pork) | Bangkok | 2003 |
| 6 | AF-ARCO-HD-17 | 130 | 34 | 12 | 2 | 34 | 58 | 46 | 38 | Human diarrheal stool | Trang | 2006 |
| 7 | AF-ARCO-HD-19 | 582 | 38 | 35 | 26 | 20 | 165 | 51 | 4 | Human diarrheal stool | Trang | 2006 |
| 8 | AF-ARCO-HD-88 | 130 | 34 | 12 | 2 | 34 | 58 | 46 | 38 | Human diarrheal stool | Trang | 2006 |
| 9 | AF-ARCO-HD-56 | 612 | 30 | 5 | 5 | 30 | 615 | 50 | 40 | Human diarrheal stool | Chiang Rai | 2008 |
| 10 | AF-ARCO-HD-57 | 612 | 30 | 5 | 5 | 30 | 615 | 50 | 40 | Human diarrheal stool | Chiang Rai | 2008 |
| 11 | AF-ARCO-HD-74 | 615 | 40 | 17 | 2 | 63 | 54 | 325 | 31 | Human diarrheal stool | Bangkok | 2008 |
| 12 | AF-ARCO-ND-58 | 616 | 3 | 3 | 30 | 15 | 598 | 330 | 4 | Human non-diarrheal stool | Chiang Rai | 2008 |
| 13 | AF-ARCO-ND-59 | 585 | 173 | 41 | 19 | 6 | 599 | 77 | 263 | Human non-diarrheal stool | Chiang Rai | 2008 |
| 14 | AF-ARCO-ND-60 | 617 | 80 | 67 | 49 | 30 | 524 | 263 | 267 | Human non-diarrheal stool | Chiang Rai | 2008 |
| 15 | AF-ARCO-HD-63 | 613 | 285 | 66 | 1 | 20 | 601 | 326 | 261 | Human diarrheal stool | Nakhon Ratchasima | 2009 |
| 16 | AF-ARCO-ND-65 | 583 | 25 | 194 | 127 | 32 | 52 | 42 | 20 | Human diarrheal stool | Pisanulok | 2009 |
| 17 | AF-ARCO-HD-70 | 166 | 50 | 40 | 19 | 45 | 165 | 68 | 48 | Human diarrheal stool | Surajthani | 2009 |
| 18 | AF-ARCO-ND-64 | 618 | 292 | 32 | 152 | 34 | 46 | 327 | 36 | Human non-diarrheal stool | Surajthani | 2009 |
| 19 | AF-ARCO-ND-66 | 130 | 34 | 12 | 2 | 34 | 58 | 46 | 38 | Human non-diarrheal stool | Surajthani | 2009 |
| 20 | AF-ARCO-ND-67 | 592 | 39 | 196 | 180 | 37 | 73 | 322 | 264 | Human non-diarrheal stool | Surajthani | 2009 |
| 21 | AF-ARCO-ND-68 | 619 | 293 | 42 | 3 | 20 | 596 | 331 | 4 | Human non-diarrheal stool | Pisanulok | 2009 |
| 22 | AF-ARCO-ND-69 | 620 | 42 | 25 | 7 | 44 | 616 | 332 | 2 | Human non-diarrheal stool | Surajthani | 2009 |
| 23 | AF-ARCO-ND-71 | 576 | 30 | 5 | 9 | 30 | 120 | 35 | 4 | Human non-diarrheal stoool | Surajthani | 2010 |
| 24 | AF-ARCO-ND-72 | 621 | 3 | 31 | 1 | 20 | 545 | 326 | 261 | Human non-diarrheal stool | Bangkok | 2013 |
| 25 | AF-ARCO-HD-73 | 614 | 81 | 62 | 26 | 144 | 597 | 328 | 260 | Human diarrheal stool | Bangkok | 2014 |
| 26 | AF-ARCO-HD-90 | 591 | 291 | 195 | 173 | 198 | 602 | 318 | 222 | Human diarrheal stool | Chonburi | 2016 |
Boldface entries represent new alleles and STs
The minimal spanning tree (MST) of seven housekeeping genes loci (3,341 bp) was constructed online at https://online.phyloviz.net to find a relationship among the 26 studied isolates, using the 120 isolate database (retrieved Jun 19, 2017, from http://pubmlst.org/arcobacter/), five ATCC and two reference strains. The reference strains consisted of A. butzleri ATCC 49616, A. skirrowii ATCC 51400, A. cryaerophilus ATCC 49615, Arcobacter cibarius LMG 21996, Arcobacter thereius LMG 24486, Campylobacter jejuni ATCC 700819, and Helicobacter pylori ATCC 26695. Overall, all STs of A. butzleri were clustered in one group, whereas the other species were split up and linked between ST111 of A. butzleri and A. skirrowii ATCC 51400. Among 26 Arcobacter isolates, the ST-94, ST-130 and ST-166 (Table 4) are dected in raw chicken and human diarrheal stool which related to the mixed sources of samples including human diarrheal stool and non-diarrheal stool and food samples obtaining from the Arcobacter database (http://pubmlst.org/arcobacter/). In the present study, ST, sources of origins, location, or year were not related, however few isolates from the human diarrheal stool and food samples (chicken offal or meat and pork offal or meat) shared identical ST(s).
In accordance with the MST, 26 Arcobacter isolates from this study and entire 867 Arcobacter isolates obtained from the Arcobacter database (retrieved Jun 19, 2017, from http://pubmlst.org/arcobacter/) (Fig 1) was constructed online at https://online.phyloviz.net. A total of 20 source categories were found in the worldwide Arcobacter MLST database. Taken together, only species-related including A. butzleri, A. cryaerophilus, A. skirrowii, A. cibarius, and A. thereius obtained from the database formed the clusters. No association of sources and genetic profiles of isolates were observed for this organism.

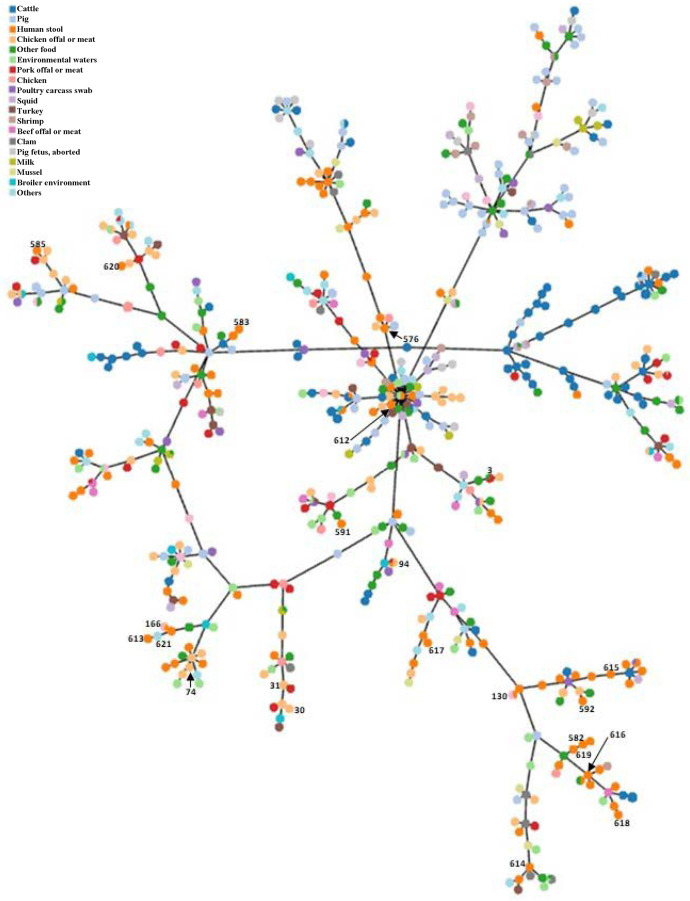
The Minimum Spanning Tree (MST) of all Arcobacter isolates in pubmlst database.
This tree was constructed based on the concatenated sequences of seven housekeeping genes loci (3,341 bp) of 867 Arcobacter isolates obtained from the present study (n = 26) and the database (n = 841). The number beside the node indicates ST in the present study.
In a previous study, the prevalence of Arcobacter spp. showed that the overall percentage of resistance to ciprofloxacin ranged from 5.7–14.8% and 5.6–19.2% for erythromycin [45]. In this study, we found a lower resistance rate to ciprofloxacin at 11.9% (10/84) and erythromycin at 3.6% (3/84) in A. butzleri isolates. The resistance rate to ciprofloxacin was 8.0% (2/25) of the A. butzleri isolated from human diarrheal stool samples and 11.4% (4/35) of the A. butzleri isolated from food samples, which was consistent with previous reports in Thailand [17]. A. butzleri isolates from human diarrheal stool samples showed lower resistance to ciprofloxacin at 3.3% (2/61) in Belgium [22] and 7.4% (2/27) in Spain [46], whereas resistance to ciprofloxacin was significantly higher in the USA than those in Asia (P < 0.001) [45]. Results from our study showed that A. butzleri isolates from human diarrheal stool samples had low resistance to erythromycin at 8.0% (2/25) and none of the A. butzleri isolates from food samples were resistant to erythromycin. However, previous studies showed that isolates from the human diarrheal stool and food samples were erythromycin-resistant at 4–21% and 0–12%, respectively [19, 22, 46–50]. A. butzleri showed 100% (84/84) susceptibility to gentamicin, kanamycin, streptomycin, and tetracycline in the present study. These results are similar to the previous reports which suggested that the aminoglycosides (gentamicin, kanamycin, and streptomycin) and tetracyclines (tetracycline) can be used as alternative drugs of choice [22, 26, 47, 49]. A previous study showed 93.8% (75/80) multidrug resistance in A. butzleri isolates [50] and 68.9% (440/638) in Arcobacter spp. [45]. Nevertheless, no multidrug resistance was observed in our study. Antimicrobial susceptibility in this study indicated that fluoroquinolones and macrolides are currently suitable treatments for Arcobacter infections in Thailand.
This study is the first to report the detection of ten virulence genes, including cadF, cj1349, ciaB, hecA, hecB, mviN, pldA, tlyA, irgA, and iroE of Arcobacter spp. isolates in Thailand. The presence of A. butzleri virulence genes showed similar results to Karadas et al. [26] who studied ten virulence genes to investigate the potential pathogenic A. butzleri isolated from food samples, and water. Almost all A. butzleri isolates possessed genes cadF, cj1349, ciaB, mviN, pldA, and tlyA, and rarely possessed hecA, hecB, irgA, and iroE [21, 27, 35, 46, 51–53]. In particular, genes hecA 36% (9/25), hecB 36% (9/25), and irgA 24% (6/25) were detected in human isolates at a higher rate than those from food samples [5.7% (2/35), 8.6% (3/35), and 2.9% (1/35), respectively] (p < 0.05). This result is consistent with previous studies [25, 35] implying that these genes might play an important role associated with the human host.
Genetic diversity of Arcobacter spp. was determined by MLST. The MLST of 26 A. butzleri isolates, 140 alleles, and 23 STs were identified, with 16 novel STs and 22.9% (32/140) new alleles being reported. Miller et al. [39] reported no association between STs from clinical, food, and environmental samples with a host or geographical source. Additionally, MLST revealed the genetic diversity of A. butzleri isolates from various samples and showed no association of alleles and STs with animal fecal samples [54], products of animal origin [55], food and contact surfaces [40]. Moreover, ST-617 from our study in Thailand was clustered with samples from the University Hospital Sant Joan de Reus (n = 3), the University Hospital Joan XXIII (n = 4) in Spain, and one STs from the USA [46]. Furthermore, A. butzleri isolates with ST-94 and ST-166 were found in both human diarrheal stool samples and chicken offal or meat samples in Thailand. The highest of STs in this study was ST-130, the result was similar to the high STs in Thailand (there were four isolates in each ST-56, ST-94, ST-117, and ST-130). The ST-130 was previously identified in A. butzleri isolated from human diarrheal stool sample in Vietnam (2002) and human non-diarrheal stool sample in Thailand (2002) [39] whereas three isolates of ST-130 in our study were isolated from two human diarrheal stool samples in Trang province (2006) and one human non-diarrheal stool sample at Surajthani province (2009), Thailand. Our MLST, ST-94 and ST-166 presented that raw chicken is a possible source of Arcobacter transmission (http://pubmlst.org/arcobacter/). However, no distinct correlation was observed between the origin of the sample and the geographical location. Also, the results from previous studies showed the persistence of the same ST from the same source, indicating possible cross-contamination between food and environmental sites [56–58]. In the present study, only 26 isolates were analyzed, the range of allelic density (number of alleles/number of strains) was 65.4% (17/26) at the gltA locus and 88.5% (23/26) at the glyA locus, whereas worldwide allelic density for glnA locus is 17.9% (155/867) and 48.4% (420/867) for glyA locus. The highest allelic density of A. butzleri was observed at 88.5% (23/26) for glyA and followed by 84.6% (22/26) for pgm. These findings coincided with the MLST study that the highest allelic density of A. butzleri was 68% (21/31) for glyA and followed by 54% (13/24) for pgm in the Northern part of Spain [55], and 28.2% (11/39) for glyA and 25.6% (10/39) for pgm in the United Kingdom [54]. This report of the high allelic density at the glyA and pgm loci is consistent with the first MLST study for A. butzleri [39]. Furthermore, the allelic density of 26 A. butzleri isolates in Thailand showed high diversity that ranged from 65.4% (17/26) of gltA to 88.5% (23/26) of glyA.
Antimicrobial resistant strains of A. butzleri in meats should be monitored for contamination and for antimicrobial resistance strains in food products. These pathogenic virulence markers such as hecB, hecA, and irgA have the potential to be developed for rapid diagnostic detection in human diarrheal stool. The glyA and pgm loci are important for studying the genetic diversity of Arcobacter spp. The collection and analysis of a larger sample size of A. butzleri isolates will generate a more comprehensive epidemiological understanding of this microorganism that is emerging as an important foodborne illness.
We acknowledge the Department of Bacterial and Parasitic Diseases Department, AFRIMS, Bangkok, for providing Arcobacter isolates.
Disclaimer: Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58