
The authors have declared that no competing interests exist.
Trichomonas vaginalis is a common protozoan parasite, which causes trichomoniasis associated with severe adverse reproductive outcomes. However, the underlying pathogenesis has not been fully understood. As the first line of defense against invading pathogens, the vaginal epithelial cells are highly responsive to environmental stimuli and contribute to the formation of the optimal luminal fluid microenvironment. The cystic fibrosis transmembrane conductance regulator (CFTR), an anion channel widely distributed at the apical membrane of epithelial cells, plays a crucial role in mediating the secretion of Cl− and HCO3−. In this study, we investigated the effect of T. vaginalis on vaginal epithelial ion transport elicited by prostaglandin E2 (PGE2), a major prostaglandin in the semen. Luminal administration of PGE2 triggered a remarkable and sustained increase of short-circuit current (ISC) in rat vaginal epithelium, which was mainly due to Cl− and HCO3− secretion mediated by the cAMP-activated CFTR. However, T. vaginalis infection significantly abrogated the ISC response evoked by PGE2, indicating impaired transepithelial anion transport via CFTR. Using a primary cell culture system of rat vaginal epithelium and a human vaginal epithelial cell line, we demonstrated that the expression of CFTR was significantly down-regulated after T. vaginalis infection. In addition, defective Cl− transport function of CFTR was observed in T. vaginalis-infected cells by measuring intracellular Cl− signals. Conclusively, T. vaginalis restrained exogenous PGE2-induced anion secretion through down-regulation of CFTR in vaginal epithelium. These results provide novel insights into the intervention of reproductive complications associated with T. vaginalis infection such as infertility and disequilibrium in vaginal fluid microenvironment.
Trichomonas vaginalis is a common sexually transmitted parasite that colonized the urogenital mucosa and causes trichomoniasis, a neglected sexually transmitted infection associated with multiple adverse reproductive outcomes in humans. However, the underlying mechanisms remain largely unknown. The epithelial cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel conducting both Cl− and HCO3−, which participates in the regulation of luminal fluid microenvironment conducive to the success of reproductive events. Prostaglandin E2 (PGE2), a bioactive molecule abundant in human seminal fluid, has been demonstrated to exhibit a robust pro-secretory action by activating CFTR in the female genital tract epithelial cells such as endometrial epithelium. These discoveries motivated the authors to investigate the effect of T. vaginalis infection on exogenous PGE2-induced transepithelial transport of electrolytes in vagina. Here, we found that in rat vaginal epithelium, luminal administration of PGE2 elicited a response of Cl− and HCO3− secretion mediated by cAMP-activated CFTR. However, T. vaginalis infection impaired transepithelial anion transport evoked by PGE2, which is probably related to the defective expression and function of CFTR. These outcomes may complement and expand our knowledge of the complex interaction between T. vaginalis and the infected host, providing a novel therapeutic strategy for disequilibrium in vaginal fluid microenvironment and infertility induced by T. vaginalis infection.
The vaginal mucosa is covered with protective stratified squamous epithelial cells served as the sentinels of vaginal defense against potential invading offenders [1]. In the female genital tract, epithelial cells also play vital roles in regulating luminal fluid microenvironments, such as pH, osmolarity and ionic milieu, which is conducive to the success of reproductive events [2]. Vaginal epithelium actively mediates electrolyte transport via multiple ion channels and transporters [2]. Among these ionic-transport proteins, the celebrated cAMP-dependent cystic fibrosis transmembrane conductance regulator (CFTR) is the major anion channel distributed widely in the apical membrane of epithelial cells [3]. The CFTR channel mediates transepithelial Cl− and HCO3− transport in both female and male genital tracts including the vagina, playing crucial roles in various reproductive events such as the maintenance of luminal fluid microenvironment homeostasis [4–9]. Conversely, the absence or dysfunction of CFTR results in alterations in the reproductive tract luminal fluid microenvironment and a higher incidence of infertility [10]. The expression and activity of CFTR are dynamically regulated by hormones, neurotransmitters and bioactive signaling factors in the genital tract microenvironment. As one of the predominant prostaglandins in the semen of fertile men, prostaglandin E2 (PGE2) functions as a critical regulator in diverse reproductive events, such as sperm maturation, ovulation, fertilization, embryo development and early implantation [11–14]. In endometrial epithelium, PGE2 has been shown to stimulate CFTR-dependent anion secretory activity [15–17]. However, little is known about the role of the seminal PGE2 in mediating electrolyte transport across the vaginal epithelium.
Trichomonas vaginalis is a flagellated parasite commonly colonizing the vagina and causes trichomoniasis, one of the most prevalent sexually transmitted infections in humans [18,19]. T. vaginalis usually harbors T. vaginalis virus and Mycoplasma hominis, which disrupts the equilibrium of lactobacilli-dominant vaginal microbiota and synergistically augments the pro-inflammatory responses in both reproductive tracts including the vagina and prostate [20–23]. Trichomoniasis has been demonstrated to be associated with severe adverse reproductive outcomes, such as infertility and multiple pregnancy complications [24–26], whilst the underlying mechanisms remain largely unclear. Recent studies showed that infection with pathogens including Campylobacter jejuni [27] and Toxoplasma gondii [28] impaired the function of epithelial Cl− secretion mediated by CFTR, suggesting a close correlation between pathogen infection and aberrant function of CFTR in host epithelial cells. As the prerequisite to colonization, T. vaginalis adheres to the host vaginal epithelial cells and initiates the inflammatory response [18,23,29,30]. Our previous work has revealed that in human vaginal epithelial cells, T. vaginalis infection elicited the down-regulation of CFTR in a cysteine protease-dependent manner, thereby mediating epithelial inflammation via intracellular Cl− signaling pathways [30]. These observations indicated the putative impairment of ion transport pathways and the imbalanced vaginal luminal fluid microenvironment after T. vaginalis infection. Therefore, this study aims to investigate the effect of T. vaginalis infection on the CFTR-dependent anion secretion of vaginal epithelium induced by exogenous PGE2 and elucidate the underlying mechanisms.
Animal care and experimentation were performed following the guidelines described by the Sun Yat-sen University Animal Use Committee (Guangzhou, China). All procedures were approved by the Sun Yat-sen University Animal Use Committee (Guangzhou, China).
T. vaginalis strain CPO 02 was obtained as a kind gift from Prof. Zhao-Rong Lun (School of Life Sciences, Sun Yat-sen University, Guangzhou, China), and was cultured as previously described [30]. Briefly, the T. vaginalis trophozoites were cultured in Diamond’s Trypticase Yeast Extract Maltose (TYM) medium (Huankai Microbial, China), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS, Tianhang Biotechnology, China), penicillin/streptomycin (100 U/mL/100 μg/mL, Hyclone, USA) at 37°C in an atmosphere of 5% CO2 [31]. Only the parasites in a logarithmic phase of growth were used for assays in this study.
Female Sprague-Dawley (SD) rats, weighing 200–250 g, were purchased from the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China). The rats received a subcutaneous injection of 0.5 mg β-estradiol (Sigma Aldrich, USA) every second day to initiate a persistent estrus. The rats were subsequently infected intravaginally with 3×107 T. vaginalis trophozoites once a day in the next consecutive two days, whereas the control group rats were pipetted intravaginally with an equal volume of phosphate-buffered saline (PBS, pH 7.4). The rats were euthanized 24 h following the last infection, and the excised vaginal mucosa was used for the measurement of short-circuit current (ISC).
Female SD rats were euthanatized by CO2 asphyxia and the freshly excised vaginal tissues were washed and cut into small pieces under antiseptic conditions. The finely minced tissues were subsequently digested enzymatically twice (1 h for each time) with type I collagenase (0.5 mg/mL, C0130, Sigma-Aldrich, USA) dissolved in DMEM/F12 (Gibco, USA) at 37°C, with gentle agitation. The digested tissues were further dissociated and dispersed by repeatedly pipetting and centrifuged at 500 g for 30 s. Afterward, the isolated cell clusters were resuspended and washed twice with DMEM/F12 (Gibco, USA) medium containing 10% FBS (Gibco, USA). The fragments were collected and placed in the six-well plate, then humidified in keratinocyte serum-free medium (K-SFM, Gibco, USA) supplemented with bovine pituitary extract (50 μg/mL, Gibco, USA), recombinant epidermal growth factor (5 ng/mL, Gibco, USA), 100× insulin-transferrin-selenium solution (1×, Gibco, USA), cholera toxin (Sigma Aldrich, USA) and penicillin/streptomycin (100 U/mL/100 μg/mL, Hyclone, USA), in an atmosphere of 5% CO2 at 37°C. After 24 h, the fragments were washed and cells were cultured with the supplemented K-SFM medium. On day 5, the cells were used for immunofluorescence staining or incubated with live T. vaginalis (1×106) for Western blot assay.
Human vaginal epithelial cell line VK2/E6E7 cells was purchased from Beijing ZhongYuan Ltd (China) and were cultured in K-SFM supplemented with bovine pituitary extract (50 μg/mL, Gibco, USA), recombinant epidermal growth factor (0.1 ng/mL, Gibco, USA), 1% (v/v) penicillin-streptomycin (Hyclone, USA) and 0.4 mM CaCl2 (Guangzhou Chemical Pharmaceutical Factory, China) at 37°C with 5% CO2 in a humidified atmosphere.
To measure the transepithelial ISC, in vitro Ussing chamber experiments equipped with vaginal tissues of female SD rats was carried out as described previously [7,8]. Briefly, the freshly isolated rat vaginal mucosae were mounted vertically between two halves of the Ussing chambers, with an available permeation area of 0.45 cm2. Each side of the mucosal sheet was bathed symmetrically with Krebs-Henseleit (K-H) buffer bubbled continuously at 37°C with carbogen (95% O2, 5% CO2) to maintain a constant pH of 7.4. The normal K-H solution (pH 7.4) was composed as follows (in mM): 117 NaCl, 4.7 KCl, 1.2 MgSO4·7H2O, 24.8 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, and 11.1 D-glucose (Guangzhou Chemical Pharmaceutical Factory, China). Two pairs of Ag/AgCl electrodes were used to measure the basal transepithelial potential exhibited by different epithelia. The electrodes were coupled to a multichannel voltage-current clamp (MODEL VCC MC6, Physiologic Instruments, USA) and connected to each chamber by the agar-salt bridges made of 3 M KCl and 3% (wt/vol) agar. The ISC (in μA) was recorded continuously when the voltage of the tissue was clamped at 0 mV by the automatic voltage clamp amplifier. When the ISC was stable, different stimulations were pipetted into the apical side or basolateral side of the epithelium as needed. The changes in ISC (ΔISC) were recorded and normalized for the opening area of the Ussing chamber (in ΔμA/cm2).
In ion substitution experiments, gluconate was used to replace Cl− in Cl−-free K-H solution, whereas N-2-hydroxyethylpiperazine-N-2-ethane sulphonic acid (HEPES, Mbchem Technology, China) was used to replace NaHCO3 in HCO3−-free K-H solution, which was gassed with 100% O2 throughout the experiment.
The primary cultured rat vaginal epithelial cells or the VK2/E6E7 cells were seeded on sterile glass coverslips. On day 5, the primary cells were used to certify the location of keratin and CFTR. For T. vaginalis infection, the primary cells were infected with 1 × 106 T. vaginalis for 3 h and the VK2/E6E7 cells were infected with 2 × 105 T. vaginalis for 3 h before the immunofluorescence assay. The samples were washed with pre-cold PBS and then fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and blocked with 3% bovine serum albumin (BSA) for 1 h at room temperature. The cells were then incubated at 4°C overnight with mouse anti-Pan-Keratin (#4545, Cell Signalling Technology, USA) or mouse anti-CFTR antibody (ab2784, Abcam, UK), followed by Alexa Fluor 488-labeled donkey anti-mouse IgG (A-21202, Thermo Fisher Scientific, USA) for 1 h at room temperature. The nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, C1006, Beyotime, China). The fluorescence was examined by laser scanning confocal microscopy (TCS-SP5, Leica, Germany).
Western blot was performed as described previously [30]. The primary antibody against CFTR (1:500, ab2784) was purchased from Abcam (UK), and the antibody against β-actin (1:1000, #4970) was purchased from Cell Signalling Technology (USA).
The measurement of intracellular Cl− was performed as previously described [30]. Briefly, the primary cultured rat vaginal epithelial cells or the VK2/E6E7 cells were grown on glass cover slips with or without T. vaginalis infection for 3 h. The changes of fluorescence intensity of the Cl− indicator dye, MQAE, was recorded using an imaging system (Olympus, IX83, Tokyo, Japan).
All statistical data were summarized as the mean ± standard deviation (S.D). Student’s two tailed t test was used for pairwise comparisons, and one-way ANOVA with Bonferroni was used to test for multiple comparisons. Data were analyzed using Origin Pro 8.0 software (OriginLab, USA), and P values < 0.05 were considered significantly different. The numerical data used in all figures are included in S1 Data.
To explore the potential effect of seminal PGE2 on transepithelial electrolyte transport in the vagina, we measured the ISC using the Ussing chamber system. Apical administration of PGE2 (50 nM) induced a remarkable ISC response characterized by a rapid increase and a long duration (Fig 1A). In addition, luminal PGE2-induced ISC response was in a concentration-dependent manner with a half-maximal effective concentration (EC50) of 24.9 nM (Fig 1B). Thus, a concentration of 50 nM was chosen for the subsequent experiments.

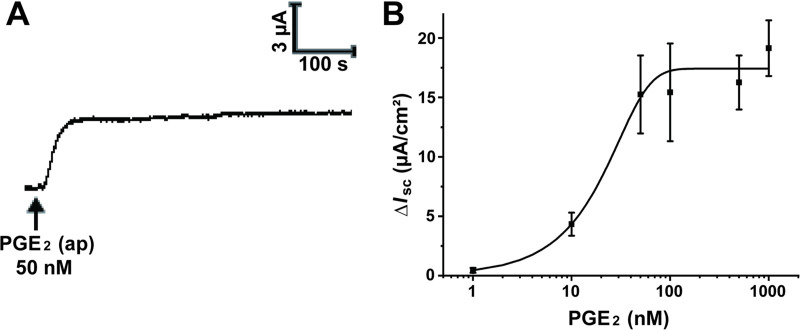
Apical administration of prostaglandin E2 (PGE2) stimulated an increase in short-circuit current (ISC) across rat vaginal epithelium.
(A) Representative trace showing the effect of the apical (ap) application of PGE2 (50 nM) on ISC response in rat vaginal epithelium. (B) The concentration-dependent curve of PGE2 induced ISC responses with a half-maximal effective concentration (EC50) of 24.9 nM. Symbols and bars indicate the means ± S.D. (n = 3–5).
An increase in ISC response represents net anion secretion or net cation absorption. Our previous study has shown that amiloride, a potent inhibitor of the epithelial Na+ channel (ENaC), significantly decreased the basal ISC of rat vaginal epithelial cells [8], indicating the important role of ENaC-mediated Na+ influx in vaginal epithelial ion transport. Therefore, we initially investigated whether seminal PGE2 promoted the absorption of Na+ and thereby increased the ISC response. As is shown in S1 Fig, luminal pretreatment with amiloride (100 μM) failed to affect the ISC response induced by PGE2, excluding the involvement of ENaC in this process. On the contrary, the PGE2-elicited ISC responses were markedly suppressed in Cl−-free, HCO3−-free or both Cl− and HCO3−-free solution (S2 Fig), revealing that luminal administration of PGE2 in vaginal epithelium stimulated transepithelial secretion of Cl− and HCO3−.
The CFTR channel reportedly mediates the transepithelial anion transport across the apical membrane of epithelium, including the vaginal epithelium [7,8]. We next sought to validate the involvement of CFTR in the ISC response induced by the luminal administration of PGE2. Both the non-selective Cl− channel blocker DPC (1 mM) and the selective CFTR blocker CFTRinh-172 (10 μM) remarkably inhibited the luminal PGE2-elicited increase of ISC (S3 Fig), suggesting that the ISC response was mediated by activation of CFTR. Considering that CFTR is activated by elevation of intracellular cAMP, we further investigated the cellular mechanism underlying seminal PGE2-induced CFTR activation. Pretreatment with either forskolin (20 μM), an activator of adenylate cyclase, or MDL-12330A (10 μM), an inhibitor of adenylate cyclase, potently abolished the ISC response (S3 Fig), which implied that luminal PGE2 facilitated anion secretion by activating CFTR via adenylate cyclase-cAMP signaling pathway.
Finally, we also verified the involvement of Na+-K+-2Cl− cotransporter (NKCC), which mediates Cl− uptake across the basolateral membrane and accumulate Cl− intracellularly [32,33]. Notably, the apical PGE2-stimulated ISC response was significantly diminished by bumetanide (100 μM), an inhibitor of NKCC (S4 Fig), confirming the critical role of NKCC in luminal PGE2-induced ISC response by supplying Cl−.
T. vaginalis infection is associated with an abnormal vaginal luminal fluid microenvironment. To investigate the effect of T. vaginalis infection on the vaginal transepithelial anion secretion induced by luminal PGE2, we established a rat model of T. vaginalis infection. As illustrated in Fig 2, compared with the control group, T. vaginalis infection remarkably attenuated the ISC response elicited by apical administration of PGE2, revealing that T. vaginalis infection impaired transepithelial anion secretion in rat vaginal epithelium.

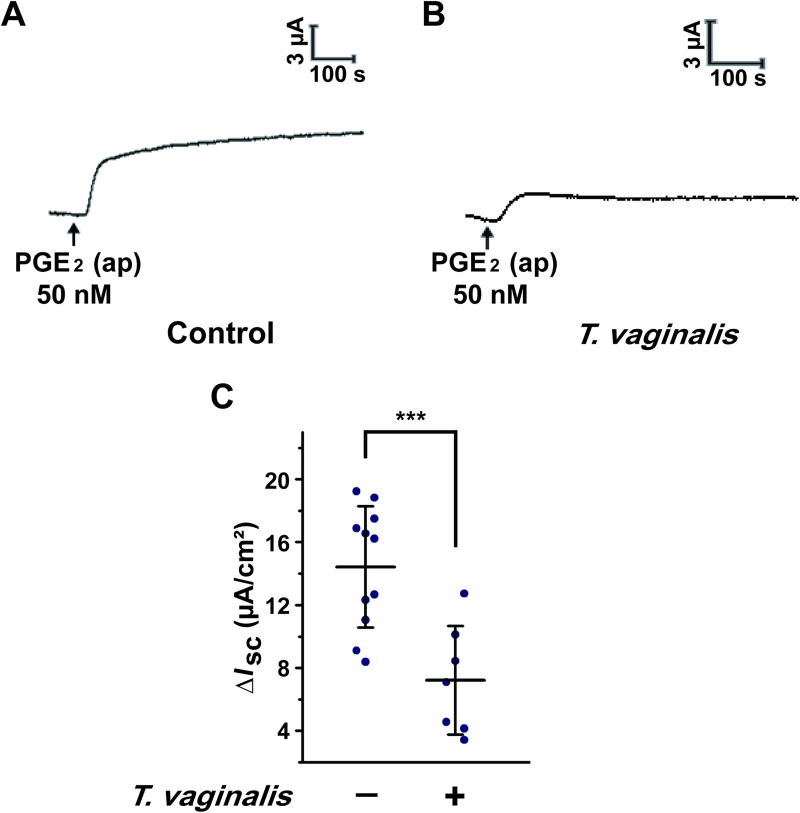
Trichomonas vaginalis infection impaired the anion transport induced by prostaglandin E2 (PGE2) in rat vaginal epithelium.
(A-B) Representative trace showing the short-circuit current (ISC) response induced by apical (ap) PGE2 (50 nM) with (B) or without (A) intravaginal T. vaginalis infection in rats. (C) Statistical analysis showing the effect of T. vaginalis infection on the ISC currents induced by apical PGE2 (50 nM) in rat vaginal epithelium. Symbols and bars indicate the mean ± S.D. (n = 7–11, *** P < 0.001).
In the light of the crucial role of CFTR in mediating anion secretion induced by luminal administration of PGE2 in vaginal epithelium, we then tested the effect of T. vaginalis infection on the expression and function of CFTR. We successfully established a primary rat vaginal epithelial cell culture system. As shown in S5 Fig, the primary cultured vaginal epithelial cells retained the epithelial morphology in light microscopy, with positive expression of CFTR and pan-keratin, a marker for epithelial cells. After T. vaginalis infection, however, the expression of CFTR was significantly down-regulated in primary cultured vaginal epithelial cells by using Western blot and immunofluorescence staining analysis (Fig 3A–3D). Furthermore, we also evaluate the effect of T. vaginalis infection on CFTR function using the intracellular Cl− measurement technique. Treatment with CFTRinh-172 (10 μM) elicited a decrease of MQAE fluorescence in the primary cultured rat vaginal epithelial cells, which represented an increase of intracellular Cl− concentration owing to the dysfunction of CFTR as described previously [30]. Nevertheless, the decrease of MQAE fluorescence elicited by CFTRinh-172 was significantly restrained after T. vaginalis infection (Fig 3E–3G), indicating that T. vaginalis impaired the Cl− transport function of CFTR in rat vaginal epithelial cells. Similar results were observed in the human vaginal epithelial VK2/E6E7 cells (Fig 4). Taken together, these results demonstrated that T. vaginalis infection triggered defective CFTR expression and function in vaginal epithelium, which might be the probable cause of the decreased PGE2-elicited ISC response after T. vaginalis infection.

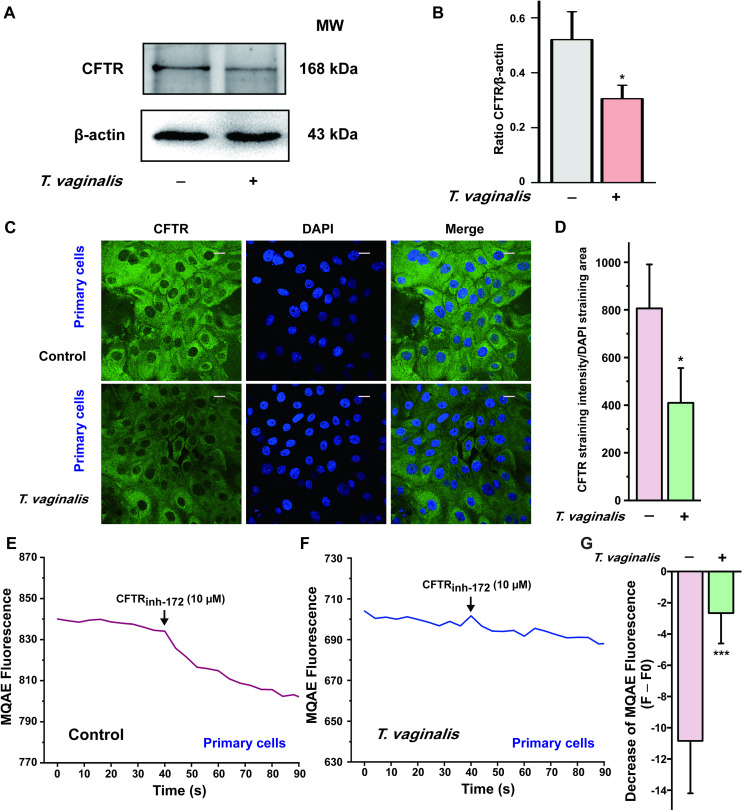
Trichomonas vaginalis infection induced down-regulation of cystic fibrosis transmembrane conductance regulator (CFTR) in the primary cultured rat vaginal epithelial cells.
(A) Representative blots showing the expression of CFTR in primary cultured vaginal epithelial cells infected with live 1 × 106 T. vaginalis for 3 h, using β-actin as a loading control. MW, molecular weight. (B) Statistical analysis of Western blot (CFTR/β-actin ratio) showing the effect of T. vaginalis infection on the expression of CFTR. Symbols and bars indicate the mean ± S.D. (n = 3, * P < 0.05 versus the non-infected group). (C) Immunofluorescence images showing the expression of CFTR in primary cultured rat vaginal epithelial cells, in the absence or presence of 1 × 106 T. vaginalis infection, with (D) the corresponding quantification analysis (n = 3, * P < 0.05 versus the non-infected group). Scale bar = 20 μm. (E) Representative trace showing the change of MQAE fluorescence elicited by CFTRinh-172 (10 μM) in primary cultured rat vaginal epithelial cells. (F) Representative trace showing the change of MQAE fluorescence elicited by CFTRinh-172 (10 μM) after 1 × 106 T. vaginalis infection for 3 h. (G) Statistical analysis showing the change of MQAE fluorescence intensity elicited by CFTRinh-172 (10 μM), with or without T. vaginalis infection. Symbols and bars indicate the mean ± S.D. (n = 31–42 cells for each group, *** P < 0.001 versus the non-infected group).

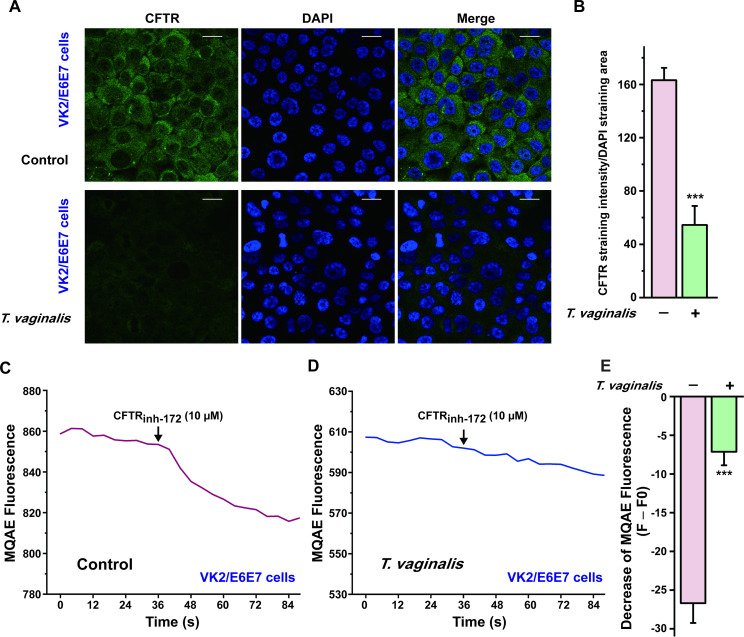
Trichomonas vaginalis infection induced down-regulation of cystic fibrosis transmembrane conductance regulator (CFTR) in human vaginal epithelial VK2/E6E7 cells.
(A) Immunofluorescence images showing the expression of CFTR in VK2/E6E7 cells, in the absence or presence of 2×105 T. vaginalis infection for 3 h, with (B) the corresponding quantification analysis (n = 3, *** P < 0.001 versus the non-infected group). Scale bar = 20 μm. (C) Representative trace showing the change of MQAE fluorescence elicited by CFTRinh-172 (10 μM) in VK2/E6E7 cells. (D) Representative trace showing the change of MQAE fluorescence elicited by CFTRinh-172 (10 μM) after 2×105 T. vaginalis infection for 3 h. (E) Statistical analysis showing the change of MQAE fluorescence intensity elicited by CFTRinh-172 (10 μM), with or without T. vaginalis infection. Symbols and bars indicate the mean ± S.D. (n = 39–41 cells for each group, *** P < 0.001, versus the non-infected group).
T. vaginalis is a common sexually transmitted eukaryotic parasite, which adheres to vaginal epithelial cells and causes trichomoniasis [18,23,29]. Although polarized epithelial cells in the female reproductive tract have been recognized as the sentinels of immune protection [1], the mechanisms underlying the host-parasite interaction are still not clearly understood. In this study, we established a rat model of T. vaginalis infection and elucidated that T. vaginalis infection impaired the transepithelial anion secretion triggered by luminal PGE2 via down-regulation of CFTR (Fig 5).

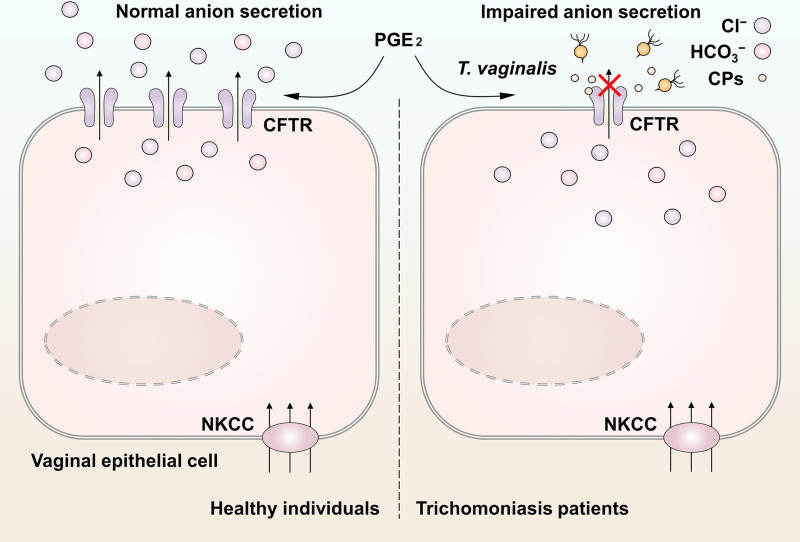
Schematic model of the impaired anion secretion triggered by Trichomonas vaginalis infection in vaginal epithelium.
Seminal prostaglandin E2 (PGE2) facilitated anion secretion by activating cystic fibrosis transmembrane conductance regulator (CFTR) in vaginal epithelium. The basolateral Na+-K+-2Cl− cotransporter (NKCC) guaranteed the maintenance of steady anion secretion by supplying Cl−. After T. vaginalis infection, the CFTR was markedly down-regulated via cysteine proteases secreted by the pathogens, which restrained the exogenous PGE2-induced anion secretion. This may be a cause of the abnormal vaginal fluid microenvironment in patients with trichomoniasis.
PGE2 is synthesized from arachidonic acid via the cyclooxygenase pathway in various cell types [34]. In fertile men, a high level of PGE2 with a concentration of approximate 70 mg/L was detectable in the semen [11,35]. Seminal prostaglandins have been shown to protect sperm from immunological damage in the male genital tract and actively regulate sperm maturation and sperm motility [12,14,36]. A lower level of PGE2 was observed in the seminal plasma of infertile men with genital tract infection than that in fertile men, revealing the important role of PGE2 in male reproductive health [37]. In the female reproductive system, PGE2 also plays a pivotal role in various physiological events, including ovulation, fertilization, embryo development, early implantation, and provides a tolerogenic immune microenvironment [13,14]. Previous research has demonstrated that PGE2 induced a potent and sustained increase of ISC in the female genital tract epithelial cells such as endometrial epithelium [16,17], suggesting that exogenous PGE2 from the ejaculated semen might be implicated in mediating ion transport processes in vagina mucosa after coitus. Notably, our results showed that the luminal administration of PGE2 elicited anion (mainly Cl− and HCO3−) secretion across the rat vaginal epithelium. This response was presumably via activation of the cAMP-dependent CFTR channel, which is consistent with previous observations in human bronchial epithelial cells [38]. Moreover, basolateral NKCC was also involved in PGE2-elicited anion transport, which supported the apical Cl− secretion via cellular supply of Cl− [5,32,39,40]. Transepithelial anion secretion is responsible for contributing to the formation of the optimal luminal fluid microenvironment in the vagina. On one hand, Cl− secretion provides the osmotic driving force for passive H2O transport [39], which leads to vaginal lubrication [7,8]. On the other hand, HCO3− secretion to the vaginal lumen might be conducive to regulating luminal pH homeostasis and sperm functions [41,42]. An optimal luminal vaginal pH is essential for fertilization since sperm are susceptible to the acidic pH after deposition [43]. Thus, our results indicated that during semen deposition in the vagina, the seminal PGE2 promoted anion secretion across the vaginal epithelial cells, which might be indispensable for fluid secretion and regulation of luminal pH and sperm motility.
Recent studies have demonstrated the impairment of transepithelial Cl− transport after infection with pathogens including C. jejuni [27], T. gondii [28], and influenza virus [44]. Here in our study, we verified that T. vaginalis infection significantly inhibited anion secretion elicited by the luminal administration of PGE2 in rat vaginal epithelium. As the major channel mediating anion transport, the apically located CFTR was reportedly down-regulated after pathogenic infection in host epithelial cells [27,44,45]. Consistent with these observations, we showed that T. vaginalis infection triggered down-regulation of CFTR in primary cultured rat vaginal epithelial cells, which may probably be the cause of impaired anion secretion. Our previous work has revealed that the cysteine proteases secreted by T. vaginalis degraded CFTR protein in human vaginal epithelial VK2/E6E7 cells [30]. We speculated that T. vaginalis infection-induced impairment of transepithelial anion secretion may also be attributed to the effects of cysteine proteases secreted by the parasites. Previous studies have highlighted the linkage between defect of CFTR and impaired host defense function of epithelial cells and neutrophils [46,47]. Here, our findings extended the previously recognized involvement of cysteine proteases in T. vaginalis immune-evasive behaviors, which was not solely associated with the degradation of antibodies [23], but also mediated host-defense failure via degradation of CFTR expressed in vaginal epithelial cells and neutrophils.
In addition to inflammation, multiple complications including infertility, are associated with trichomoniasis. Clinical data showed that the prevalence of T. vaginalis infection in infertile women was significantly higher than that in the control group [48]. Additionally, trichomoniasis-related fertility disorders may be ascribed to the phagocytosis of sperm cells during their journey along the female reproductive tract [25,49]. Previous investigations showed that a PGE2-rich microenvironment protects sperm from phagocytosis [50]. Our findings indicated that the optimal luminal fluid microenvironment modulated by PGE2 could be destroyed by T. vaginalis infection. This might have, at least partially, accounted for the protective role of PGE2 and the pathogenesis of trichomoniasis-related sperm phagocytosis. As the sexually transmitted protozoan, T. vaginalis may be transmitted from both male or female carriers to their partners through sexual intercourse. Considering the evidence of the acute and chronic inflammation in the prostate after T. vaginalis infection [20,51], we speculated that defective CFTR expression and function may also exist in the upper genital tract epithelium of men infected with T. vaginalis, although further studies are required. Additionally, sexual transmission of T. vaginalis complicates the scenarios of T. vaginalis-PGE2-CFTR interactions, leading to different short-term impact and long-term outcomes, especially when co-infected with other bacterial pathogen or in symbiosis with T. vaginalis virus and M. hominis [22,23]. These situations synergistically worsen the inflammatory damage to the genital tract. It should be noted that new evidence supports the atypical locations of trichomonads including T. vaginalis and zoonotic trichomonads in the human respiratory tract [52,53]. In the light of our previous work that CFTR dysfunction elicited chronic airway inflammation via Cl−-sensing kinase [54], the presented data as we showed here may have far-reaching implications beyond non-viral sexually transmitted diseases since acquired defects in CFTR might also be implicated in pulmonary trichomoniasis.
In conclusion, this study revealed that T. vaginalis infection impaired the PGE2-elicited anion transport via down-regulation of CFTR in vaginal epithelium, confirming the crucial role of CFTR in the host-parasite interaction. Our results provide valuable insights into a better understanding of the pathogenesis of trichomoniasis and offer a novel therapeutic strategy for T. vaginalis infection via restoration of epithelial CFTR function.
We thank Dr. Jiehong Huang (School of Life Sciences, Sun Yat-sen University, Guangzhou, China), Dr. Jia-Wen Xu (School of Life Sciences, Sun Yat-sen University, Guangzhou, China) and Dr. Dong-Dong Gao (School of Life Sciences, Sun Yat-sen University, Guangzhou, China) for their helpful comments and suggestions. We also thank Ms. Chu-Ying Zhang (School of Life Sciences, Sun Yat-sen University, Guangzhou, China) for her technical support.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54