
The authors have declared that no competing interests exist.
Cilia play critical roles during embryonic development and adult homeostasis. Dysfunction of cilia leads to various human genetic diseases, including many caused by defects in transition zones (TZs), the “gates” of cilia. The evolutionarily conserved TZ component centrosomal protein 290 (CEP290) is the most frequently mutated human ciliopathy gene, but its roles in ciliogenesis are not completely understood. Here, we report that CEP290 plays an essential role in the initiation of TZ assembly in Drosophila. Mechanistically, the N-terminus of CEP290 directly recruits DAZ interacting zinc finger protein 1 (DZIP1), which then recruits Chibby (CBY) and Rab8 to promote early ciliary membrane formation. Complete deletion of CEP290 blocks ciliogenesis at the initiation stage of TZ assembly, which can be mimicked by DZIP1 deletion mutants. Remarkably, expression of the N-terminus of CEP290 alone restores the TZ localization of DZIP1 and subsequently ameliorates the defects in TZ assembly initiation in cep290 mutants. Our results link CEP290 to DZIP1-CBY/Rab8 module and uncover a previously uncharacterized important function of CEP290 in the coordination of early ciliary membrane formation and TZ assembly.
Dysfunction of cilia leads to various human genetic diseases, including many caused by defects in transition zones (TZs), the “gates” of cilia. A study in Drosophila reveals that the cilia TZ core protein CEP290 coordinates early ciliary membrane formation and TZ assembly; the N-terminus of CEP290 recruits DZIP1, which in turn recruits Rab8 and CBY to promote early ciliary membrane formation.
Cilia are microtubule-based organelles that extend from the surface of most eukaryotic cells. These protrusions are important for diverse cellular functions, including cell signaling, sensory perception, cell motility, and extracellular fluid movement [1–4]. In humans, almost every cell has at least 1 cilium. Due to the ubiquitous distribution and important functions of cilia, ciliary dysfunction results in a wide variety of human genetic disorders, collectively termed ciliopathies [5,6].
Cilia arise from the distal ends of basal bodies (BBs) that are derived from mother centrioles. Depending on the cell type, cilia form through 1 of 2 pathways: the intracellular pathway and the extracellular pathway [7,8]. Recent studies on mammalian cells have revealed that, in both pathways, ciliogenesis begins with the docking of Myo-Va–associated small vesicles to mother centriole distal appendages (DAs) [9]. These small vesicles, termed preciliary vesicles (PCVs), then fuse into a large ciliary vesicle (CV) in a process mediated by the membrane-shaping proteins EH domain-containing protein 1 (EHD1) and EHD3 [10]. Then, the Rab11-Rabin8-Rab8 signaling cascade mediates the formation of the early ciliary shaft membrane [9–12]. Perhaps at the same time, the centriole cap protein CP110 is removed, followed by the subsequent assembly of the transition zone (TZ) which is the fundamental ciliary base structure for gating the ciliary compartment [13,14], and then the elongation of the axoneme which is mediated by the evolutionarily conserved intraflagellar transport (IFT) machinery [15].
The TZ is characterized by Y-link–like structures under electron microscopy, which connect proximal axonemal microtubules to the ciliary base membrane and may organize the ciliary necklace composed of annular intramembrane particles on the TZ membrane surface. It has been proposed that the TZ functions as a diffusion barrier to gate the ciliary compartment [16–18]. Dozens of proteins have been identified as TZ components from various model organisms [6,14,19]. Elegant genetic studies on C. elegans have grouped known TZ proteins into 3 functional modules, namely, Meckel–Gruber syndrome (MKS), Nephronophthisis (NPHP), and CEP290 [20–22]. Although great progress has been made in understanding the molecular composition and hierarchical assembly of TZs, little is known about how TZ assembly is initiated after basal body docking, or about how early ciliary shaft membrane formation is coordinated with TZ assembly.
CEP290, an important TZ component, is evolutionarily conserved from unicellular Chlamydomonas to humans [20,21,23–25] and is one of the most intriguing cilia genes. Mutations in CEP290 cause several ciliopathies ranging from nonsyndromic retinal degeneration, i.e., Leber Congenital Amaurosis (LCA), to syndromic disorders including, Senior–Loken syndrome (SLS), Joubert syndrome (JBTS), MKS, and Bardet–Biedl syndrome (BBS) [26]. Over 100 CEP290 mutations have been identified in patients, and these mutations cause a wide variety of phenotypes, ranging from isolated blindness to embryonic lethality [26]. The broad spectrum of diseases and phenotypes associated with CEP290 mutations highlight the multiple and critical roles of CEP290 in cilia. However, the molecular mechanisms of CEP290 in TZ assembly are still not fully understood.
Here, we discover that CEP290 is essential for TZ assembly initiation in Drosophila. Complete deletion of CEP290 blocks ciliogenesis at the stage of TZ assembly initiation in both ciliated sensory neurons and spermatocytes, 2 main ciliated cell types of Drosophila. Further studies reveal that the N-terminus of CEP290 directly interacts with DAZ interacting zinc finger protein 1 (DZIP1) and recruits DZIP1 to the TZ. Interestingly, deletion of DZIP1 results in a phenotype similar to cep290 deletion mutants. Furthermore, we demonstrate that DZIP1 functions upstream of CBY and Rab8 to mediate ciliary membrane assembly. We propose that ciliary membrane assembly is a prerequisite for TZ assembly and that CEP290 coordinates the formation of early ciliary membranes with the assembly of the TZ.
To determine the domain that mediates the TZ localization of Drosophila CEP290, we constructed several green fluorescent protein (GFP)-tagged CEP290 truncation constructs and assessed their subcellular localization in various types of cilia in Drosophila: auditory cilia, olfactory cilia, and spermatocyte cilia. It has been proposed that CEP290 bridges axonemal microtubules and ciliary membranes, where its C-terminus contains a microtubule binding domain and N-terminus possesses a highly conserved membrane-binding amphipathic α-helix motif (Fig 1A) [23,27,28]. Consistent with these findings, the C-terminal fragment of CEP290 containing the microtubule binding domain (CEP290-C, from aa 1385 to the end) localized to the TZ in all examined cilia in Drosophila (Fig 1A–1E), and the N-terminal fragment of CEP290 (CEP290-N, comprising aa 1–650) alone was also located at the TZ, but the middle fragment (CEP290-M, aa 651–1384) was not (Fig 1A–1E). The fact that CEP290-N alone targets to the TZ suggests that the N-terminus of CEP290 has a TZ target signal independent of the C-terminal microtubule binding domain. Notably, wild-type (WT) flies with overexpression of the N-terminus of CEP290 showed defective motility (Fig 1F), a phenotype commonly associated with cilia dysfunction.

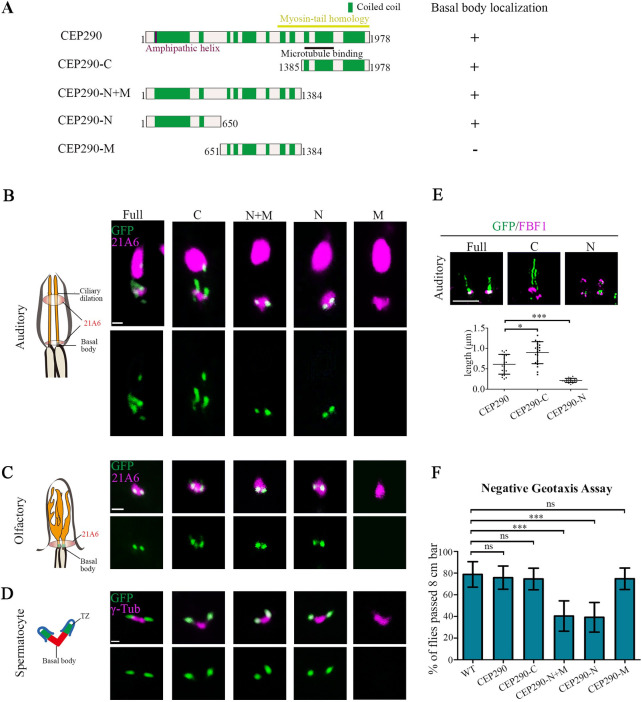
Both the N-terminal and C-terminal truncation mutants of CEP290 localized to TZs in Drosophila.
(A) Schematic representations of CEP290 truncations used in (B–E). (B) Localization patterns of various GFP-tagged CEP290 truncations in auditory cilia. 21A6 marks the ciliary base (red). Both CEP290-N and CEP290-C localized to the ciliary base, but CEP290-M did not. Bar, 1 μm. (C) The localization of various GFP-tagged CEP290 truncations in olfactory cilia. 21A6 marks the ciliary base (red). Both CEP290-N and CEP290-C localized to the ciliary base, but CEP290-M did not. Bar, 1 μm. (D) The localization of various GFP-tagged CEP290 truncations in spermatocytes. γ-Tubulin was used to label the BBs (red). Both CEP290-N and CEP290-C localized to the ciliary base, but CEP290-M did not. Bar, 1 μm. (E) 3D-SIM images of the localization of CEP290, CEP290-N, and CEP290-C in auditory cilia (upper panel), and the corresponding quantification of signal length (lower panel). FBF1 was used to indicate the ciliary base. n = 20. Bar, 1 μm. (F) Analysis of the moving behavior of flies overexpressing of CEP290 truncations. Climbing assay was used to assess the fly motility. Motility of flies with overexpression of CEP290-N and CEP290-N+M were significantly compromised. Uncropped images for panels B and C can be found in S1 Raw Image. Numerical data for panel E and F can be found in the file S1 Data. 3D-SIM, three-dimensional structured illumination microscopy; BB, basal body; CEP290, centrosomal protein 290; FBF1, Fas binding factor 1; GFP, green fluorescent protein; ns, not significant; WT, wild-type.
The length of the TZ in different types of cilia is variable in Drosophila. TZs of auditory cilia are significantly longer than those of olfactory cilia and spermatocyte cilia [27]. TZ in auditory cilia contains 2 longitudinal subdomains: The proximal one comprises all TZ components, whereas the distal one comprises CEP290 only [27]. We did see that the CEP290 signal in auditory cilia was longer than that in other cilia (Fig 1B). However, we observed that the distribution of CEP290-N signal was significantly more restricted than the full-length CEP290 signal (Fig 1B), suggesting that CEP290-N is restricted toward the proximal part of the TZ. On the other hand, we observed that the signal of CEP290-C was significantly longer than that of CEP290 (Fig 1B), which was also seen in olfactory cilia (Fig 1C). Different distributions of CEP290, CEP290-N, and CEP290-C in TZs of auditory cilia were further confirmed by three-dimensional structured illumination microscopy (3D-SIM) (Fig 1E). These results suggest that the N-terminus of CEP290 might have an inhibitory effect on the localization of its C-terminus.
The fact that a short N-terminal truncation mutant of CEP290 was targeted to the TZ suggests that the previously reported cep290 mutant (cep290mecH, lacking the C-terminal sequence from aa 1471 to the end) may be not a null mutant [24]. To better understand the function of CEP290, especially its N-terminus, we employed CRISPR-mediated genome engineering and generated a putative null mutant, cep2901 (c.469-1174Del), in which site-specific deletion leads to a frameshift, leaving only 156 aa of the N-terminus (Fig 2A and S1A Fig). In order to compare the phenotype differences between the cep2901 mutants and C-terminus deletion mutants, we additionally generated cep290ΔC (c.4156delG), in which a deletion results in a frameshift and C-terminus (aa 1386-end) loss (Fig 2A and S1B and S1C Fig). As expected, immunofluorescence staining of CEP290 in spermatocytes with an antibody against its N-terminal showed that the N-terminus of CEP290 was completely lost in cep2901 mutants, but it was still present in cep290ΔC mutants (S1D Fig). Notably, compared with WT, the signal intensity of CEP290 in cep290ΔC mutant was significantly reduced (S1D Fig). This is consistent with the transcript level of cep290 in cep290ΔC mutant, which was also significantly lower than that of WT (S1B Fig), probably due to nonsense-mediated mRNA decay.

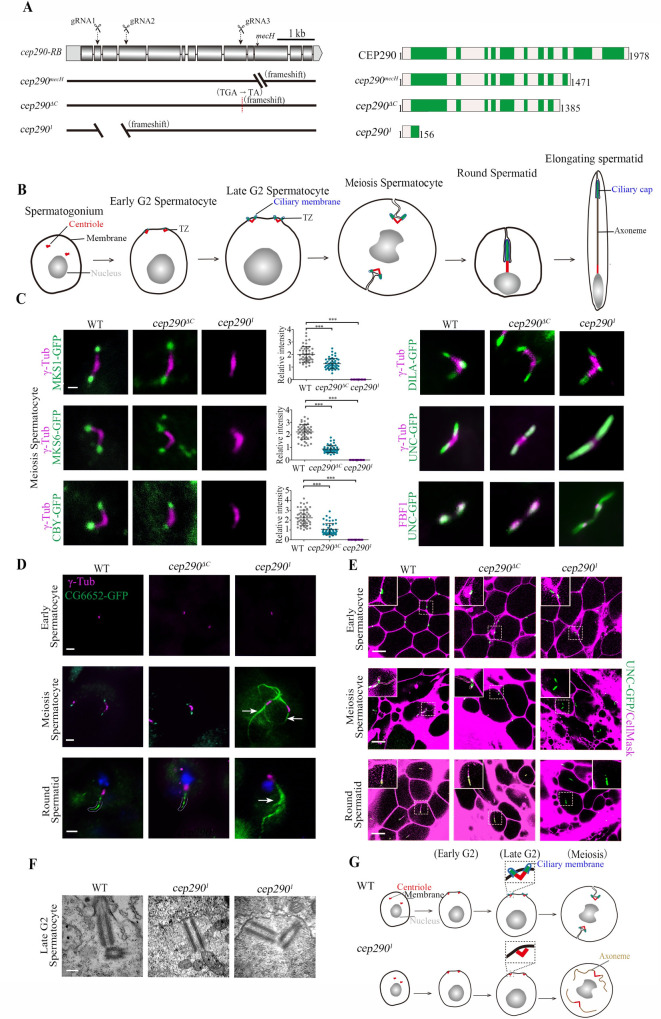
CEP290 is essential for TZ assembly initiation in spermatocytes.
(A) Generation of cep290 knockout flies. Schematics show the genomic (left) and protein (right) structures of CEP290 and its mutants. The gRNA target sites are shown. cep290ΔC (c.4156delG) lacks the C-terminal region from aa 1386 to the end, and cep2901 has a deletion in cDNA from nt 469 to 1,174, resulting in a frameshift and leaving only 156 aa. (B) Schematic diagram of dynamic changes in cilia/TZs during different stages of spermatogenesis. Cilia grow from both mother and daughter centrioles in Drosophila spermatocytes. V-shaped centriole pair docks to the plasma membrane in early G2 phase, and then assemble cilia/TZs. During meiosis, primary cilia are internalized along with BB, as BB needs to be used to assemble spindle for meiosis. During the process of internalization, cilia and the plasma membrane remain connected. After meiosis, spermatids elongate and mature. At this stage, cilia, also known as ciliary caps of spermatids, are extended and move away from the BB. (C) Representative images of the localization of TZ-related proteins in WT, cep290ΔC mutants, and the cep2901 mutants. Compared with WT flies, the signal of MKS1-GFP, MKS6-GFP, and CBY-GFP was significantly reduced in cep290ΔC mutants but completely lost in cep2901 mutants. The corresponding quantitative relative fluorescence intensities were shown on the right. Numerical data can be found in the file S1 Data. n = 50 centrioles over 5 flies. Targeting of DILA and UNC was not affected in cep290ΔC mutants and the cep2901 mutants, but UNC-GFP abnormally extended above the TF in cep2901 mutants. Bar, 1 μm. (D) Representative images of the localization of CG6652-GFP in spermatocytes and spermatids in the indicated genetic background. CG6652 marks the primary ciliary axoneme and extends abnormally (arrows) in cep2901. γ-Tubulin (red) was used to label the centrosomes. Bars, 2 μm. (E) Live imaging of BB docking in WT flies, cep290ΔC mutants, and cep2901 mutants. The PM was labeled with CellMask, and the BBs were indicated by UNC-GFP. In cep290ΔC mutant, no defects were observed in the connection between the BBs and the PM. In cep2901 mutants, the BBs could initially migrate to the PM, but the connection between the BBs and the membrane disassociated in later stages. Bars, 10 μm. (F) EM images showing that BBs are close to the membrane in cep2901 mutants but that no ciliary bud is observed. Bar = 200 nm. (G) Schematic diagrams of BBs and cilia in different stages of spermatocytes in WT flies and cep2901 mutants. The centriole pair migrates and docks to the PM in early spermatocytes, and then the TZ is assembled, which stabilizes the connection between the PM and BBs. In cep2901 mutant, the centriole pair initially migrates to the plasma membrane, but the TZ fails to form, leading to disassociation of the BB from the PM in later stages, and causing abnormal elongation of the axoneme. BB, basal body; CEP290, centrosomal protein 290; DILA, Dilatory; gRNA, guide RNA; PM, plasma membrane; TZ, transition zone; WT, wild-type.
Similar to previously reported alleles [24,29], both cep2901 and cep290ΔC were severely uncoordinated during walking and flying, and they had severe defects in both touch sensitivity and hearing sensation (S1E Fig), typical phenotypes of cilia deficiency mutants. Importantly, all these defects could be rescued by expression of UAS-cep290 under the pan-neural driver elav-GAL4 (S1E Fig).
First, we focused on cilia in spermatocytes. Drosophila spermatocyte ciliogenesis has several unique features (Fig 2B): (1) Cilia grow from both mother and daughter centrioles [30]; (2) cilia have only a TZ region, with no axoneme above the TZ [31]; (3) cilia are assembled on the cell surface in the early G2 phase but are internalized into the cytosol along with BBs in later stages, as BBs need to be used to assemble the spindle for meiosis [32,33]; and (4) during spermatid elongation after meiosis, cilia (also known as ciliary caps at this stage) extend and move away from BBs [24].
To analyze cilia/TZs formation in spermatocytes, we examined the localization of TZ core proteins (MKS1 and MKS6) and TZ-related proteins (CBY and DILA/CEP131). CBY and Dilatory (DILA) cooperate to build the TZ in Drosophila [34], but their localization in the TZ is very different; CBY completely colocalizes with MKS1 and MKS6 near the TZ membrane in spermatocytes, whereas DILA is inside the lumen of the TZ axoneme. Consistent with previous reports [24], we observed that all these proteins were present at the tips of BBs in cep290ΔC mutant, although the signal intensity of MKS1, MKS6, and CBY was significantly reduced (Fig 2C). However, surprisingly, we observed that MKS1, MKS6, and CBY were completely lost from the tips of BBs in cep2901 mutants (Fig 2C), while the distribution of DILA was normal. These results indicate that CEP290 is essential for TZ assembly.
Strikingly, we observed that, compared with that in WT flies and cep290ΔC mutants, the signal of Uncoordinated (UNC, the homolog of mammalian OFD1) was aberrantly elongated in cep2901 mutants (Fig 2C). Colabeling of UNC with the transition fiber (TF) protein Fas binding factor 1 (FBF1) indicated that the elongation occurred above the TF (Fig 2C), suggesting that the ciliary axoneme, rather than the centriole, was elongated. To confirm this, we labeled primary cilia axonemes with CG6652-GFP, which specifically labels axoneme in cilia of spermatocytes and ciliary caps of round spermatids [34]. Indeed, as shown in Fig 2D, axonemes extended abnormally in cep2901 mutants, but not in WT flies or cep290ΔC mutants.
Abnormal spermatocyte ciliary axoneme elongation has previously been observed in cby;dila double mutants and has been attributed to defects in basal body docking and the absence of a TZ membrane cap [34]. Accordingly, we observed that the association between BBs (as shown by UNC-GFP) and the plasma membrane (labeled with CellMask, Invitrogen, Carlsbad, California, United States of America) was defective in later spermatocytes and spermatids in cep2901 mutants, but not in WT flies or cep290ΔC mutants (Fig 2E). However, in the early spermatocyte stage, we observed that BBs were still attached/close to the membrane (Fig 2E). To further confirm this result, we employed transmission electron microscopy (TEM). Consistent with our immunofluorescence observations, BBs were able to migrate to the plasma membrane in cep2901 mutants, but no cilia-like structures and TZs formed (Fig 2F). Taken together, we proposed that in cep2901 mutants, BBs initially migrate to the plasma membrane, but the TZ assembly initiation is completely blocked, such that primary cilia/TZs never form. During the process of BB internalization in later stages, due to the absence of the ciliary cap, the stable association between the BB and the cell membrane was unable to be maintained, and the BB’s microtubules were abnormally elongated within the cytoplasm (Fig 2G).
In addition to the initiation of TZ assembly, spermatogenesis was also very different between cep2901 mutants and cep290ΔC mutants. As previously reported [24], cep290ΔC mutants had mild defects in spermatid development and male fertility (S1F and S1G Fig); however, sperm cysts were severely defective in elongation in cep2901 mutants, although the overall sizes of their testes were comparable (S1F Fig). Subsequently, no mature sperms were produced, and males were completely infertile in cep2901 mutants (S1G Fig). TEM examination of testes showed that flagellar axonemes were almost completely lost in cep2901 mutants (S1H Fig). Similar severe defects in the spermatid axoneme were also observed in TZ absent mutants [34], but the underlying mechanism remains poorly understood.
Next, we asked whether CEP290 is also involved in TZ assembly initiation in sensory neurons. Previously, EM data have shown that the auditory cilium in cep290mecH mutants lacks the TZ and ciliary axoneme [27]. Consistent with this, complete loss of cilia was observed in cep2901 and cep290ΔC mutants by both immunofluorescence and electron microscope (EM) assays (Fig 3A and 3B). Our EM data showed that BBs were very close to the plasma membrane, but no apparent TZs formed in either cep2901 or cep290ΔC mutants (S1I Fig). Although, under EM, we did not find any obvious differences in cilia base structures between cep2901 and cep290ΔC mutants; we found that, similar to that of spermatocyte cilia, the localization of TZ components in auditory cilia were drastically different in different cep290 mutants. All TZ proteins examined were present at the BB in cep290ΔC mutants, although their signal intensities were severely reduced compared to WT. However, remarkably, these signals were completely lost in cep2901 mutants (Fig 3C). Similar results were also observed in olfactory cilia (Fig 3C). These results suggest that in sensory neurons, the CEP290 N-terminus may be essential for initial TZ assembly, whereas TZ elongation or maturation may involve a tissue-specific requirement for the C-terminus of CEP290.

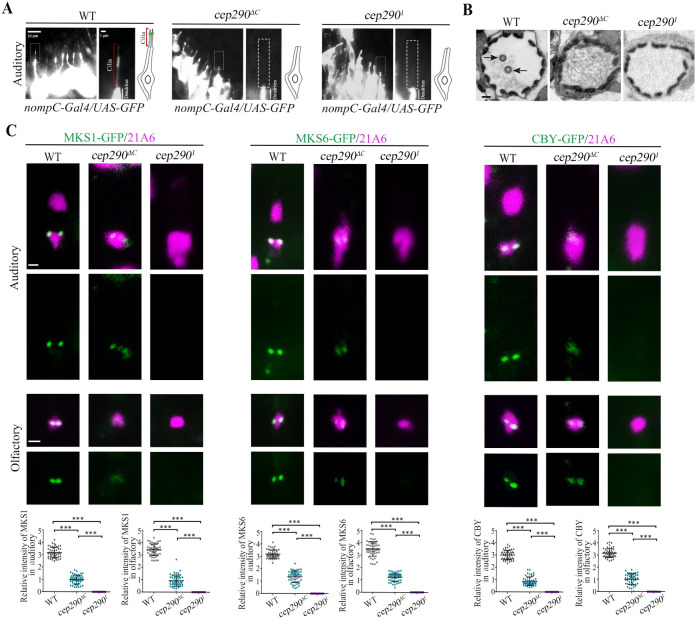
CEP290 is critical for TZ assembly initiation in sensory neurons.
(A) Cilium morphology in auditory cilia as labeled by nompC-Gal4; UAS-GFP in WT flies and cep2901 mutants. Cilia were completely lost in cep2901 mutants. (B) EM analysis of cross sections of auditory cilia. In WT flies, each scolopidium possessed 2 ciliary axonemes (arrows), but no ciliary axonemes were observed in the scolopidia of cep290 mutants. Bar, 200 nm. (C) Localization of various TZ proteins in auditory cilia and olfactory cilia in WT flies, cep290ΔC mutants, and cep2901 mutants, and corresponding relative fluorescence intensity quantitation. In cep290ΔC mutant, TZ proteins, MKS1-GFP, MKS6-GFP, and CBY-GFP were still present at the ciliary base, although the signals were severely reduced. However, these signals were completely lost in cep2901 mutants. 21A6 (red) was used to label the ciliary base. Uncropped images for panel C can be found in S1 Raw Image. Numerical data for panel C can be found in the file S1 Data. The bars and error bars represent the means and SDs, respectively. n = 50 basal bodies over 5 flies. Scale bars, 1 μm. CEP290, centrosomal protein 290; EM, electron microscope; SD, standard deviation; TZ, transition zone; WT, wild-type.
Taken together, our results indicate that CEP290 is essential for the initiation of TZ assembly in both sensory neurons and spermatocytes. Of note, without a robust antibody to survey expression to validate levels of CEP290 protein expression within our alleles, we cannot fully unpick how much of our phenotypes are due solely to reduced levels versus requirement for specific domains. We suspect it is likely a combination of both.
To elucidate the role of CEP290 in TZ assembly initiation, we employed a yeast two-hybrid assay to search for TZ-related proteins that interact with the N-terminus of CEP290 and identified DZIP1. Drosophila DZIP1 is the ortholog of mammalian DZIP1 and DZIP1L (S2A–S2D Fig), both have been implicated in ciliogenesis [35–38]. In our yeast two-hybrid assay, DZIP1 interacted with the N-terminus of CEP290, but did not interact with its C-terminus (Fig 4A). The interaction was further confirmed by the glutathione S-transferase (GST) pull-down assay (Fig 4B and S3A Fig). Both the N-terminus and C-terminus of DZIP1 interacted with the CEP290 N-terminus. DZIP1-N (aa 1–293) interacted with both CEP290 N (aa 401–650) and CEP290 N (aa 651–887); DZIP1-C (aa 294–737) interacted with CEP290 N (aa 651–887) (S3B Fig). To further confirm the interaction, we expressed GFP-tagged CEP290 and HA-tagged DZIP1 in cultured Drosophila S2 cells and performed coimmunoprecipitation (Co-IP) using GFP-trap beads. Western blots of the GFP-pulldowns confirmed the binding between CEP290 and DZIP1 (Fig 4C).

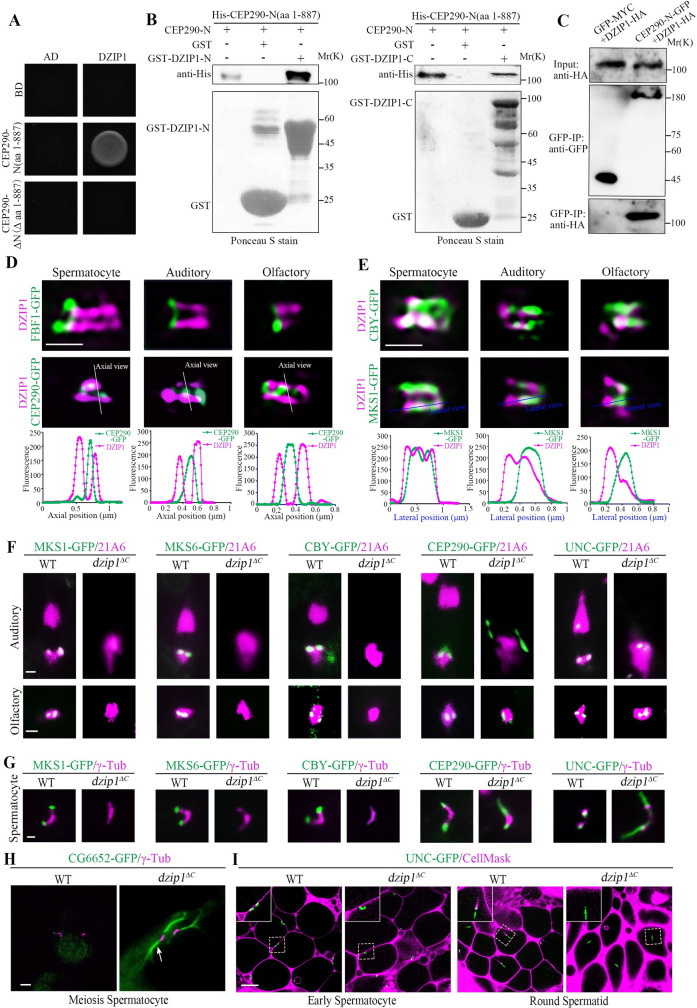
DZIP1 interacts with the N-terminus of CEP290, and DZIP1 deletion mutants mimic the phenotype of cep2901 mutants.
(A) Yeast two-hybrid assay results showing that DZIP1 interacts with CEP290-N (aa 1–887), but not with CEP290-ΔN (Δ aa 1–887). (B) GST pull-down assay confirmed the interaction between CEP290-N and DZIP1 in vitro. Both DZIP1-N (aa 1–293) and DZIP1-C (aa 294–737) interacted with CEP290-N. Upper panel, blotting with anti-His antibodies. Lower panel, Ponceau S staining of GST, GST-DZIP1-N, or GST-DZIP1-C. (C) CEP290-N and DZIP1 were coimmunoprecipitated in S2 cells. S2 cells were transiently transfected with CEP290-N-GFP and DZIP1-HA; 48 h later, cells were subjected to Co-IP using GFP-trap beads. (D, E) 3D-SIM images of the localization of DZIP1 on TZs in different types of cilia. (D) In all cilia, DZIP1 localized distal to the TF protein FBF1 and surrounded the C-terminal GFP-tagged CEP290. (E) In spermatocytes, DZIP1 overlapped with MKS1 and CBY. In sensory neurons, DZIP1 localized above FBF1, below MKS1, and partially overlapped with MKS1. Bars, 500 nm. (F) The TZ proteins MKS1-GFP, MKS6-GFP, and CBY-GFP were completely lost from the ciliary base in sensory neurons in dzip1ΔC mutants. CEP290-GFP and UNC-GFP were still present at the ciliary base. Notably, the signal of CEP290 was abnormally expanded in auditory cilia. 21A6 (red) marks the ciliary base. Bar, 1 μm. (G) MKS1, MKS6, and CBY were almost completely lost in spermatocytes of dzip1ΔC mutants. CEP290 and UNC were still present, but the signals were longer in mutants than in WT flies. γ-Tubulin was used to label the BBs (red). Bar, 1 μm. (H) Representative images of aberrantly elongated and branched spermatocyte ciliary axonemes (white arrow) labeled by CG6652-GFP in dzip1ΔC mutants. γ-Tubulin was used to label the BBs (red). Bar, 2 μm. (I) Live imaging of the connection between BBs and the PM in WT flies and dzip1ΔC mutants. The membrane was labeled with CellMask, and the BBs were indicated by UNC-GFP. In dzip1ΔC mutants, the BBs migrated to the PM in the early spermatocyte stage but failed to maintain the cilium-PM connection in subsequent processes. Bar, 10 μm. Numerical data for panels D and E can be found in the file S1 Data. Uncropped images for panel F can be found in S1 Raw Image. Uncropped immunoblots for panels B and C can be found in S2 Raw Image. 3D-SIM, three-dimensional structured illumination microscopy; BB, basal body; CBY, Chibby; CEP290, centrosomal protein 290; Co-IP, coimmunoprecipitation; DZIP1, DAZ interacting zinc finger protein 1; FBF1, Fas binding factor 1; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, the hemagglutinin (HA) tag; MKS1, Meckel syndrome type 1; MKS6, Meckel syndrome type 6; PM, plasma membrane; TZ, transition zone; UNC, Uncoordinated; WT, wild-type.
While the role of Drosophila DZIP1 in ciliogenesis was unknown when we started this project, a recent study showed that DZIP1 was localized to the TZ and was required for ciliogenesis in Drosophila [29]. Consistent with this report, we observed that Drosophila DZIP1 localized to the basal body in all cilia examined (S4A and S4B Fig). In spermatocytes, DZIP1 was found to localize to the tips of BBs. In spermatids, like TZ proteins, DZIP1 migrated along with the ciliary cap to the flagellar tip. To more accurately determine the subcellular localization of DZIP1, we employed 3D-SIM and observed that in all types of cilia, DZIP1 localized above the TF protein FBF1, surrounded the C-terminal GFP-tagged CEP290 which labels the center of the TZ [27] (Fig 4D). In spermatocytes, DZIP1 overlapped completely with the TZ protein MKS1 and CBY along TZ membranes (Fig 4E). In spermatids, DZIP1 was enriched at the ring centriole like CBY, but did not extend distally along the whole ciliary cap like MKS1 and MKS6 (S4C Fig). In sensory neurons, there was an obvious gap between the TF protein FBF1 and the TZ component MKS1 (S4D Fig). We observed that DZIP1 was above FBF1, but clearly below MKS1 and partially overlapped with MKS1, indicating that DZIP1 occupied the gap region between FBF1 and MKS1, and further extended distally, partially colocalizing with MKS1 (Fig 4E). Therefore, there are tissue-specific differences in DZIP1-related ciliary base structures. Overall, Drosophila DZIP1 is a TZ protein interacting with the N-terminus of CEP290.
To investigate the functional relationship between CEP290 and DZIP1, we generated a deletion mutant of dzip1ΔC (c.1069-1529Del), in which the C-terminus (aa 357-end) of DZIP1 is lost (S5A–S5C Fig). Consistent with the recent report [29], dzip1 mutant exhibited typical cilia mutant phenotypes. The flies were completely uncoordinated, unable even to stand, and were severely deficient in touch and hearing (S5D Fig). Introducing a WT DZIP1 cDNA transgene into the mutants fully rescued these deficiencies (S5D Fig), indicating that DZIP1 was the gene responsible for these defects.
Interestingly, we found that our dzip1 mutants mimicked the phenotype of cep2901 mutants. In sensory cilia of dzip1 mutants, TZ components MKS1, MKS6, and CBY were completely absent from the TZ, but the recruitment of CEP290 and UNC was not affected (Fig 4F). And cilia were almost completely lost in auditory cilia of dzip1ΔC mutant as demonstrated by both immunofluorescence and EM assays (S5E and S5F Fig). In spermatocytes of dzip1 mutants, MKS1, MKS6, and CBY were almost completely lost but CEP290 persisted (Fig 4G). Furthermore, similar to cep2901 mutants, ciliary axonemes extended aberrantly in dzip1 mutants, as shown by UNC-GFP and CG6652-GFP (Fig 4G and 4H). Additionally, deletion of DZIP1 resulted in the separation of the BBs and the plasma membrane in late spermatocytes (Fig 4I). All these observations are consistent with the recent report [29], indicating that like CEP290, DZIP1 is essential for TZ assembly initiation.
cep2901 and dzip1 mutants have similar phenotypes, and DZIP1 is dispensable for the recruitment of CEP290 (Fig 4F and 4G). Interestingly, the DZIP1 signal was completely lost in both spermatocyte cilia and sensory neuron cilia in cep2901 mutants, but it is still existed in the unc, dila, and cby mutants (Fig 5A and 5B). It has been reported that CEP290 is lost in cby;dila double mutants [34]; accordingly, no DZIP1 was observed in this double mutants (Fig 5A and 5B). Notably, in cep290ΔC mutants, DZIP1 was present in all types of cilia, suggesting that the remaining N-terminus of CEP290 has a role in recruiting DZIP1 to the TZ. However, compared with WT, the signal intensity of DZIP1 decreased significantly in sensory cilia in cep290ΔC mutants, and it extended abnormally into the whole cilia/TZs in spermatids (Fig 5B and 5C), suggesting that the CEP290 C-terminus may have tissue-specific roles in regulating the localization of DZIP1.

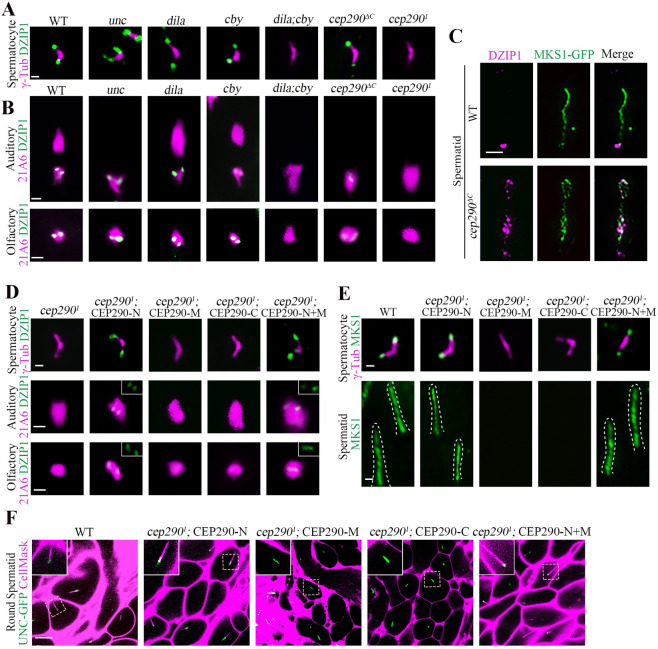
The N-terminus of CEP290 recruits DZIP1 to regulate TZ assembly.
(A) Localization of DZIP1 in spermatocytes in the indicated genetic background. DZIP1-GFP was completely lost in cep2901 mutants and cby;dila double mutants, but existed in other mutants, including cep290ΔC mutants. γ-Tubulin (red) was used to label the BBs. Bar, 1 μm. (B) Localization of DZIP1 in sensory neurons in the indicated genetic background. In cep2901 mutants, DZIP1-GFP was completely lost. In cep290ΔC mutants, DZIP1 was present, but the signal was severely reduced in sensory neurons. 21A6 staining indicates the ciliary base in sensory neurons. Uncropped images can be found in S1 Raw Image. Bars, 1 μm. (C) The C-terminus of CEP290 is required to limit the DZIP1 signal at the ring centriole in spermatids. Bar, 1 μm. (D) Overexpression of the CEP290 N-terminus (aa 1–650) ameliorated the localization defects of DZIP1 in cep2901 mutants. Bars, 1 μm. (E) Overexpression of the CEP290 N-terminus rescued the localization of MKS1 in spermatocytes and spermatids in cep2901 mutants. Bars, 1 μm. (F) Overexpression of CEP290 N-terminus restored the connection between the BBs and the membrane in cep2901 mutants. CellMask was used to label the membrane, and UNC-GFP indicates the BBs. Bar, 10 μm. BB, basal body; CEP290, centrosomal protein 290; DZIP1, DAZ interacting zinc finger protein 1; MKS1, Meckel syndrome type 1; TZ, transition zone.
Because the N-terminus of CEP290 directly interacts with DZIP1 (Fig 4A–4C), we hypothesized that CEP290-N may directly recruit DZIP1 to regulate TZ assembly. If so, expressing the N-terminus of CEP290 should ameliorate the defects in TZ assembly initiation in cep2901 mutants. To test this hypothesis, we expressed CEP290 truncations in cep2901 mutants individually. Interestingly, we found that overexpression of CEP290-N restored the signal of DZIP1 on the TZ in cep2901 mutants, but the middle and C-terminal fragments did not (Fig 5D). Remarkably, we observed the return of MKS1 signal in both spermatocytes and spermatids, indicating that overexpression of the N-terminus correspondingly attenuated the defects in TZ formation in cep2901 mutants (Fig 5E). Accordingly, the BB docking defects in late spermatocytes were also ameliorated after overexpression of CEP290-N (Fig 5F). Notably, the flies with overexpression of CEP290-N were still uncoordinated; this is because that CEP290 has other roles independent of its N-terminal and DZIP1; CEP290 is not only required for the initiation of ciliogenesis and TZ assembly, but also required for TZ maturation and axoneme formation in sensory neurons.
Lapart and colleagues reported that Drosophila DZIP1 directly interacts with CBY and function upstream of CBY-FAM92 module to regulate the TZ assembly [29]. However, dzip1 mutant has much more severely defective phenotypes than cby or fam92 single mutants. Ciliogenesis is completely blocked in our dzip1 mutants, but it is partially affected in cby or fam92 single mutants [29,39]. These results suggest that defects caused by deletion of DZIP1 cannot be attributed to CBY-FAM92 pathway only, other binding factors of DZIP1 should also be involved.
It has been reported that mammalian DZIP1 interacts with ciliary membrane formation regulator Rab8 [40]. Rab8 is a core regulator of membrane vesicle trafficking whose role in ciliary membrane formation has been extensively studied [9,41]. To determine if DZIP1 interacts with Rab8 in Drosophila, we performed the GST pull-down assay. Interestingly, we found that the N-terminus of DZIP1 interacted with Rab8, while the C-terminus of DZIP1 did not (Fig 6A). Consistent with what has been reported in mammals [40], DZIP1 preferentially interacted with the Rab8GDP-mimicking mutant Rab8T22N. To further confirm the interaction, we transiently transfected Drosophila S2 cells with DZIP1-GFP and Rab8-HA. GFP-pulldowns from cell extracts confirmed that DZIP1 did interact with Rab8 (Fig 6C). Therefore, Rab8 is a binding factor of DZIP1 in Drosophila.

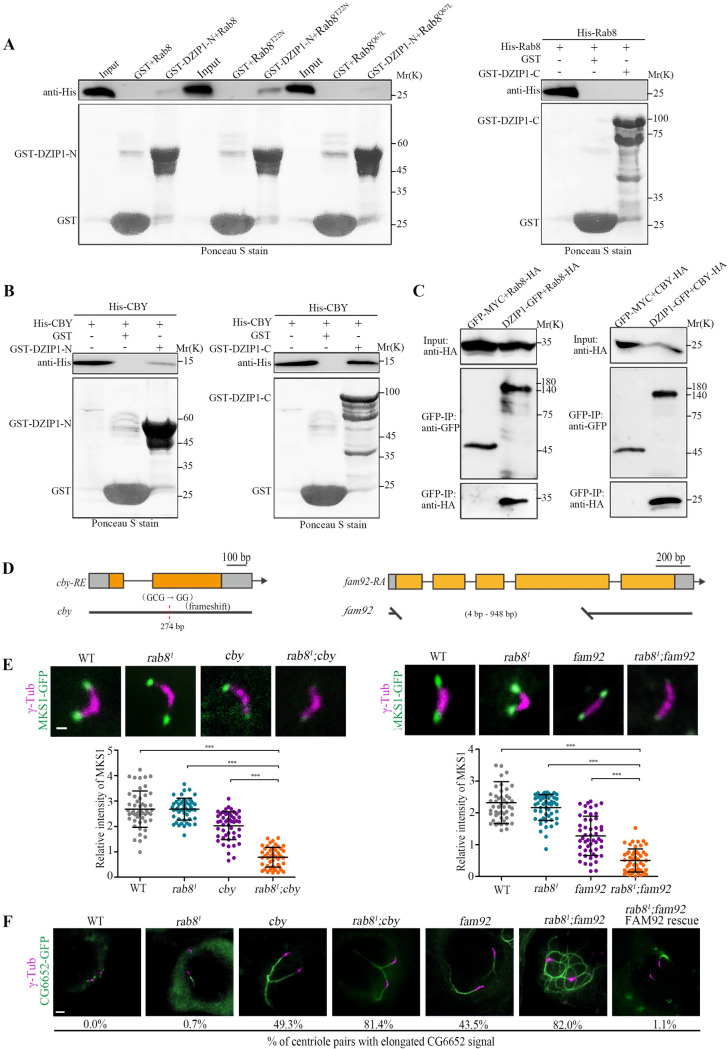
DZIP1 recruits CBY and Rab8 to promote TZ assembly.
(A) The N-terminus of DZIP1 interacted with Rab8 in a GST pull-down assay, but the C-terminus did not. And DZIP1 preferentially bound to Rab8T22N. (B) DZIP1 interacted directly with CBY in a GST pull-down assay. (C) DZIP1-GFP and Rab8-HA, DZIP1-GFP, and CBY-HA were coimmunoprecipitated in cultured Drosophila S2 cells. (D) Schematics of cby and fam92. cby (c.121delC) are predicted to encode a truncated protein lacking the C-terminal region from aa 41 to the end. fam92 (c. 4-789Del) deletes a large portion (262 out of 395 amino acids) of FAM92 protein. (E) Localization of MKS1-GFP in spermatocytes in the indicated genetic background (upper panel) and the corresponding quantitative relative fluorescence intensities (lower panel). Compared with any single mutants, MKS1-GFP was significantly reduced in rab81; cby double mutants and in rab81; fam92 double mutants. The bars and error bars represent the means and SDs, respectively. n = 50 centrioles over 5 flies. Scale bars, 1 μm. (F) Images of CG6652-GFP signals in late spermatocytes in WT flies, rab81 mutants, cby mutants, fam92 mutants, rab81; cby double mutants, and rab81; fam92 double mutants. Compared to WT, rab81, cby, and fam92, the percentage of centriole pairs with abnormally elongated CG6652 signal increased significantly in cby; rab8 double mutant and in rab81; fam92 double mutants. Overexpression of FAM92 rescued the elongated CG6652 phenotype of fam92, rab81 double mutants. n = 2,000 over 10 flies for rab8 mutants, n = 500 over 5 flies for others. Scale bars, 2 μm. Uncropped immunoblots can be found in S2 Raw Image. Numerical data can be found in the file S1 Data. CBY, Chibby; DZIP1, DAZ interacting zinc finger protein 1; FAM92, Family with sequence similarity 92; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, the hemagglutinin (HA) tag; MKS1, Meckel syndrome type 1; SD, standard deviation; TZ, transition zone; WT, wild-type.
The function of Rab8 in Drosophila ciliogenesis has not been investigated yet. We observed that Rab8 localized to the ciliary base in both auditory neurons and olfactory neurons. And consistent with what is observed in mammals, the Rab8GTP-mimicking mutant RAB8Q67L was frequently observed inside cilia (S6A and S6B Fig). Interestingly, DZIP1 and CEP290 were necessary for the localization of Rab8 in both auditory and olfactory cilia (S6C–S6E Fig). However, we were disappointed to find that auditory cilium morphology looked normal in rab81 mutants (S6F Fig), supported by the behavior readouts that no defects were observed in larval hearing and touch sensitivity (S6G Fig). However, we did observe that a small proportion of spermatocyte centriole pairs have elongated CG6652-GFP signal in every rab8 mutant fly we checked (Fig 6F). Elongated CG6652 signal in spermatocytes is usually attribute to ciliary cap/TZ defects. So, Rab8 is indeed involved in ciliogenesis in Drosophila, even though the effect is relatively weak, probably due to the redundancy with other factors, as has been reported for Rab8 and Rab10 in mammalian ciliogenesis [42].
Multiple reports indicated that the CBY-FAM92 module facilitate ciliary membrane formation and BB docking [43,44]. Since both CBY-FAM92 module and Rab8 are involved in ciliary membrane formation, we hypothesized that these 2 DZIP1 binding partners may jointly promote early ciliary membrane formation and ciliogenesis in Drosophila. To test our hypothesis, we first confirmed the interaction between CBY and DZIP1 in Drosophila (Fig 6B and 6C). Then, we created a cby deletion mutants (Fig 6D), and then generated the cby; rab8 double mutants. Interestingly, we found that the signal of the TZ component MKS1 was dramatically decreased, whereas the percentage of centrioles with abnormally elongated CG6652 signal was significantly increased in spermatocytes of cby; rab8 double mutant, compared with either cby or rab8 single mutants (Fig 6E and 6F).
Furthermore, as CBY and FAM92 function in a same pathway to regulate ciliogenesis. The TZ localization of CBY and FAM92 is interdependent, and fam92 mutants mimic the phenotype of cby mutants. We wondered whether deletion of rab8 could also aggravate the phenotype of fam92 mutants. Then, we created fam92 deletion mutants and generated the fam92; rab8 double mutants. Indeed, as expected, MKS1 signal decreased dramatically, and the percentage of centrioles with abnormally elongated CG6652 signal increased significantly in spermatocytes of fam92; rab8 double mutant (Fig 6E and 6F).
All these results strongly indicated that CBY and Rab8 do function downstream of DZIP1 and jointly contribute to TZ formation.
Ciliogenesis begins with the BB docking, followed by the subsequent formation of the early ciliary shaft membrane and assembly of the TZ, which together form the ciliary bud. However, how early ciliary membrane formation coordinates with TZ assembly is still not clear. In this work, we link the TZ core component CEP290 to the DZIP1-CBY/Rab8 ciliary membrane formation module and uncover a novel function of CEP290 in coordinating early ciliary membrane formation and TZ assembly. We propose that the N-terminus of CEP290 recruits DZIP1, which then recruits Rab8 and CBY to promote early ciliary membrane formation (Fig 7), whereas the C-terminus of CEP290 associates with axonemes and regulates TZ elongation and/or maturation. Our observation that TZ assembly is completely blocked in dzip1 mutants suggests that early ciliary membrane formation is a prerequisite for TZ assembly.

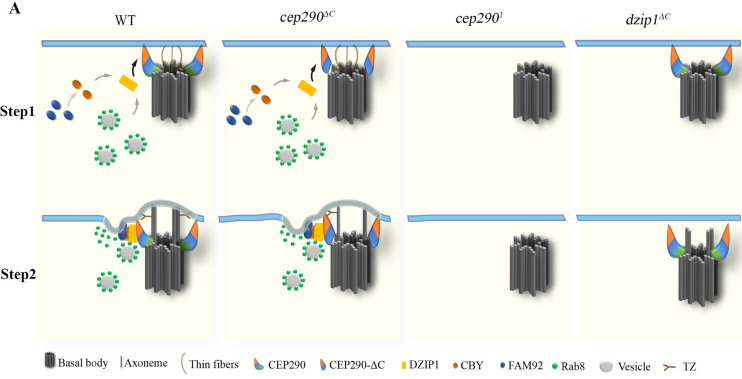
Model for the initiation of TZ assembly in Drosophila.
Proposed model for the initiation of TZ assembly based on data from Drosophila spermatocytes. Thin fibers with unknown components were observed to mediate the early interaction between the BB and the PM in Drosophila spermatocytes [50]. CEP290 localizes at the distal end of centriole, with its C-terminus toward the centriole microtubules. After the BB migrates to the membrane, the N-terminus of CEP290 binds to the membrane and recruits DZIP1, which then recruits CBY and Rab8. CBY together with FAM92 to remodel the membrane and facilitate Rab8-mediated ciliary membrane formation, and then the TZ assembly begins. In cep290ΔC mutant, the remaining N-terminus of CEP290 is functional; therefore, the transition zone assembly is initiated. In cep2901 and dzip1 mutant, ciliogenesis is blocked at the stage of early ciliary membrane formation. BB, basal body; CBY, Chibby; CEP290, centrosomal protein 290; DZIP1, DAZ interacting zinc finger protein 1; FAM92, family with sequence similarity 92; PM, plasma membrane; TZ, transition zone.
In agreement with previous reports [24,27], TZs fail to form in sensory cilia in cep290ΔC mutants, but they still form in spermatocyte cilia, suggesting that CEP290 has a tissue-specific functional difference in TZ assembly of sensory cilia and spermatocyte cilia, 2 types of ciliated cells in Drosophila. This difference may be correlated with the significant reduction of DZIP1 signal in sensory cilia but not in spermatocyte cilia in cep290ΔC mutants (Fig 5A and 5B). It has been reported that ciliary base structures are different in sensory neurons and spermatocytes [27]. Our SIM analysis of the TZ protein localization showed that there is an obvious gap, where DZIP1 is located, between TF and TZ core components in sensory neurons, but no gap exists between TF and TZ core components in spermatocytes (Fig 4D and 4E). All these structural differences may contribute to the tissue-specific functional difference of CEP290 in Drosophila. In addition, the TZ in Drosophila sensory neurons are longer than most cells and are suggested to be homologous to the specialized TZ, “connecting cilium” [45]. It is also possible that CEP290 plays a specific role in the formation of this kind of TZ. Interestingly, mammalian CEP290 may also have a specific role in the formation of “connecting cilium” of photoreceptors. In cep290 null mice, photoreceptor cells lack cilia, while ependymal cells retain cilia [46]. In CEP290 patients, cilia defects are often evident in photoreceptors [26,47]. Therefore, there may be an evolutionary conservative mechanism of CEP290 in the formation of “connecting cilium” which is worthy of further investigation.
From our TEM analysis, we observed that BBs are attached/very close to the plasma membrane in cep2901 mutant, similar to what Jana and colleagues has proposed [27]. However, technically, it is very difficult to determine whether BBs are docked or just apposed to the plasma membrane. In mammalian cells, the emerging model of the BB is as follows: Myo-Va–associated preciliary vesicles dock to distal appendages of the mother centriole, fuse into a large ciliary vesicle, and then the large ciliary vesicle associated with the BB migrates to and fuses with the plasma membrane [9]. However, to date, no evidence indicates that this mechanism is conserved in invertebrates. In Drosophila, the centriole pericentrin-like protein (PLP) regulates the migration of centriole/BB [48,49]. But how the centriole attaches to the plasma membrane remains unclear. Recently, thin fibers with unknown components were observed to mediate the early interaction between the BB and the plasma membrane in Drosophila spermatocytes [50]. In sensory neurons, there are Drosophila-specific structures called dense fibers that connect the proximal centriole (daughter centriole) to the membrane [51,52]. It is likely that those thin fibers in spermatocytes and dense fibers in sensory neurons are involved in the initial BB membrane docking. More work will be needed to understand the mechanisms of BB docking and the role of CEP290 in BB docking in Drosophila.
By using the simple Drosophila model, we demonstrated that Rab8 and CBY-FAM92 module function downstream of DZIP1 and jointly regulate TZ assembly initiation. The fact that rab8; cby double mutants and rab8; fam92 double mutants show synthetic defects suggests that they have synergetic roles in ciliogenesis. It is tempting to speculate that CBY-FAM92 module promotes membrane remodeling and then facilitate the Rab8 and its yet unknown compensatory mechanism regulating the ciliary membrane formation. The data available so far suggest that the function of DZIP1-Rab8 module and DZIP1-CBY-FAM92 module in ciliogenesis is largely conserved from Drosophila to mammals [53]. The interaction between DZIP1 and Rab8, between DZIP1/DZIP1L and CBY, and between CBY and FAM92 are conserved [36,37,40,43], and all of them have been implicated in ciliogenesis. Similar to what we observed in Drosophila, Dzip1 or Dzip1l knockout (KO) mice have much more severe defects than Cby1 KO mice or Fam92a KO mice, supporting the notion that CBY-FAM92 is not the solely downstream pathway of DZIP1 or DZIP1L [35,36,44,54]. However, there are 2 DZIP1 orthologs (DZIP1 and DZIP1L), 2 FAM92 orthologs (FAM92A and FAM92B), and 3 CBY orthologs in vertebrates, the existence of so many paralogs makes it very complicated to study their genetic relationship.
For the first time, using the Drosophila model, we demonstrated that TZ core protein CEP290 directly interacts with DZIP1 and controls TZ assembly initiation by regulating DZIP1-mediated early ciliary membrane formation. It has been reported that mammalian DZIP1/DZIP1L interacts with Rab8 and CBY [36,37,40], and mammalian DZIP1L is required for ciliary bud formation and TZ assembly. Mother centrioles with docked vesicles but no ciliary buds have been observed in Dzip1l mutant mice [36]. Thus, the function of DZIP1/DZIP1L in early ciliary membrane formation is likely conserved in mammals [53]. Although there is no reports connecting CEP290 to DZIP1 in mammals yet, it has been suggested that mammalian CEP290 is involved in ciliary vesicle formation [55], and defects in early ciliary membrane formation have been observed in fibroblasts of CEP290-deficient patients [47]. In addition, our preliminary data show that human CEP290 interacts with DZIP1L (S7 Fig). Therefore, it is highly possible that the function of CEP290-DZIP1 module in TZ assembly initiation is conserved in mammals. It will be interesting to investigate and confirm our overall model for TZ assembly initiation in mammalian cells.
The fly strains used in the study are as follows: w1118 (FBal0018186), elav-GAL4 (BDSC 458), unc2 (BDSC 7424), rab81 (BDSC 26173), Df(3L)ED228 (BDSC 8086), DJ-GFP (BDSC 5417), UASp-YFP-Rab8T22N (BDSC 9780), UASp-YFP-Rab8Q67L (BDSC 9781), and UASp-YFP-Rab8 (BDSC 9782); CG6652-GFP, UNC-GFP, MKS1-GFP and MKS6-GFP were provided by Dr. Bénédicte Durand; nompC-Gal4; UAS-GFP was provided by Dr. Wei Zhang. The flies were raised on standard media at 25°C.
Transgenic lines were generated by the Core Facility of Drosophila Resources and Technology, Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Chinese Academy of Sciences (CAS). To construct the FBF1-GFP, CBY-GFP, and DZIP1-GFP plasmids, the entire coding sequences (CDSs) with their promoter regions were separately cloned and inserted into the HindIII and BamHI sites of PJFRC2 vectors using a one-step cloning method. To construct the UAS-DZIP1-GFP and UAS-CEP290-GFP plasmids, we cloned the entire CDSs into the BglII and BamHI sites of PJFRC2 vectors. The DILA-GFP, truncated CEP290, and truncated CEP290-GFP plasmids were prepared similarly. We first subcloned the ubiquitin promoter region from pWUM6 into the HindIII and BglII sites of PJFRC2 vector or PJFRC2-APEX vector, and then individually cloned each gene cDNA sequence into the NotI and BamHI sites of the vector. Transgenes of CEP290, DILA, and UNC were inserted at the attP site of the 25C6 locus on chromosome 2, and transgenes of FBF1 and DZIP1 were inserted at the attP site of 75B1 locus on chromosome 3. All CDS fragments were amplified from cDNA reverse transcribed from total RNA. The primers used are listed in S1 Table.
The CRISPR/Cas9 system was used to generate the deletion mutants. Briefly, CRISPR targeting sites were designed using Target Finder (http://tools.flycrispr.molbio.wisc.edu/targetFinder). Customized single-guide RNA (gRNA) oligos were synthesized and then cloned into the BbsI site of the pEASY-PU6-BbsI-chiRNA vector. All the constructed plasmids were then injected into vasa-Cas9 embryos by the Core Facility of Drosophila Resources and Technology, SIBCB, CAS following a standard protocol. The mutant fly lines were screened via PCR. Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) was used to analyze the transcription level. The rp49 gene was used as an internal reference. The primers used are listed in S1 Table.
For staining of sensory cilia or testes, pupae, or juveniles were collected 36–48 h after puparium formation (APF). The collected flies were dissected and washed with 1× PBS. The dissected tissues were then placed on slides and squashed with coverslips. The slides were submerged in liquid nitrogen for 1 min, after which coverslips were removed. After incubation in cold methanol (−20°C) for 15 min followed by cold acetone (−20°C) for 10 min, the slides were washed 2 times for 10 min each in 1× PBS at room temperature, and then blocked for at least 1 h in blocking buffer (0.3% Triton X-100, 3% bovine serum albumen in PBS) at room temperature and stained with primary antibodies overnight in a moisture chamber at −4°C. After washing with 1× PBS, the slides were incubated with secondary antibodies for 3 h at room temperature.
Testes from juvenile flies were dissected in PBS and placed on slides. The testes were stained in CellMask Deep Red (Invitrogen, Carlsbad, CA, USA) solution (40 μg/mL) for 5 min at room temperature.
Rabbit antibodies against fly DZIP1 (aa 451–737) and CEP290 (aa 292–541) were produced by YOUKE Biotech (Shanghai, China). Mouse anti-GFP (1:200, 11814460001, Roche, Basel, Switzerland), rabbit anti-GFP (1:500, ab290, Abcam), mouse anti-γ-tubulin (1:500, T5326, Sigma-Aldrich), and mouse anti-21A6 (1:200, AB528449, DSHB) were also used. The secondary antibodies (1:1000 dilution) were goat anti-mouse Alexa Fluor 488/594 or goat anti-rabbit Alexa Fluor 488/594.
Most slides were visualized using a fluorescence microscope (Nikon Ti, Tokyo, Japan) or a confocal microscope (Olympus FV1000a, Tokyo, Japan) with a 100 × (1.4 NA) oil-immersion objective. SIM images were taken using a Delta Vision OMX SR (GE Healthcare, Buckinghamshire, United Kingdom) with a 60 × (1.42 NA) oil-immersion objective. All data analysis was performed using ImageJ (National Institutes of Health, Bethesda, USA). For quantification of signal intensity of TZ proteins, the fluorescence intensity is calculated by subtracting the sum of background signals of the same size from the sum of the signals in the region of interest, n = 50 centrioles or BBs over 5 flies.
Testis and antenna samples were prepared according to published protocols. Briefly, tissues were fixed in 2.5% glutaraldehyde buffer for at least 24 h and post fixed in OsO4. The samples were dehydrated using a diluted ethanol series and embedded in epoxy resin. Ultrathin sections (approximately 70 nm) were cut using an Ultracut S ultramicrotome (Zeiss, Jena, Germany). The samples were stained with uranyl acetate and lead citrate. Electron microscopy observations were made with Hitachi H-7650 transmission electron microscope (Tokyo, Japan) at 80 kV.
Single newly enclosed males were crossed with a virgin w1118 females for 3 days. The fertility ratio was quantified, and the progeny were counted.
For the adult climbing assay, 10 flies aged 3 to 5 d were placed in a graduated cylinder. At the beginning of each test, the flies were gently tapped to the bottom of the cylinder. The flies that climbed above the 8-cm height mark in 10 s were then counted, and counting was repeated 10 times. The data for each genotype are the averages of 5 biological replicates.
The larval touch assay was performed as previously described [52]. A single third instar larva was gently touched on its thoracic segments with a slim eyelash during bouts of linear locomotion on an agar plate. A score was assigned according to the response of the larva: A score of 0 indicated that the larva did not respond and continued to crawl, a score of 1 indicated that the larva hesitated or immediately stopped, a score of 2 indicated that the larva anteriorly retracted with or without anterior turns, a score of 3 indicated that the larva retracted with 1 full wave of the body, and a score of 4 indicated that the larva retracted with 2 or more full waves of the body. The score for each larva was determined 5 times, and the results were summed. In each group, 10 larvae were tested, and there were 5 groups for each genotype.
The larval hearing assay was processed as previously described [56]. In each assay, 5 third instar larvae were placed on an agar plate. Larvae were observed for contraction responses before and during a tone of 1k Hz at 30 s intervals, repeated 5 times. Five groups were tested for each genotype. The data are presented as box plots.
DZIP1 was introduced into the pGBKT7 vector (Clontech, Mountain View, CA, USA), and CEP290-N (aa 1–887) and CEP290-ΔN (aa 888–1978) were individually inserted into the pGADT7 vector (Clontech). The constructs were subsequently transferred into strain AH109 (Takara Bio, Tokyo, Japan) by the LiCl-polyethylene glycol method. Yeasts were grown on SD-Leu-Trp plates at 30°C. After 3 to 4 d of incubation, positive colonies were tested on SD-Ade-Leu-Trp-His plates and incubated for 5 d at 30°C.
To construct the DZIP1-GFP, CEP290-N-GFP (aa 1–878), DZIP1-HA, CBY-HA, and Rab8-HA plasmids, we cloned the CDSs into the Drosophila Gateway vector PAWG or PAWH, respectively. GFP was cloned into pAWM as a control. One microgram of total DNA was transfected into S2 cells or HEK293 cells in a 60-mm plate with Effectene (Qiagen, Duesseldorf, Germany). After 48 h, the cells were lysed in ice-cold Co-IP lysis buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40, 1× protease inhibitor) at 4°C for 45 min and centrifuged at 20,000 × g for 10 min at 4°C. The supernatants were added to anti-GFP nanobody agarose beads (Chromotek, Planegg-Martinsried, Germany), and the beads were tumbled end over end for 1 h at 4°C. After western blotting, the polyvinylidene fluoride (PVDF) membrane was incubated with anti-GFP antibody (Roche) and anti-HA antibody (Invitrogen) or anti-Flag antibody (Sigma). Full scans of the western blots are presented in S1 Raw Image.
To generate the GST-DZIP1-N (aa 1–293), GST-DZIP1-C (aa 294–737), His-CEP290-N (aa 1–887), His-CEP290-ΔN (aa 888–1978), His-CBY, His-Rab8, His-Rab8T22N, and His-Rab8Q67L plasmids, we cloned the CDSs into the pET28a or pGEX-4 T-1 vector. The constructs were transformed into BL21 (DE3) E. coli. The proteins were expressed and purified using His resin or Glutathione Sepharose. The purified His-CEP290-N, His-CEP290-ΔN, His-CBY, His-Rab8, His-Rab8T22N, and His-Rab8Q67L proteins were incubated with either GST, GST-DZIP1-N, or GST-DZIP1-C in binding buffer (25 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.5% Triton X-100, 1 mM dithiothreitol, 10% glycerol and protease inhibitors) overnight at 4°C. After western blotting, the PVDF membrane was incubated with anti-His antibodies (Invitrogen) and stained with Ponceau S. Full scans of the western blots are presented in S1 Raw Image.
The results are presented in all figures as the means and SDs. Statistical analyses were performed with two-tailed unpaired Student t tests. (GraphPad Prism 5, Version X; La Jolla, CA, USA; ns, P > 0.05; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
We thank Dr. Bénédicte Durand from Université Claude Bernard Lyon 1 and Dr. Wei Zhang from Tsinghua University for strains. We thank core facilities of Drosophila Resource and Technology (SIBCB, CAS), confocal imaging core facilities (SIPPE, CAS), and EM core facilities (SIPPE, CAS) for their technical support.
| 3D-SIM | three-dimensional structured illumination microscopy |
| APF | after puparium formation |
| BB | basal body |
| BBS | Bardet–Biedl syndrome |
| CDS | coding sequence |
| CEP290 | centrosomal protein 290 |
| Co-IP | coimmunoprecipitation |
| CV | ciliary vesicle |
| CBY | Chibby |
| DA | distal appendage |
| DILA | Dilatory |
| DZIP1 | DAZ interacting zinc finger protein 1 |
| EHD1 | EH domain-containing protein 1 |
| EM | electron microscope |
| FBF1 | Fas binding factor 1 |
| GFP | green fluorescent protein |
| gRNA | guide RNA |
| GST | glutathione S-transferase |
| IFT | intraflagellar transport |
| JBTS | Joubert syndrome |
| LCA | Leber Congenital Amaurosis |
| MKS | Meckel–Gruber syndrome |
| NPHP | Nephronophthisis |
| PCV | preciliary vesicle |
| PLP | pericentrin-like protein |
| PVDF | polyvinylidene fluoride |
| RT-PCR | reverse transcription-polymerase chain reaction |
| SLS | Senior–Loken syndrome |
| TEM | transmission electron microscopy |
| TF | transition fiber |
| TZ | transition zone |
| UNC | Uncoordinated |
| WT | wild-type |
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56