
The authors wish it to be known that, in their opinion, the first two authors should be regarded as Joint First Authors.
- Altmetric
Nucleic Acids Res.2020 Nov 4;48(19):10940-10952. https://doi.org/10.1093/nar/gkaa756
The authors would like to apologize for an error in Figure 4C of their article. During figure assembly and manuscript preparation, the Western blot image for input of PHF8pS854 was accidentally used for both the ‘input’ and ‘pulldown’. The original blots and corrected figure appear below. This error does not affect the results and conclusion of the article. The original article has been updated.
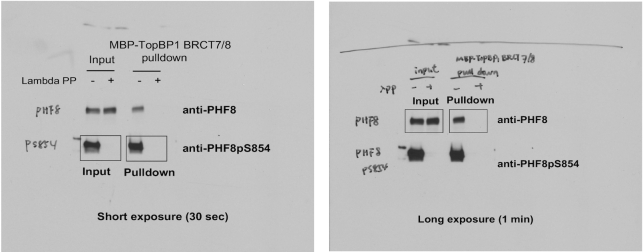
Left: Original blot 1 for Figure 4C. Right: Original blot 2 for Figure 4C.

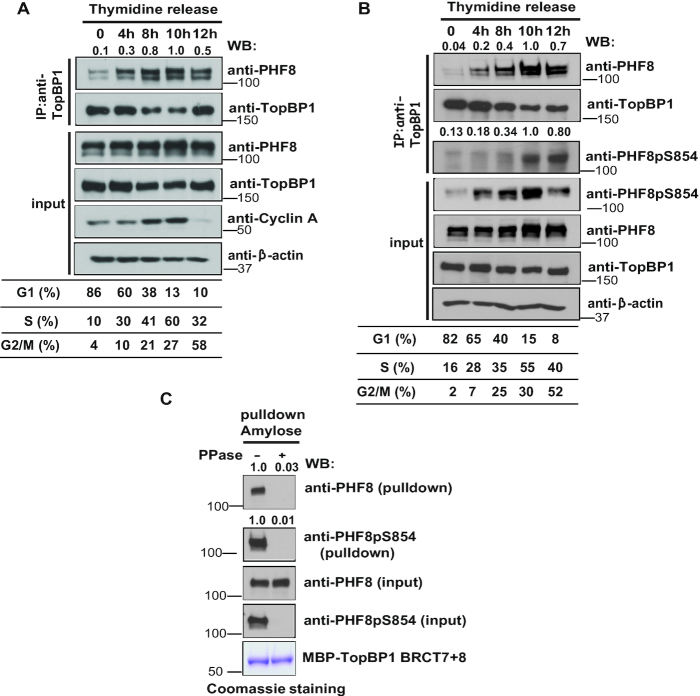
Interaction between TopBP1 and PHF8 is cell-cycle and phosphorylation dependent. (A) The interaction between TopBP1 and PHF8 is cell-cycle dependent. HeLa cells were synchronized by double thymidine block, released in fresh medium without thymidine, and collected at the indicated time points. Cell lysates were prepared, and immunoprecipitation and immunoblotting experiments were performed with the indicated antibodies. Samples were taken at the indicated time points and analyzed by fluorescence-activated cell sorting. (B) Phosphorylation of PHF8 at the S854 site is cell-cycle regulated. HeLa cells were synchronized by double thymidine block, and then released in fresh medium without thymidine and harvested at the indicated time points. Cell lysates were prepared, and immunoblotting experiments were performed using antibodies as indicated. Samples were taken at the indicated time points and analyzed by fluorescence-activated cell sorting. (C) TopBP1–PHF8 interaction is phosphorylation dependent. HEK-293T cells were lysed with NTEN buffer. Beads coated with bacterially expressed MBP-TopBP1 BRCT7+8 fusion protein were incubated with clear cell lysates that were mock treated or treated with protein phosphatase. Immunoblotting experiments were carried out with the indicated antibodies.
Notes
The research work was done at Cleveland Clinic.
 CK2 kinase-mediated PHF8 phosphorylation controls TopBP1 stability to regulate DNA replication
CK2 kinase-mediated PHF8 phosphorylation controls TopBP1 stability to regulate DNA replication