
- Altmetric
- INTRODUCTION
- CURRENT TREATMENT OF IMPLANT-ASSOCIATED S. AUREUS BIOFILM
- NANOTECHNOLOGY AS A NOVEL APPROACH FOR THE TREATMENT OF S. AUREUS BIOFILMS
- NANOSYSTEMS PRECLINICAL VALIDATION: BIOFILM MODELS
- LIPID-BASED NANOSYSTEMS
- POLYMERIC-BASED NANOSYSTEMS
- METALLIC-BASED NANOSYSTEMS
- SILICA-BASED NANOSYSTEMS
- QUANTUM DOTS
- CONCLUDING REMARKS
- FUNDING
Staphylococcus aureus (S. aureus) is considered by the World Health Organization as a high priority pathogen for which new therapies are needed. This is particularly important for biofilm implant-associated infections once the only available treatment option implies a surgical procedure combined with antibiotic therapy. Consequently, these infections represent an economic burden for Healthcare Systems. A new strategy has emerged to tackle this problem: for small bugs, small particles. Here, we describe how nanotechnology-based systems have been studied to treat S. aureus biofilms. Their features, drawbacks and potentialities to impact the treatment of these infections are highlighted. Furthermore, we also outline biofilm models and assays required for preclinical validation of those nanosystems to smooth the process of clinical translation.
Staphylococcus aureus biofilms are a major cause for implant-associated infections. The lack of an effective treatment and the escalating numbers of antibiotic-resistance events are prompting the research toward alternative nano-based strategies.
INTRODUCTION
Staphylococcus aureus is a Gram-positive commensal bacterium mostly present in the normal bacterial flora of the human skin and mucous membranes (WHO 2014). This bacterium is able to establish a mature 3D biofilm, where bacterial cells attached to a surface or to other cells are embedded within a protective polymeric extracellular matrix (Donlan and Costerton 2002; Lister and Horswill 2014) (Fig. 1). After the implantation of a medical device into the human body, proteins, which are present at the surgical wound site (e.g. fibronectin), adhere to the implants surface (Bhattacharya et al. 2015). These proteins are easily recognized by microbial surface components as adhesive matrix molecules, providing an opportunity for bacteria to establish a biofilm. This mode of bacterial growth plays a key role in antibiotic resistance, mainly due to an insufficient drug penetration into the biofilms matrix (Anderson and O'Toole 2008). Besides, the bacteria within the biofilm adopt a dormant lifestyle, contributing to a poor response to antibiotics (Anderson and O'Toole 2008). The complex structure of biofilms provides a unique opportunity to the survival and virulence of bacteria in the human body and ultimately to severe pathological conditions (Parsek and Singh 2003). Biofilms have a high genetic and phenotypic diversity, with less metabolically active bacterial cells in the lower regions of the structure that are well adapted to persist in hostile environments (Srivastava and Bhargava 2016). In the biofilm matrix, antibiotics are mostly restrained to the surface of the biofilm and are unable to reach the bacterial cells in the deeper layers of the structure (Srivastava and Bhargava 2016). Additionally, altered microenvironments, such as low pH and low oxygen, may compromise the efficacy of antibacterial agents (del Pozo and Patel 2007). Enzymes embedded in the biofilm matrix are also capable of destroying antibacterial agents, contributing to a low efficacy of these agents. At a genetic level, biofilms structure facilitates horizontal gene transfer, which promotes the spread of antimicrobial resistance (del Pozo and Patel 2007). Due to these factors, biofilms can be up to 1000 times more tolerant to antibiotics compared to planktonic bacteria (Srivastava and Bhargava 2016). The biofilm matrix also protects bacteria from the immune system by hindering the recognizable antigens present in bacterial cells (Parsek and Singh 2003). The detachment of single cells or clusters from the biofilm structure also contributes to the dissemination of bacterial cells, which may lead to infections in other parts of the body (Srivastava and Bhargava 2016).

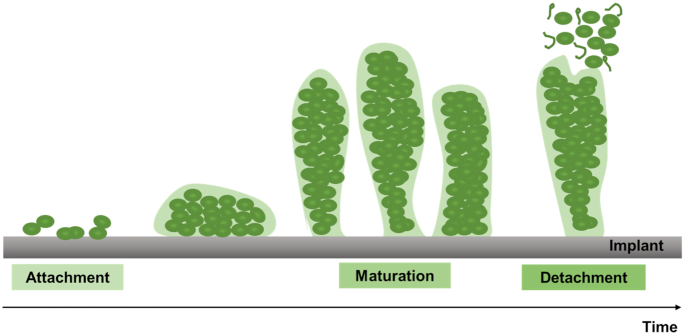
The development of a biofilm involves three main phases: initial attachment, maturation and final detachment. The initial attachment is mediated by host matrix proteins that cover the implant surface immediately after its insertion (Otto 2008). Thus, microbial surface components are able to recognize and bind to these matrix proteins, enabling bacterial colonization. Subsequently, the biofilm grows until it reaches a phase of maturation, adopting a 3D appearance, mainly due to intercellular aggregation mediated by adhesive proteins and exopolymers. Finally, detachment of single cells or cell clusters from the biofilm structure occurs, allowing dissemination and colonization of other sites of the host (Otto 2008).
Implantable medical devices have a massive global impact, in the order of hundreds of millions (Arciola, Campoccia and Montanaro 2018). Each year, more than a million cardiovascular electronic devices are implanted worldwide (McIntyre and Healey 2017). In the USA alone, >1 million knee and hip arthroplasty procedures are performed annually (Andersen et al. 2013). Despite the high use in the clinic, there are still complications associated to these devices, leading to their failure. In the past decades, different bacterial biofilms have been found to be a major cause of several infections associated to medical devices, such as orthopedic implants, catheters and pacemakers (Fig. 2). Among the most common biofilm-associated infections are osteomyelitis, endocarditis and urinary tract infections (Del Pozo 2018). These infections represent an economic burden for Healthcare Systems, leading to an increase in hospital stays as well as hospital-associated mortality (Bhattacharya et al. 2015). Onche et al. (2011)) estimated the costs associated with the removal of infected orthopedic implants to be $613.15 (USD) per patient, considering hospital costs and loss of work days. More recently, Otto (2014) reported that device-related biofilm infections add >$1 billion (USD) to hospitalization costs in the USA each year.

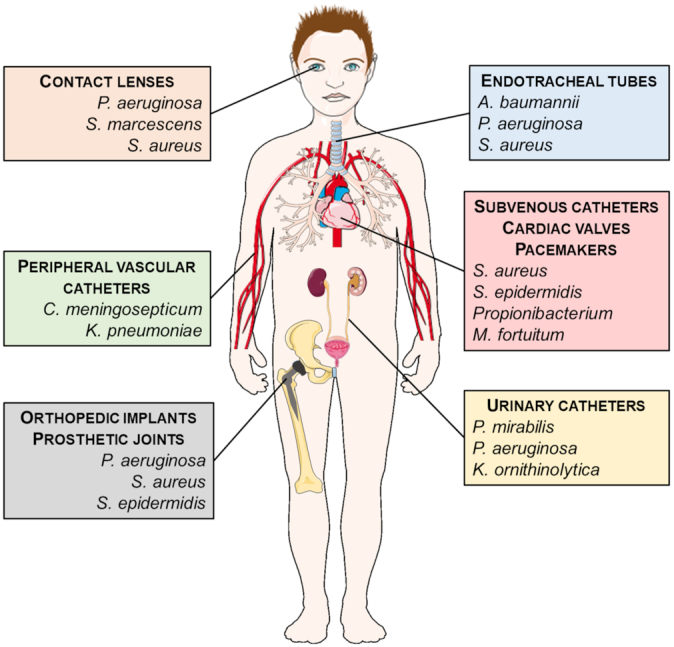
Medical devices associated to biofilm infections and the most prevalent bacterial species for each device (Nafee 2015; Srivastava and Bhargava 2016). A. baumannii, Acinetobacter baumannii; C. meningosepticum, Chryseobacterium meningosepticum; K. ornithinolytica, Klebsiella ornithinolytica; K. pneumoniae, Klebsiella pneumoniae; M. fortuitum, Mycobacterium fortuitum; P. aeruginosa, Pseudomonas aeruginosa; P. mirabilis, Proteus mirabilis; S. aureus, Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; S. marcescens, Serratia marcescens. Figure adapted with the permission of Servier Laboratories.
Nowadays, S. aureus is recognized by the Nosocomial Infections Surveillance System as one of the most frequent causes of nosocomial and community-acquired infections (Otto 2008; Lister and Horswill 2014). Among these, implant-associated infections are being highlighted due to the increasing number of multiple-antibiotic resistance cases and the high demand for indwelling prosthetic devices (Archer et al. 2011).
The global health and economic impact of S. aureus biofilm implant-associated infections represent a critical concern due to the current lack of an effective treatment and the increasing numbers of antibiotic-resistance bacterial strains. Thus, the World Health Organization considers S. aureus as a high priority pathogen for which new therapies should be developed (Tacconelli et al. 2018). This review provides an overview of the current therapy and its limitations, biofilm models available for preclinical validation of new therapeutic approaches and nanosystems as tools for the treatment of S. aureus biofilm implant-associated infections.
CURRENT TREATMENT OF IMPLANT-ASSOCIATED S. AUREUS BIOFILM
Once a mature S. aureus biofilm is established at the surface of an implant, only a few treatment alternatives exist. The solution for these infections usually requires physical removal of the implant and surrounding infected tissue combined with antibiotic therapy (Bhattacharya et al. 2015).
The treatment of biofilm implant-associated infections commonly implies a surgical procedure, ranging from debridement with implant retention to implant removal (Bhattacharya et al. 2015). The first procedure is less invasive and less time-consuming than surgical removal of the implant. Unfortunately, retention of implants frequently leads to failure, requiring revision surgery combined with antibiotic therapy to solve the infection (Bhattacharya et al. 2015). Thus, replacement of the infected implant by a new one is currently the best option to eradicate a long-lasting mature biofilm.
Nowadays, due to the increasing impact of bacterial resistance, there is no consensus on the antibiotic that should be used (Sousa et al. 2010). Thus, the guidelines for antibiotic therapy are specific for the type of infection and depend on the antibiotic susceptibility of the S. aureus strains (Osmon et al. 2013).
Methicillin-resistant S. aureus (MRSA) has evolved from methicillin-susceptible S. aureus (MSSA) by acquisition of the gene mecA (WHO 2014). This gene mediates the production of a beta-lactamase enzyme that inactivates both beta-lactamase-stable drugs (e.g. methicillin and cloxacillin) and beta-lactamase inhibitors (e.g. sulbactam). Since its discovery, MRSA strains widely spread through all regions of the world. In 2014, the World Health Organization reported that 86% of the clinical isolates of S. aureus were resistant to methicillin (MRSA). Patients infected with MRSA have an increased mortality rate and require more healthcare resources than MSSA-infected patients, representing a high health and economic burden (WHO 2014). Furthermore, the treatment of MRSA infections may require second-line antibiotics, which are more expensive and have higher toxicity at a systemic level. Currently, antibiotics such as vancomycin and daptomycin are among the most commonly used drugs to treat MRSA infections (WHO 2014).
Vancomycin is a widely administered glycopeptide antibiotic for both MRSA and S. aureus biofilm-associated infections (Foster 2017). However, recent cases of vancomycin-intermediate and vancomycin-resistant S. aureus strains led to the need of combining vancomycin with other drugs, such as rifampicin or linezolid (Bhattacharya et al. 2015). More recently, antibiotics such as daptomycin are being suggested for the treatment of biofilm-associated infections once only few nonsusceptible bacterial isolates were reported (Gonzalez-Ruiz, Seaton and Hamed 2016). Daptomycin is a cyclic lipopeptide molecule characterized by a fast bactericidal effect against a wide range of Gram-positive pathogens, including multiresistant staphylococci isolates (Sader et al. 2014; Bhattacharya et al. 2015; Gonzalez-Ruiz, Seaton and Hamed 2016). The mechanism of action of daptomycin involves calcium-dependent insertion of the compound into the bacterial cytoplasmic membrane, promoting a fast disruption of the bacterial membrane (Gonzalez-Ruiz, Seaton and Hamed 2016; Foster 2017). However, the use of this antibiotic to eradicate bacterial biofilms requires several high-dose administrations (Gonzalez-Ruiz, Seaton and Hamed 2016). Similar to other antibiotics, daptomycin faces the challenge of efficiently penetrating the biofilms matrix and reaching the bacterial cells. Consequently, the antibiotic is unable to reach bacterial cells deep within the biofilm structure at therapeutic concentrations, which may lead to antibiotic resistance phenomena. A detailed overview of the mechanisms of resistance to currently used antibiotics can be found elsewhere (Foster 2017).
To overcome the challenge of poor antibiotic penetration within the biofilms matrix, some research has been focusing on matrix disrupting agents. For instance, Siala et al. (2016) suggests the combination of an antifungal as a matrix inhibitor with antibiotics for an innovative approach to treat bacterial biofilms. However, the use of antifungals might affect the human fungal flora, which may limit the clinical use of this therapeutic approach.
Besides bacterial resistance, other drawbacks are also associated to the treatment against bacterial biofilms. Antimicrobial drugs are commonly administered through the oral route, with a poor bioavailability and efficacy of drugs (Pinto et al. 2017). Additionally, the systemic distribution of antibiotics leads to a lower concentration at the target site. Consequently, the treatment of bacterial infections usually requires high and repetitive doses, which may lead to adverse side effects. The efficacy of antimicrobial drugs can also be compromised by intrinsic factors, such as poor cellular penetration, limited intracellular retention, decreased intracellular activity and inefficient subcellular distribution (Pinto et al. 2017).
Overall and considering the high costs and discomfort of removal surgery and the increasing numbers of antibiotic resistance, the current available treatments are far from being ideal. Thus, novel effective therapies are urgently needed.
NANOTECHNOLOGY AS A NOVEL APPROACH FOR THE TREATMENT OF S. AUREUS BIOFILMS
The limitations and the inefficiency of the current therapy available against S. aureus biofilm implant-associated infections are prompting the research toward alternative strategies. Nanotechnology is a promising approach to treat biofilm-associated infections. Nanoparticles (NPs) are emerging as potential drug delivery systems due to their ability to maintain a sustained drug release at the target site, minimizing side effects and improving the therapeutic efficacy (Lopes et al. 2015; Nafee 2015). Additionally, some nanoparticles are being extensively studied for their intrinsic antimicrobial activity (Nafee 2015). Due to the characteristics of NPs, the use of nanotechnology in antimicrobial therapy decreases the potential development of bacterial resistance (Lopes et al. 2015).
In the last few decades, several nanosystems have been approved for clinical use in a variety of diseases, while many other nanoparticle formulations are under clinical trials (Zhang et al. 2010). A market analysis revealed that 67 nanodevices were identified in the market by January 2012, out of which 33 were associated to nanotherapeutics (Etheridge et al. 2013). In this analysis, 11 approved nanosystems with antimicrobial properties were reported (Etheridge et al. 2013). According to the BCC Research report, the market value of the worldwide nanomedicine industry was $214.2 billion (USD) in 2013 and $248.3 billion (USD) in 2014 (Evers 2015). By the year 2019, it is projected that the global market of nanomedicine reach $528 billion (USD) (Evers 2015).
NANOSYSTEMS PRECLINICAL VALIDATION: BIOFILM MODELS
The preclinical validation of nanosystems is a crucial step for further clinical trials and commercialization. Biofilm models are an important tool to evaluate the antibiofilm activity of nanosystems. Several in vitro, ex vivo and in vivo biofilm models have been developed for that purpose. Extensive reviews about biofilm models can be found in the literature (Peterson et al. 2011; Lebeaux et al. 2013).
In vitro biofilm models are widely used in research due to their simplicity and low cost (Fig. 3). These models mimic important characteristics of biofilms, such as gradients of nutrients and production and release of extracellular matrix (Lebeaux et al. 2013). Static systems (e.g. colony biofilm and calgary biofilm device) are fast and simple methods, amenable to high throughput screening. However, these models have limited nutrient flow and aeration. On the other hand, dynamic systems (e.g. flow cells) have constant replacement of nutrients and removal of dead cells and metabolic byproducts (Lebeaux et al. 2013). Additionally, it is possible to control environmental parameters of the systems and to perform nondestructive visualization of the biofilms by microscopy (Heydorn et al. 2000; Lebeaux et al. 2013). Nevertheless, dynamic systems are not well adapted for high throughput analysis and require specialized equipment and technical support (Lebeaux et al. 2013). More recently, complex systems based on the interactions between bacteria and the host were developed. These systems, microcosms, consider the complexity and heterogeneity of the in vivo environment.

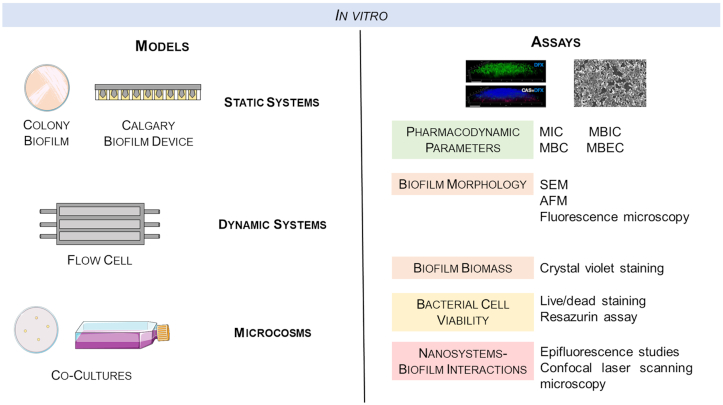
Schematic representation of in vitro biofilm models and the most common assays performed to evaluate the efficiency of antimicrobial agents against bacterial biofilms (Chen et al. 2012; Siala et al. 2016). AFM, atomic force microscopy; MBC, minimum bactericidal concentration; MBEC, minimum biofilm eradication concentration; MBIC, minimum biofilm inhibitory concentration; MIC, minimum inhibitory concentration; SEM, scanning electron microscopy. Microscopic images were reprinted with permission from Siala et al. 2016 (left) and Chen et al. 2012, American Chemical Society (right).
Several in vitro assays based on pharmacodynamic parameters, biofilm morphology and biomass, cell viability and NPs–biofilm interactions have been extensively used on biofilm models (Fig. 3). The determination of the minimum inhibitory concentration (MIC) and of the minimum bactericidal concentration (MBC) are key pharmacodynamic parameters to study the efficiency of nanosystems against planktonic bacteria (Pinto et al. 2017). On the other hand, to assess the efficacy of nanosystems against bacteria within a biofilm structure, minimum biofilm inhibitory concentration (MBIC) and the minimum biofilm eradication concentration (MBEC) are used (Macia, Rojo-Molinero and Oliver 2014).
Besides these parameters, other in vitro studies can be performed to elucidate the potential of nanosystems for eradication of biofilms. For instance, biofilm morphological changes can be detected by microscopy techniques, namely, scanning electron microscopy (Meng et al. 2016; Siala et al. 2016), atomic force microscopy (Salunke et al. 2014) and fluorescence microscopy (Hou et al. 2017). The biofilm biomass can also be determined by in vitro assays, such as the crystal violet staining method (Boda et al. 2015; Thomas et al. 2015). Despite the importance of evaluating morphological changes and biofilm biomass, it is also essential to assess bacterial cell viability within the biofilm structure. For that purpose, a live/dead staining (Boda et al. 2015) or a resazurin assay (Guo et al. 2017a) can be performed. Additionally, epifluorescence studies are commonly used to evaluate the interactions between nanosystems and biofilms (Rivero Berti et al. 2016). The penetration of nanosystems within the biofilm can also be examined by confocal laser scanning microscopy (Siala et al. 2016).
In an effort to consider the complex environment of in vivo models in a controlled experimental condition, ex vivo models were developed (Fig. 4). Ex vivo models consist on extracting tissues or organs from an organism and place them in an artificial environment for further procedures (Lebeaux et al. 2013). These models are extremely useful for imaging and analysis of bacterial colonization of the organ or tissue of interest (Lebeaux et al. 2013).

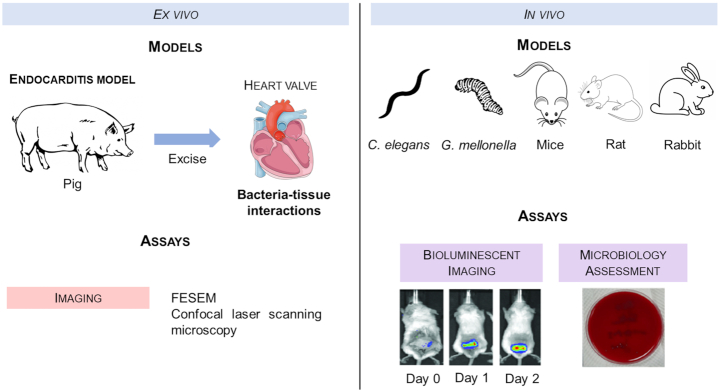
Schematic representation of ex vivo and in vivo biofilm models and respective examples of assays to evaluate the efficiency of antimicrobial agents against bacterial biofilms (Siala et al. 2016; Fang et al. 2017). C. elegans, Caenorhabditis elegans; FESEM, field emission scanning electron microscopy; G. mellonela, Galleria mellonela. Images from experimental assays were reprinted with permission from Siala et al. 2016 (left) and Fang et al. 2017 (right).
Although in vitro and ex vivo studies are useful tools, they are still far from mimicking the in vivo conditions. Several in vivo models have been developed to study the toxicity and efficacy of antibacterial agents against biofilms. The most commonly used are mice (Meng et al. 2016) and rat (Sharma, Gupta and Gupta 2016). However, lower eukaryotes, such as Caenorhabditis elegans (Mizdal et al. 2018; Kannappan et al. 2019) and Galleria mellonela (Souza dos Santos et al. 2019), are attractive alternative biofilm infection models due to their simple methodology, which is an advantage for high throughput screenings. Thus, in vivo efficiency assays are required to validate the in vitro outcome. For instance, Siala et al. (2016) used in vivo bioluminescent live imaging to evaluate the activity of antimicrobial agents in mice for 7 days. Additionally, microbiology assessment can be performed after sacrificing the in vivo models (Fang et al. 2017).
In the past decades, several nanotechnology-based systems that aimed to eradicate S. aureus biofilm-associated infections were studied using the previously mentioned in vitro and in vivo models. These novel nanosystems, their advantages and their limitations are going to be detailed in this review. An overview of the lipid, polymeric, metallic, magnetic, silica-based and quantum dots (QDs) systems that have been reported in the literature is provided.
LIPID-BASED NANOSYSTEMS
Liposomes
Liposomes are vesicles composed of a phospholipid bi- or multilayer with an aqueous core (Huh and Kwon 2011; Lopes et al. 2014). These vesicles are widely used delivery systems for antimicrobial agents due to their lipid bilayer structure that mimics the cell membrane (Huh and Kwon 2011). Consequently, they are able to easily fuse with infectious microbes, such as S. aureus (Huh and Kwon 2011). Additionally, both hydrophobic and hydrophilic drugs can be encapsulated within the biocompatible and biodegradable structure of liposomes (Lopes et al. 2014). Due to their advantages, liposomes are, by far, the main delivery system in the market (Huh and Kwon 2011). In this context, several studies are being developed with these carriers for S. aureus biofilms eradication (see Table 1).

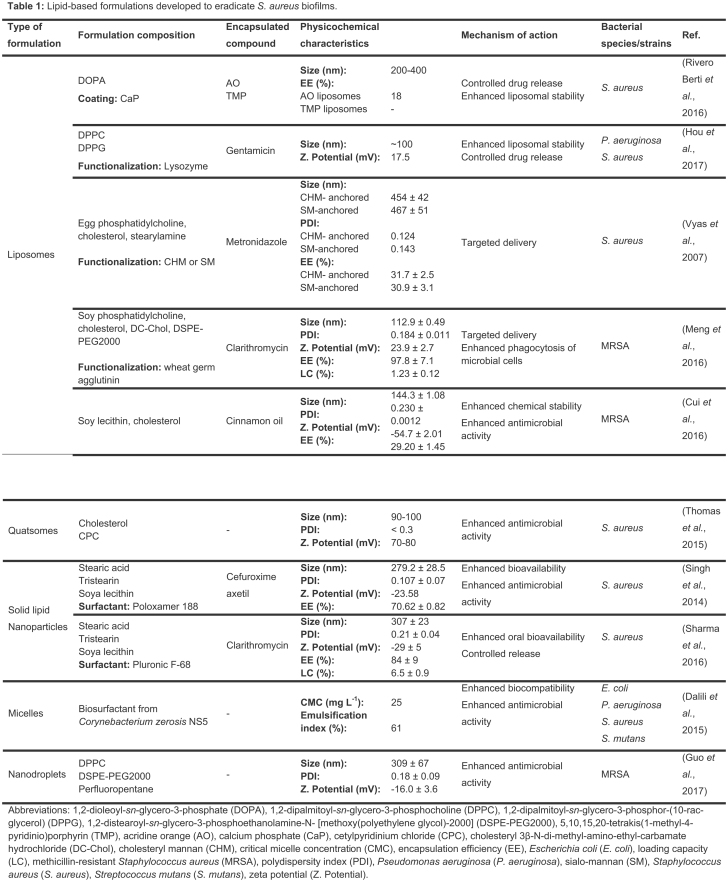
Despite the promising results of liposomes as delivery systems to eradicate bacterial biofilms, their poor stability is still a concern. To overcome this limitation, surface modification of liposomes with stabilizer ligands is being investigated. For instance, calcium phosphate was used as a coating to improve the delivery capacity and the mechanical stability of liposomes (Rivero Berti et al. 2016). In this study, two dyes, acridine orange and 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin (TMP), were loaded into the aqueous core of liposomes. Furthermore, the interaction of the calcium phosphate-coated liposomes and S. aureus was evaluated. Microscopy images showed that the calcium phosphate-coated vesicles remained adhered to the bacterial cell walls, even after intense washing treatment. Epifluorescence studies showed stained biofilms after a 2 h incubation with the liposomes. According to the authors, this indicates that upon contact of S. aureus biofilms and calcium phosphate-coated liposomes, there is a controlled release of the fluorophores (Rivero Berti et al. 2016). More recently, Hou et al. (2017) designed a gentamicin-loaded liposomal formulation with lysozyme surface functionalization. The cationic lysozyme was associated to the negatively charged liposome through electrostatic attraction. Besides stabilizing the vesicle, the functionalization also facilitated the interactions of the liposomes with the negatively charged matrix of the bacterial biofilm. The functionalization with lysozyme led to a zeta potential change from −54.5 to 17.5 mV. Additionally, it prevented undesirable drug release during storage. Besides, lysozyme-associated liposomes showed higher efficiency at preventing biofilm formation and disrupting preformed biofilms, when compared with gentamicin or lysozyme alone (Hou et al. 2017).
In the past few years, several ligands were used at the surface of liposomes to promote a targeted delivery of antimicrobial agents to biofilms. Hydrophobic derivates of mannan, such as cholesteryl mannan and sialo-mannan, were investigated by Vyas et al. (2007) when anchored at the surface of metronidazole-loaded liposomes. These ligands were obtained from the conjugation of mannan (hydrophilic) with hydrophobic anchors, which enables the polysaccharide to interact with the vesicle membrane. The main purpose of using mannan as ligands was to promote receptor-mediated uptake of liposomal contents into the vicinity of the biofilms. When tested against S. aureus biofilms, the mannosylated liposomes showed a percentage of bacterial growth inhibition of 98% for sialo-mannan-anchored, 86% for cholesteryl mannan-anchored and 70% for plain liposomes (Vyas, Sihorkar and Jain 2007). Similarly, in vivo results using a carboxymethylcellulose pouch infection model showed a higher efficiency of sialo-mannan-anchored liposomes (>3 log10 reduction) when compared with other formulations (Vyas, Sihorkar and Jain 2007). These findings suggest that sialo-mannan ligand mediates the interactions between the liposomes and the biofilm, leading to the release of the encapsulated drug, metronidazole, at the target site (Vyas, Sihorkar and Jain 2007).
In addition to the importance of targeting to increase drug release near the biofilm, ligands can simultaneously be used to promote an immune response. For instance, liposomes can be modified with wheat germ agglutinin (WGA) (Meng et al. 2016). WGA recognizes N-acetylglucosamine present in the biofilm matrix, promoting the interaction of liposomes with the bacterial cell walls and subsequent drug release (Cerca et al. 2006; Meng et al. 2016). Moreover, WGA contributes for an enhanced antibacterial activity of the immune system by binding to the surface of macrophages (Corradin, Buchmuller-Rouiller and Mauel 1991; Meng et al. 2016). This leads to enhanced phagocytosis of bacterial cells and upregulated expression of several cytokines (Corradin, Buchmuller-Rouiller and Mauel 1991; Meng et al. 2016). Nonmodified were compared with WGA-modified liposomal clarithromycin for antibiofilm activity against MRSA biofilms (Meng et al. 2016). The MIC values of the plain drug and the drug-loaded nonmodified liposomes were 64 µg mL−1. This result shows that the entrapped drug in nonmodified liposomes could not enhance the antibacterial activity against MRSA, when compared to the free drug. However, drug-loaded WGA-modified liposomes showed a significant lower MIC value (16 µg mL−1). Regarding eradication of in vitro preformed biofilm, only WGA-modified liposomes led to a significant decrease of biofilm, compared to the negative control (Meng et al. 2016). In vivo assays, where mice were injected intraperitoneally with 1 × 107 CFU, revealed a decrease of 16- to 24-fold of the viable bacteria that remained in the kidneys and the spleens of infected animals after being treated with WGA-modified liposomes, compared to the control group (Meng et al. 2016). Additionally, in vitro cellular uptake studies showed that phagocytosis of MRSA treated with WGA-modified liposomes was significantly higher than with the nonmodified vesicles. The targeted efficiency of WGA was further confirmed by in vivo biodistribution studies, in which the modified liposomes revealed a high affinity to the abdominal cavity. Therefore, liposomes modified with WGA showed an enhanced targeted delivery and immune response for therapeutic applications of abdominal infections (Meng et al. 2016).
Although several reports in the literature are focused on antibiotics encapsulation, natural compounds have recently been highlighted as promising alternatives to conventional antibacterial drugs, due to the increasing bacterial resistance phenomenon observed for the latter ones. Cui et al. (2016) designed liposomes to encapsulate cinnamon oil, a natural essential oil with antibacterial properties obtained from a plant resource. This compound interacts with microbial membranes due to its hydrophobicity, leading to their destruction (Mathew and Abraham 2006). Nevertheless, the chemical instability of cinnamon oil hinders its application for health purposes (Cui et al. 2016). Thus, cinnamon oil was encapsulated into liposomes to reduce its chemical instability and to improve its antibacterial activity. To assess its in vitro antibiofilm efficiency, the liposomal formulation was incubated for 24 h with MRSA biofilms previously established on different material surfaces (stainless steel, gauze, nylon membrane and nonwoven fabrics). In all tested materials, a significant reduction of MRSA was observed when compared with free cinnamon oil. Specifically, the treatment of biofilms formed on stainless steel with free cinnamon oil reduced the amount of MRSA viable cells by 1.49 logs, while the treatment with loaded liposomes reduced this number by 2.45 logs (Cui et al. 2016). Microscopic analysis showed reduced thickness and sizes of MRSA biofilms after treatment with the liposomal formulation (Cui et al. 2016). Thus, the improved chemical stability of cinnamon oil after encapsulation into liposomes led to an enhancement of its antibiofilm activity.
Quatsomes
Inspired by liposomes, other structures, such as quatsomes, were developed for delivery purposes. Quatsomes are unilamellar vesicles composed of quaternary ammonium surfactants and sterols (Grimaldi et al. 2016). These structures are stable during a long storage time and homogenous regarding size, lamellarity and membrane organization. Similar to liposomes, both hydrophilic and hydrophobic drugs can be encapsulated within quatsomes structure. Thus, these novel systems are a promising alternative to overcome the low stability of structures such as liposomes (Grimaldi et al. 2016).
Quatsomes obtained from the self-assembly of equimolar ratios of cetylpyridinium chloride (CPC) and cholesterol were developed by Thomas et al. (2015) aiming the eradication of S. aureus biofilms (see Table 1). CPC is an antiseptic quaternary ammonium compound, composed of a lipophilic tail and a positively charged head group (Gilbert and Moore 2005). This composition facilitates the interaction of CPC with the negatively charged bacterial surface, leading to bacterial cell lysis (Gilbert and Moore 2005). In this study, the CPC quatsomes were compared with CPC micelles for their antibacterial activity (Thomas et al. 2015). CPC quatsomes showed in vitro a dose-dependent antibacterial effect, with >99% biofilm killing at the CPC concentration of 0.5% (Thomas et al. 2015). At lower concentrations, CPC quatsomes revealed a significantly lower efficiency than CPC micelles. In fact, CPC micelles showed 80–90% of efficiency against S. aureus biofilms at a CPC concentration of 0.05% and 0.1%. Higher CPC concentrations resulted in almost complete bacteria killing within the biofilm. The authors suggest that this effect may result from the penetration of the micellar formulation into the biofilm, while CPC quatsomes are mostly present at the surface of the biofilm. To evaluate the safety of the both CPC micelles and quatsomes, epithelial toxicity studies using lactate dehydrogenase were performed. At all CPC concentrations tested (from 0.05% to 1.0%), both formulations showed no cytotoxicity on the NuLi-1 human airway epithelial cell line. In conclusion, the main goal of this research work is to highlight the potentiality of quatsomes as a safe new drug delivery system (Thomas et al. 2015). Despite their potential, further research to optimize quatsomes for drug delivery purposes is required.
Solid lipid nanoparticles
Lipid nanoparticles have been recently highlighted due to their ability to enable a controlled drug release and to be cost effective and easily scaled-up (Battaglia and Gallarate 2012; Severino et al. 2012). Lipid NPs are usually composed of a matrix of physiological or physiologically related lipids characterized by their versatility, biocompatibility and biodegradability (Pardeike, Hommoss and Müller 2009; Das and Chaudhury 2011; Battaglia and Gallarate 2012). In addition, these NPs are able to incorporate either hydrophobic or hydrophilic drugs (Abed and Couvreur 2014). Different types of lipid NPs have been engineered, such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (Ali Khan et al. 2013). Until this moment, in the context of biofilm treatment, only SLNs studies have yet been reported. SLNs are composed of a matrix of lipids in a solid state at both room and body temperatures (Severino et al. 2012).
Nowadays, antibiotics such as clarithromycin and cefuroxime axetil have been widely used against a vast variety of pathogens, including S. aureus. However, both antimicrobial agents have a poor systemic bioavailability (Singh et al. 2014; Sharma, Gupta and Gupta 2016). To overcome this drawback, SLNs have been engineered (see Table 1).
Singh et al. (2014) developed cefuroxime-loaded SLNs to treat S. aureus biofilms. The SLNs were produced and optimized to achieve a small particle size, a narrow polydispersity index and a high encapsulation efficiency (see Table 1). In vitro drug release studies at pH 6.8 indicated a prolonged and sustained release, since the lipid formulation showed 54% of drug diffusion in 2 h and 96% in 12 h. Under the same experimental conditions, 99% of free drug was released in the first 2 h. The optimal formulation showed an MBIC of 40 µg mL−1, whereas the free drug had an MBIC of 80 µg mL−1 (Singh et al. 2014). According to the authors, the lower MBIC suggests a superior antibiofilm activity of the drug when encapsulated into SLNs, which may be due to the nanoscale effect combined with the enhanced biofilm penetration of lipid NPs (Singh et al. 2014). Despite the promising findings, this work lacks in vivo studies for further elucidation of the formulation potential for a therapeutic application.
With the same purpose, Sharma, Gupta and Gupta (2016) designed SLNs encapsulating clarithromycin. For the formulation optimization, three different surfactants were tested: poly vinyl alcohol, Pluronic F-68 and Tween 80. The formulation containing Pluronic F-68 showed the smaller particle size with higher encapsulation efficiency. These findings support the hypothesis that Pluronic F-68 provides a higher mechanical and thermodynamic stability than poly vinyl alcohol and Tween 80, which prevents the coalescence of the particles (Lee, Choi and Park 2008; Peltonen and Hirvonen 2010; Sharma, Gupta and Gupta 2016). Consequently, Pluronic F-68 was selected for the optimization process (Sharma, Gupta and Gupta 2016). Additionally, stearic acid with tristearin was used to enhance the encapsulation efficiency during the optimization process. The optimized formulation (see Table 1) exhibited a controlled drug release behavior, with only 34% of drug diffusion after 3.5 h, while 99% of free drug was released in the same conditions. Furthermore, the optimized formulation showed to be 12 times more effective than the free drug against a S. aureus strain (MTCC86), in vitro. The efficiency of the formulation was also verified against S. aureus biofilms. Complete in vitro biofilm eradication was obtained at a drug concentration of 40 µg mL−1 for the optimized formulation, whereas 140 µg mL−1 of free drug was required to achieve the same effect (Sharma, Gupta and Gupta 2016). According to the authors, the higher efficiency of the SLNs is probably due to a sustained drug delivery profile and the adherence/adsorption of these particles to the bacterial cell walls (Sharma, Gupta and Gupta 2016). To evaluate the in vitro cytotoxicity of the SLNs, a macrophage (J774A.1) cell line was used and the SLNs showed no cytotoxicity at a drug concentration of 48 µg mL−1, which is higher than the MBIC of the formulation. In vivo studies in wistar rats showed that a dose equivalent to 10 mg per kg of body weight did not cause toxic effects after an oral administration. Moreover, the in vivo pharmacokinetic profile of the SLNs revealed an almost 5-fold improvement of clarithromycin oral bioavailability comparing to the free drug (Sharma, Gupta and Gupta 2016). The superior antibacterial activity and oral bioavailability of clarithromycin encapsulated into SLNs combined with the safety of the nanosystem may lead to an improved drug efficiency. Overall, these studies support the evidence of lipid NPs as delivery systems with potential to enhance the therapeutic effect of drugs for the treatment of bacterial biofilms.
Micelles
Micelles are structures composed of amphiphilic molecules (Letchford and Burt 2007). When in an aqueous solution, these molecules self-assemble into micelles above a specific concentration, the critical micelle concentration. In a micelle, amphiphilic molecules organize their hydrophobic regions to create an inner core, where lipophilic drugs can be loaded (Letchford and Burt 2007). Dalili et al. (2015) isolated a novel cyclic lipopeptide from Corynebacterium xerosis NS5, named coryxin which consists of a heptapeptide and a β-hydroxy fatty acid with 11 carbon atoms (see Table 1). The low critical micelle concentration of coryxin (25 mg L−1) revealed the high efficiency of this biosurfactant. Furthermore, the antibacterial and the antibiofilm efficacy of the isolated coryxin was evaluated through in vitro studies against Pseudomonas aeruginosa, Escherichia coli, S. aureus and Streptococcus mutans. The biosurfactant exhibited antimicrobial activity against all strains, with MIC values of 0.19 mg mL−1 against S. aureus and S. mutans and MIC values of 3.12 and 100 mg mL−1 for E. coli and P. aeruginosa, respectively (Dalili et al. 2015). Regarding its antibiofilm activity, coryxin (100 mg mL−1) was able to disrupt 1-day old biofilms from all tested strains, by 83%, 80%, 66% and 30% for S. aureus, Streptococcus mutans, E. coli and P. aeruginosa, respectively. According to these results, Gram-positive bacteria were more sensitive to the biosurfactant effect than Gram-negative bacteria, which the authors claim is probably due to the differences in the bacterial membrane structure (Dalili et al. 2015). Due to its nature, coryxin and other biosurfactants are biocompatible and nontoxic, contrary to chemically synthesized surfactants (Dusane et al. 2011; Dalili et al. 2015). Besides being a lipopeptide, coryxin easily modifies the bacterial surface hydrophobicity and, consequently, interferes with the bacterial adhesion to medical devices (Pecci et al. 2010; Dalili et al. 2015). Thus, the use of biosurfactants to treat S. aureus biofilms adhered to medical devices may be a promising, effective and safe alternative to the current therapy.
Nanodroplets
Besides the well-known NPs previously described, other nanotechnology-based structures have been researched. Recently, Guo et al. (2017a) designed novel stimulated phase-shift acoustic nanodroplets (NDs) in combination with vancomycin for an efficient MRSA biofilm eradication. The lipid phase-shift NDs consisted of a lipid shell of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and a liquid core of condensed perfluoropentane (Guo et al. 2017a). NDs undergo a phase transition to gaseous bubbles (acoustic droplet vaporization) when they are exposed to an ultrasound or thermal energy threshold (Kripfgans et al. 2000; Rapoport et al. 2009). It is hypothesized that the generated bubbles may cause mechanical effects in tissues or cells after cavitation (Pajek et al. 2014). The NDs treated sequentially with low-intensity pulsed ultrasound and heating (37°C) achieved vaporization and cavitation (Guo et al. 2017a). Furthermore, the combined effect of NDs and vancomycin was evaluated against established MRSA biofilms. This combined effect promoted a stronger destruction on the biofilm structure and a higher bacterial death than NDs or vancomycin alone, which was verified by microscopy and a resazurin assay. The authors suggest that NDs detach the biofilm structure, which promotes a closer contact of vancomycin with the bacteria (Guo et al. 2017a). Thus, stimulated phase-shift acoustic NDs are a potential antibiofilm strategy, when combined with traditional antibacterial agents.
POLYMERIC-BASED NANOSYSTEMS
Polymeric nanoparticles
Polymeric NPs are composed of biodegradable polymers and are characterized by a high structural integrity. Hence, they can increase the stability of any volatile drug, promoting its controlled release (Kayser, Lemke and Hernandez-Trejo 2005). Besides, they are easy to scale-up, are cost effective and have a high stability during storage (Kayser, Lemke and Hernandez-Trejo 2005, El-Say and El-Sawy 2017).
Among a wide range of polymers, poly(lactic-co-glycolic acid) (PLGA) is the most commonly used for drug delivery purposes due to its biocompatibility and biodegradability (Huh and Kwon 2011). Several studies reported the use of PLGA micro- and nanoparticles for antibiotic delivery to bacterial biofilms. In the study performed by Thomas et al. (2016), micro- and nanoparticles encapsulating ciprofloxacin were designed to overcome the low bioavailability of this drug and its tolerance by biofilms. The different surface areas of micro- and nanoparticles led to a significant difference in the loading capacity values (see Table 2). The lower loading capacity of NPs can also be associated to a high energy input required to produce these particles, which may facilitate drug leakage (Thomas et al. 2016). Both micro- and nanoparticles showed identical release profiles, with 50–60% of ciprofloxacin release in the first 24 h and complete drug release within 5 days. The MBEC was evaluated using 5-day old S. aureus and P. aeruginosa biofilms and was lower for the free drug compared to the MBEC of both micro- and nanoparticles, after 24 h incubation at 37°C (Thomas et al. 2016). For S. aureus, the MBEC values were 128 and >256 µg mL−1 for free drug and encapsulated drug, respectively. This result was expected as the particles showed a time-dependent drug release, with an incomplete release within 24 h (Thomas et al. 2016). The antibiofilm performance of free drug and encapsulated drug during a treatment of 6 days was also evaluated. The sustained drug release by both micro- and nanoparticles contributed to a higher efficiency in the eradication of S. aureus biofilms, compared to the repeated administration of free drug under the same conditions. Based on these results, the authors claim that both types of particles revealed a high potential to a local treatment of biofilms once sustained antibiotic concentrations are locally achieved and, consequently, systemic adverse effects are expected to be minimized, although this was not studied (Thomas et al. 2016).

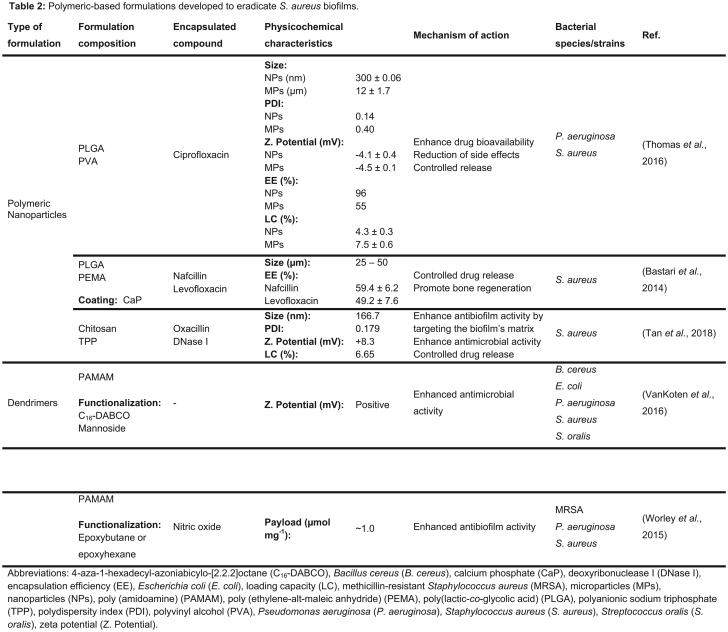
Despite that several studies reported successful eradication of bacterial biofilms using uncoated polymeric NPs, evidence shows that functionalization of their surfaces with specific ligands, making them targeted delivery systems or promoting a faster recovery from the infection, increases their efficiency (Huh and Kwon 2011). For instance, Bastari et al. (2014) showed the potential of a ceramic–polymer hybrid system for osteomyelitis, by providing a controlled drug release and promoting bone regeneration. In this study, biodegradable PLGA NPs coated with calcium phosphate to accelerate the bone healing process were developed and loaded with either nafcillin or levofloxacin (see Table 2). Due to the higher potency of levofloxacin against S. aureus and the superior loading capacity and controlled drug release of levofloxacin than nafcillin, the first formulation was used for biofilm inhibition and deterioration studies (Bastari et al. 2014). Both coated and uncoated levofloxacin-loaded NPs prevented biofilm formation for >4 weeks and completely eradicated an established biofilm after 7 days of treatment. Thus, levofloxacin-loaded NPs have a high potential for both prevention and eradication of S. aureus biofilms associated to osteomyelitis.
Besides PLGA, other polymers such as chitosan are widely used in pharmaceutical and medical applications. Chitosan is a derivate of chitin and presents biocompatible and biodegradable properties and it has been reported that chitosan also shows antibacterial activity itself (Jayakumar et al. 2010). Due to these properties, Tan et al. (2018) produced chitosan NPs for the co-delivery of an antibacterial agent (oxacillin) and a biofilm matrix disruptive agent (Deoxyribonuclease I). Deoxyribonuclease I (DNase I) was selected for this purpose since it breaks the extracellular DNA, which is one of the key components of the biofilm's matrix (Montanaro et al. 2011). The antibacterial and antibiofilm activities of the DNase-oxacillin NPs against S. aureus were evaluated. This formulation revealed an MIC value of 1 µg mL−1, while free oxacillin showed an MIC value of 0.5 µg mL−1 (Tan et al. 2018). The authors claim that this result is probably due to a controlled drug release from the NPs. However, DNase-oxacillin NPs exhibited higher antibiofilm activity than the free drug against mature biofilms. The antibiofilm effect with repeated treatment for 2 days was also assessed and the higher concentration of free drug and DNase-oxacillin NPs revealed a biofilm reduction of 92.3% and 100%, respectively. Additionally, the DNase-oxacillin NPs did not show any cytotoxic effects against a human immortalized keratinocytes (HaCaT) cell line (Tan et al. 2018).
Dendrimers
Dendrimers are hyperbranched polymers synthetized layer-by-layer around a core unit (Huh and Kwon 2011). Consequently, these structures have a high surface area to size ratio, leading to great reactivity to bacteria in vivo. Both hydrophilic and hydrophobic drugs can be loaded within the dendrimers structure. Besides, a high density of functional groups can be added to these structures for a targeted delivery (Huh and Kwon 2011).
In an attempt to inhibit and eradicate bacterial biofilms, fourth-generation poly(amidoamine) (PAMAM) dendrimers were developed by VanKoten et al. (2016) (see Table 2). These dendrimers were decorated with a quaternary ammonium compound, 4-aza-1-hexadecylazoniabicylo-[2.2.2]octane (C16-DABCO), which is a cationic surfactant with antibacterial activity (McDonnell and Russell 1999). Additionally, mannoside end groups were added to the dendrimeric structure to promote interactions with the bacterial cell wall (VanKoten et al. 2016). The antibacterial activity of these dendrimers was compared with the C16-DABCO monomer to evaluate the effect of multivalency on the MIC values. For all tested Gram-positive (Streptococcus oralis, S. aureus and Bacillus cereus) and Gram-negative (P. aeruginosa and E. coli) strains, the MIC value is at least 10-fold higher for the monomer than for the dendrimeric structure. The authors claim that this discrepancy is probably due to the dendrimers native cations that interact with surface-associated adhesion molecules from bacteria (VanKoten et al. 2016). Despite the promising results against planktonic bacteria, disruption was not observed when the dendrimers were evaluated in previously established biofilms. According to the authors, the lack of efficiency against mature biofilms is possibly due to the dendrimers’ large size, which hinders their penetration into the matrix of the biofilm and are restricted to the biofilm surface (VanKoten et al. 2016). Besides, the dendrimers showed toxicity in hemolysis assays and in a cytotoxic assay using the A549 human lung carcinoma cell line, at concentrations within the same order of magnitude as the MIC values assessed for the studied bacterial strains (VanKoten et al. 2016). The authors believe that this result indicates a broad activity of the dendrimers on both bacterial and mammalian cells. Despite the antibacterial activity of these dendrimers, their safety and antibiofilm activity need to be improved for therapeutic applications.
To overcome the low penetration efficiency of dendrimers into bacterial biofilms, nitric oxide (NO)-releasing PAMAM dendrimers were designed by Worley, Schilly and Schoenfisch (2015) (see Table 2). NO free radical has a wide range of antibacterial activity due to the production of reactive products that promote bacterial membrane disruption (Fang 1997). The designed NO-releasing PAMAM dendrimers were functionalized with butyl and hexyl chains (Worley, Schilly and Schoenfisch 2015). The efficiency of the dendrimers was evaluated against bacterial biofilms, namely of P. aeruginosa, S. aureus and MRSA. The biofilms were grown on medical-grade silicon rubber substrates and further exposed to the dendrimers for 24 h. Hexyl-modified dendrimers were undoubtedly more effective at biofilm eradication than the butyl systems. Thus, it is hypothesized by the authors that the hexyl-modified dendrimers penetrate deeper into the biofilm at a faster rate and promote higher cell membrane damage due to a longer alkyl chain (Worley, Schilly and Schoenfisch 2015). The NO release from hexyl-modified dendrimers did not show an improvement in antibiofilm efficacy. This result suggests that a significant membrane damage promoted by the hexyl chains precludes the achievement of intracellular NO to bactericidal concentrations (Worley, Schilly and Schoenfisch 2015). In the case of butyl-modified dendrimers, NO-releasing dendrimers have a higher biocidal action than those without NO. Additionally, it was verified that increasing the dendrimer generation from G1 to G2 or G3 increases the eradication efficiency of the butyl-modified dendrimers due to a higher functional group density. Regarding the bacterial strains, hexyl-modified dendrimers exhibited a higher activity against P. aeruginosa biofilms, disregarding of the dendrimer generation. Both types of dendrimers revealed similar bactericidal action against MRSA biofilms, requiring higher eradication concentrations than nonresistant S. aureus (Worley, Schilly and Schoenfisch 2015). Thus, the efficacy of the dendrimers depends on their alkyl chain length, the dendrimer generation and the bacterial strain. Nevertheless, only the G3 hexyl dendrimer system efficiently eradicated biofilms at concentrations below the inhibitory concentration at 50% viability (IC50) against L929 mouse fibroblasts cell line (Worley, Schilly and Schoenfisch 2015). Due to its efficiency and safety, this dendrimer is the most promising option for biomedical applications.
METALLIC-BASED NANOSYSTEMS
Metallic nanoparticles
Metallic NPs can be applied as drug delivery systems once they can protect the drugs until their target site and avoid the immune system activation with low cytotoxicity (Lopes et al. 2014). Additionally, many metallic NPs have been studied for their intrinsic antimicrobial effect and the unlikely development of bacterial resistance to these type of NPs (Lopes et al. 2014). A summary of the metallic NPs developed for S. aureus biofilm eradication is shown in Table 3.

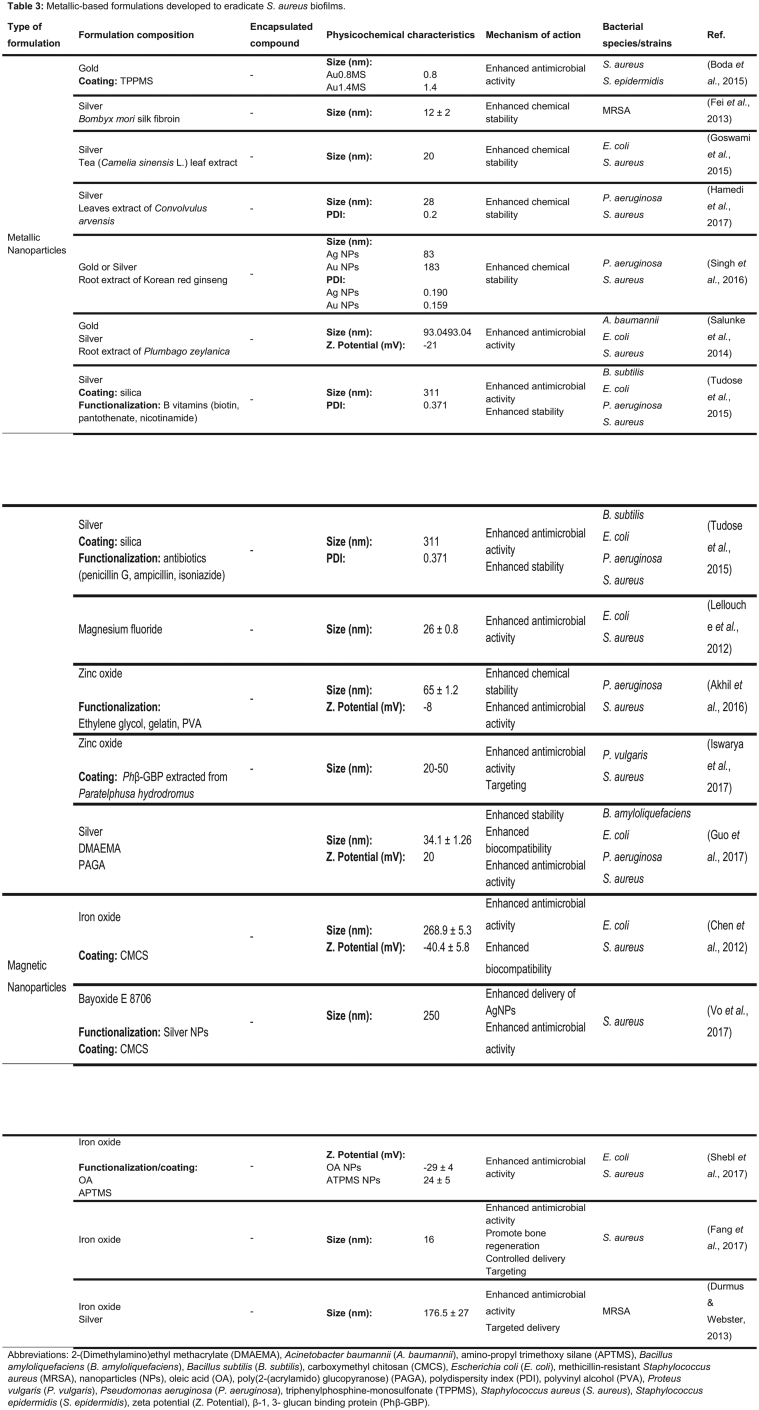
Gold and silver NPs are among the most commonly researched types of metallic NPs, with several literature articles focusing on their antimicrobial activity against planktonic and biofilm bacteria. Additionally, these NPs are easily functionalized with ligands, which contributes in enhancing their biocompatibility and chemical stability.
Boda et al. (2015) developed ultrasmall AuNPs functionalized with triphenylphosphine-monosulfonate. The triphenylphosphine-monosulfonate shell was added to the Au core of the NPs to improve their stability. The AuNPs with core diameters of 0.8 and 1.4 nm (Au0.8MS and Au1.4MS) were evaluated against S. aureus, Staphylococcus epidermidis, E. coli and P. aeruginosa in vitro. The AuNPs caused acute toxicity to planktonic bacteria, with a 5-log reduction in viable cells after 5 h of exposure (Boda et al. 2015). Regarding staphylococci, AuNPs caused membrane blebbing and cell swelling, which was observed by scanning electron microscopy, suggesting membrane damage and eventual cell lysis. Mature staphylococci biofilms of 48 h were treated with Au0.8MS and Au1.4MS, showing 80% reduction in bacterial viability when compared with the untreated bacteria (Boda et al. 2015). However, Staphylococcus epidermidis biofilms were less sensitive to the toxicity of AuNPs than S. aureus biofilms since no significant biomass reduction was obtained when such biofilms were treated. In this context, further studies regarding the efficiency of AuNPs against staphylococcal biofilms are needed.
Despite their potential for biomedical applications, chemical synthesis of metallic NPs is a concern due to the use of organic solvents, toxic compounds and the existence of hazardous by-products (Abou El-Nour et al. 2010; Singh et al. 2016). To overcome these limitations, a simple, rapid and eco-friendly methodology has emerged. The green synthesis relies on the use of compounds from microorganisms and plant extracts to reduce and to stabilize the NPs during their preparation, acting as reducing and capping agents (Abou El-Nour et al. 2010; Singh et al. 2016). In this process, a plant extract or microorganism-derived compound is added to the solvent and to the metal solution during the process (Kalpana and Devi Rajeswari 2018). Several naturally synthetized Au and Ag NPs with antibiofilm activity are reported in the literature. For instance, Fei et al. (2013) prepared AgNPs in situ using the natural polymer Bombyx mori silk fibroin as a reducing agent of Ag. Additionally, silk fibroin chains were connected to the surface of the AgNPs, which improved their stability in aqueous dispersions due to the nonionic nature of the silk fibroin (Kvitek et al. 2008; Fei et al. 2013). After their preparation, AgNPs were tested against mature MRSA biofilms obtained from a clinical isolate. At the concentration of 76.8 µg mL−1, AgNPs were able to partially disrupt the biofilm structure with a significant decrease in bacterial viability, revealing the strong bactericidal activity of these particles. A 5-fold increase of the NPs concentration resulted in a complete destruction of the bacterial biofilm (Fei et al. 2013). Similarly, Goswami et al. (2015) developed AgNPs from tea extract (Camelia sinensis L.) and evaluated their potential against bacterial biofilms. S. aureus and E. coli biofilms grew at the surface of silicone tubes and polystyrene coverslips for 24 h and were then incubated during 48 h with AgNPs, at room temperature. At the concentration of 15 µg mL−1, the AgNPs showed an inhibition rate of 89% and 75% for S. aureus and E. coli, respectively. In addition, the release of cellular contents after the treatment with AgNPs confirmed the impairment of bacterial cell–cell adhesion due to membrane damage (Goswami et al. 2015). To evaluate the biocompatibility and safety of the designed AgNPs, a hemolytic assay was conducted using goat erythrocytes. The results revealed that AgNPs induced insignificant hemolysis, being a potentially safe nanosystem for drug formulation and coating on medical devices. Nevertheless, cytotoxic studies using animal cell lines to assess NPs biocompatibility are lacking (Goswami et al. 2015). Hamedi, Shojaosadati and Mohammadi (2017) also reported the potential of green synthesis of AgNPs against S. aureus biofilms. In this study, leave extracts of Convolvulus arvensis were used. Most bacteria of the biofilm were inactivated when exposed to 20 µg mL−1 of AgNPs (Hamedi, Shojaosadati and Mohammadi 2017). AgNPs and AuNPs were also produced by Singh et al. (2016) using root extracts of the herbal medicinal plant Korean red ginseng. The developed NPs did not aggregate, revealing a long-term stability at room temperature, in aqueous solution. However, only AgNPs were tested to determine their activity against S. aureus and P. aeruginosa biofilms. For both biofilm types, a maximum biofilm degradation was observed at 4 µg mL−1 of AgNPs (Singh et al. 2016).
Bimetallic NPs (AgAuNPs) synthesized using a green methodology have also been studied for their superior antimicrobial properties. AgAuNPs synthesized using the medicinal plant Plumbago zeylanica reported a higher antibacterial effect than AgAuNPs chemically synthesized, with a biofilm disruption activity of 61–77% for all tested biofilms (Acinetobacter baumannii, E. coli and S. aureus) (Salunke et al. 2014). Despite the scientific progress, the poor disruption activity of these AgAuNPs emphasizes the need of optimization of these nanosystems for a potential therapeutic application.
An alternative strategy to stabilize metallic NPs, such as AgNPs, consists in using a silica matrix to avoid coagulation and oxidation (Ravindran, Chandran and Khan 2013; Tudose et al. 2015a; Tudose et al. 2015b). Tudose et al. (2015a) developed AgNPs coated with silica (Ag-SiO2NPs) and functionalized with vitamins B (biotin, pantothenate and nicotinamide). Ag-SiO2-biotin and Ag-SiO2-nicotinamide exhibited very low MBEC values (0.97 and 1.95 µg mL−1, respectively) against S. aureus biofilms in vitro, revealing a higher antibiofilm activity than nonfunctionalized Ag-SiO2NPs. Thus, it is hypothesized by the authors that the vitamins promoted the release of Ag ions and their intracellular accumulation and facilitated the penetration of Ag-SiO2NPs into the biofilm matrix (Tudose et al. 2015a). Furthermore, Ag-SiO2NPs were also functionalized with antibiotics (ampicillin, penicillin G and isoniazide) (Tudose et al. 2015b). Despite being a promising approach, in vitro efficacy assays showed that nonfunctionalized Ag-SiO2NPs had a similar or even higher antibiofilm activity than the ones functionalized with penicillin G and isoniazide (Tudose et al. 2015b). The authors claim that a chemical modification of the antibiotics occurred, leading to a decrease of their antibacterial efficiency. On the contrary, a synergistic effect between ampicillin and Ag-SiO2NPs was confirmed by the lower MBEC values against S. aureus and P. aeruginosa biofilms, when compared with the nonfunctionalized Ag-SiO2NPs (Tudose et al. 2015b).
Besides AuNPs and AgNPs, other types of metallic NPs are also reported in the literature for their potential antibacterial and antibiofilm activity. Lellouche et al. (2012) produced magnesium fluoride NPs and evaluated their efficiency against established S. aureus and E. coli biofilms. Biofilms 1- to 7-day old were exposed to the magnesium fluoride NPs in a concentration of 1 mg mL−1 for 24 h. The NPs promoted an 87% and 44% decrease in E. coli biofilm viability for a 1- and 7-day old biofilm, respectively. Under the same experimental conditions, S. aureus biofilm viability decreased 92% (1-day old) and 69% (7-day old) after treatment with magnesium fluoride NPs (Lellouche et al. 2012). In another example, zinc oxide NPs were synthetized by co-precipitation and evaluated for their antibacterial and antibiofilm activity against S. aureus and P. aeruginosa (Akhil et al. 2016). For both species, zinc oxide NPs showed a higher efficiency against established biofilms in the presence of light than in dark conditions. According to the authors, these results are probably due to reactive oxygen species production in the presence of light (Akhil et al. 2016). A more complex system involving zinc oxide NPs was recently reported by Iswarya et al. (2017). Zinc oxide NPs were coated with crustacean immune molecule β-1,3-glucan binding protein (Phβ-GBP) purified from the rice field crab Paratelphusa hydrodromus. The coated zinc oxide NPs exhibited antibiofilm activity against a S. aureus established biofilm by disrupting bacterial membranes and destroying the biofilm structure. The biofilm thickness was significantly reduced from 40 (control) to 10 µm after treatment with 75 µg mL−1 of NPs (Iswarya et al. 2017). The strong antibiofilm activity of these particles suggests a combinatory effect of the Phβ-GBP coat, which specifically binds to the surface of bacteria, and zinc oxide NPs, that induces reactive oxygen species release from bacterial cells (Goncalves et al. 2012; Iswarya et al. 2017).
The previously mentioned studies show highly promising alternative therapies to treat bacterial biofilms, including from S. aureus strains. Nonetheless, there is a lack of in vivo studies to further confirm the in vitro antibiofilm efficacy of the NPs. Guo et al. (2017b) studied the antibiofilm activity of polymer functional silver nanocomposites both in vitro and in vivo. In these nanocomposites, the biocompatible carbohydrate polymer poly(2-(acrylamido) glucopyranose) (PAGA) and the membrane-disrupting cationic polymer quaternizated poly(2-dimethylaminoethyl methacrylate) (PDMAEMA-C4) were used as ligands. The safety of this nanosystem was evaluated by in vitro hemolysis and cytotoxicity assays. The NPs revealed no toxic effects on red blood cells at all polymer concentrations tested (until 10 µmol L−1). On NIH3T3 cells, the nanocomposites did not induce significant cytotoxic effects below a polymer concentration of 2.5 µmol L−1. The efficacy of these nanocomposites was evaluated against several biofilms, including S. aureus (Guo et al. 2017b). For the in vivo efficiency assay, implants coated with biofilms were introduced into the mouse peritoneum and then locally treated with the nanocomposites (5 µmol L−1). After 24 h incubation, it was observed a reduced bacterial colonization on the implant, compared with the control group (Guo et al. 2017b). Although the developed nanocomposites have potential to eradicate in vivo biofilms previously established on implantable medical devices, the polymer concentration used for this study showed cytotoxicity effects on mammalian cells. Thus, the safety of this nanosystem needs to be improved for therapeutic purposes.
In the past few decades, the magnetic properties of iron oxide NPs have been studied for their diagnostic and therapeutic properties (Pankhurst et al. 2003; Wu et al. 2015). Thus, magnetic NPs (MNPs) are a potential targeted nanosystem as they can be directed or guided by a magnetic field gradient toward biological targets (Pankhurst et al. 2003). Additionally, these particles are cost effective, physically and chemically stable, biocompatible and eco-friendly (Wu et al. 2015).
Chen et al. (2012) produced MNPs coated with carboxymethyl chitosan (CMCS) to effectively eradicate bacterial biofilms applying an external magnetic field. The CMCS was selected due to its enhanced aqueous solubility and antimicrobial activity, when compared with chitosan (Chen and Park 2003). The strong bactericidal action of CMCS is a consequence of the lysis and the inhibition of the nutrients transport into bacteria (Gu et al. 2007; Eldin et al. 2008; Du et al. 2009). The designed CMCS MNPs were evaluated in vitro against S. aureus and E. coli both in planktonic and biofilm forms (Chen et al. 2012). A decrease of 26% and 30% of viable S. aureus were found after 2 h treatment with CMCS and CMCS MNPs, respectively. The viability dropped to 1% after 10 h of treatment with CMCS and CMCS MNPs. Further, both CMCS and CMCS MNPs were evaluated regarding their antibiofilm activity at concentrations that showed no cytotoxic effects on 3T3 fibroblast cells. After a 48 h incubation period, CMCS (0.34 mg mL−1) and CMCS MNPs (1.0 and 2.0 mg mL−1) did not show significant decrease in S. aureus cell viability within the biofilms in the absence of a magnetic field (Chen et al. 2012). However, the number of viable S. aureus cells exposed to CMCS MNPs decreased by 84% when an external magnetic field was applied for 5 minutes, revealing a higher penetration of the MNPs into the biofilm structure. Nevertheless, a complete biofilm eradication was not achieved due to the restricted bactericidal activity of CMCS MNPs to bacteria in the immediate surroundings. Thus, the bacteria that are not in direct contact with the particles may survive within the biofilm matrix (Chen et al. 2012). To overcome this limitation, Vo, Sabrina and Lee (2017) added AgNPs to the surface of CMCS MNPs (AgNPs-CMCS MNPs). AgNPs are able to release Ag ions and generate reactive oxygen species that effectively kill bacteria (Xu et al. 2012). On the other hand, CMCS MNPs may be a good scaffold to avoid AgNPs aggregation (Varma, Deshpande and Kennedy 2004; Huang et al. 2008; Laudenslager, Schiffman and Schauer 2008). In this study, when 3-day old S. aureus biofilms were exposed to AgNPs-CMCS MNPs for 15 minutes, under an external magnetic field, no live bacteria were observed (Vo, Sabrina and Lee 2017). Hence, a high concentration of Ag ions and reactive oxygen species were released from AgNPs under a magnetic field, which effectively killed the bacteria within the biofilm (Vo, Sabrina and Lee 2017). Therefore, the dual action of AgNPs-CMCS MNPs shows a high potential for a therapeutic application against bacterial biofilms.
The hydrophobicity and the charge modulation are characteristics with a high impact in the antibacterial and the antibiofilm activity of MNPs. Shebl, Farouk and Azzazy (2017) revealed that oleic acid-coated MNPs (hydrophobic) and amino-propyl trimethoxy silane (APTMS)-coated MNPs (positively charged) were able to destroy 94% and 89% of preformed S. aureus biofilms, respectively. These findings suggest that the negative charge of bacterial cells and the hydrophobicity of both MNPs and biofilm matrixes play a critical role in their interaction (Pagedar, Singh and Batish 2010; Hajipour et al. 2012; Krasowska and Sigler 2014). Despite being a promising strategy for biofilms eradication, both types of MNPs exhibited cytotoxicity effects in human epithelial cells (Shebl, Farouk and Azzazy 2017).
Among all magnetic nanoparticles, superparamagnetic iron oxide particles (SPIONs) have been extensively used for several medical applications, including imaging, biodetection, drug and gene delivery, among others (Hao et al. 2010). Fang et al. (2017) studied the heating effect of MNP-induced hyperthermia in in vivo S. aureus biofilms. For that purpose, an osteomyelitis rat model was stablished by implanting metallic 18 G needle into the bone marrow cavity of distal femur after injecting a bacterial suspension of S. aureus. The inserted implants were further heated to 75°C by magnetic heating, promoting a better distribution of the particles within the biofilm structure. The use of MNPs heated by hyperthermia promoted the destruction of the biofilm and enhanced the efficiency of the plain vancomycin, which was co-administrated with the MNPs. This study highlights the potential of combining antibiotics with hyperthermia to enhance antibiotic penetration within the biofilm matrix and, consequently, to eradicate bacterial biofilms (Fang et al. 2017). Besides the combination with antibiotics, SPIONs were also combined with Ag as an alternative to treat bacterial biofilms. The Ag-conjugated SPIONs were tested against MRSA, without using antibiotics (Durmus and Webster 2013). In this study, 24 h old biofilms were exposed to the developed Ag-conjugated SPIONs for 24 h. The Ag-conjugated SPIONs (1 mg mL−1) significantly decreased planktonic MRSA growth to 1.57% within the biofilm structure. Furthermore, they showed a significant biofilm biomass decrease to <47% in the presence of an external magnetic field. Thus, the combination of magnetic (SPIONS) and antibacterial properties (Ag) within the same system increases the potential of this nanosystem as a targeted therapy to eradicate MRSA biofilms, in alternative to antibiotics (Durmus and Webster 2013).
SILICA-BASED NANOSYSTEMS
Mesoporous silica NPs are promising drug delivery systems due to their biocompatibility and their stability that leads to a controlled drug release (Bharti et al. 2015). Besides, their large surface area, provided by homogenous pores, enables a high payload and an effective functionalization with a variety of groups for a targeted delivery (Bharti et al. 2015). These NPs do not have an intrinsic antimicrobial activity. However, they have a huge potential for antibiotics delivery to multidrug resistant infections once they can be designed to load multiple drugs within their structure (Miller et al. 2015). A summary of the silica-based NPs developed to eradicate S. aureus biofilms is provided in Table 4. For antibiotic delivery purposes, Gounani et al. (2019) produced carboxyl-modified mesoporous silica NPs loaded with polymyxin B and vancomycin. The antibiofilm activity of these NPs was evaluated in both P. aeruginosa and S. aureus mature biofilms. The MBIC and MBEC values of the studied NPs were surprisingly higher than free antibiotics. According to the authors, these findings suggest that NPs did not penetrate the biofilm probably due to their size and the electrostatic interactions between the NPs and the negatively charged biofilm matrix. The in vitro cytotoxicity of the NPs and the free drugs was evaluated using three human cell lines (HEPG2, HEF-2 and HEK-293) (Gounani et al. 2019). The results showed a dose-dependent decrease in cell viability and increase of reactive oxygen species production in all cell lines when exposed to the free drugs. For the same drug concentrations, the developed NPs showed no cytotoxicity effects. Additionally, an hemolysis assay confirmed the safety of the nanosystem. Based on this, the authors claim that carboxyl-modified mesoporous silica NPs loading antibiotics may lead to an enhanced biocompatibility of the drugs, reducing their adverse side effects (Gounani et al. 2019).

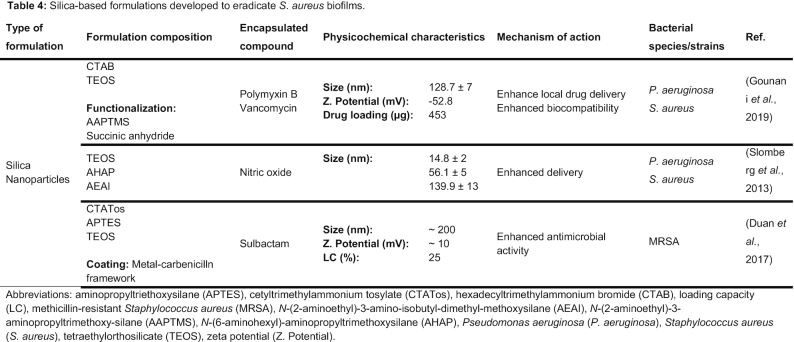
Despite the relevance of antibiotics in this fight, the increasing rates of resistance to these agents leads to the search for alternatives with equal antimicrobial potential. Slomberg et al. (2013) designed NO-releasing silica NPs to eradicate S. aureus and P. aeruginosa biofilms. The particles were evaluated according to their size (14, 50 and 150 nm) and shape (spherical and rod-like). The smaller particles were more effective against planktonic and biofilm S. aureus, compared to 50 and 150 nm particles. Regarding their shape, the spherical NPs were significantly less effective than rod-like NPs, requiring a higher concentration to eradicate the biofilms. These findings were further confirmed by confocal microscopy, where it was possible to observe that NO-releasing NPs with decreased size and increased aspect ratio exhibited better NO delivery and, consequently, enhanced bactericidal action (Slomberg et al. 2013). Despite their therapeutic potential, the NO-releasing NPs exhibited cytotoxicity effects against L929 fibroblasts at the MBEC values for S. aureus and P. aeruginosa. Thus, it is crucial to optimize this nanosystem toward lower toxicity to nontarget cells. Additionally, further studies combining NO with other antibacterial agents can be explored to reduce NO to nontoxic levels (Slomberg et al. 2013).
Combination of different drugs/compounds within the same nanosystem may lead to a synergistic antibacterial effect against resistant bacteria, such as MRSA. Duan et al. (2017) designed pH-responsive metal–carbenicillin framework-coated mesoporous silica nanoparticles (MSNs) to efficiently co-deliver β-lactam antibiotics and β-lactamase inhibitors to MRSA biofilms. Carbenicillin, the selected β-lactam antibiotic, was coordinated with Fe3+ to form the metal–carbenicillin framework to block the pores of the MSNs (Duan et al. 2017). However, bacteria are rapidly evolving to efficiently evade antibiotics, lowering their antibacterial potential. One of the most important bacterial mechanisms to resist to β-lactam antibiotics is the production of β-lactamases (Fisher Meroueh and Mobashery 2005; O'Connell et al. 2013). Thus, to improve the antibacterial efficiency of carbenicillin, the β-lactamase inhibitor sulbactam was encapsulated in the MSNs pores (Duan et al. 2017). The developed nanosystem was stable at physiological conditions (Duan et al. 2017). At the acidic pH of the bacterial infection site (pH∼5.0) there was a controlled release of the active agents due to the sensitivity of the coordination between carbenicillin and Fe3+ at low pH. Additionally, cytotoxicity and hemolysis assays confirmed the safety and nontoxic profile of the MSNs in vitro. Confocal scanning laser microscopy images showed an effective penetration and diffusion of the designed MSNs within the biofilm matrix. As expected, the enhanced penetration ability of MSNs, associated to the synergistic effects of carbenicillin and sulbactam, lead to an effective destruction of established MRSA biofilms (Duan et al. 2017). To further confirm the potential of the system, MRSA intradermally infected mouse were used as an in vivo model system. The control group treated with phosphate-buffered saline exhibited an obvious skin infection, while the group treated with the developed MSNs only showed a slight skin infection. Thus, the authors believe that the reported MSNs may be an alternative therapeutic approach for the treatment of biofilm-associated infections (Duan et al. 2017).
QUANTUM DOTS
QDs are semiconducting NPs with excellent optical properties and high photostability (Ozkan 2004). These particles have a wide ultraviolet absorption spectrum, while their emission spectrum is very narrow, enabling multiplexed imaging under a single light source. Additionally, QDs are easily functionalized, having a great potential for targeted drug delivery (Ozkan 2004). Singh et al. (2017) developed curcumin (Cur) QDs composed of zirconia to enhance Cur aqueous solubility and stability. Cur is a hydrophobic polyphenol compound with a wide spectrum of medical applications, including in antimicrobial therapy (Priyadarsini 2014). However, this compound is rapidly degraded and has a poor aqueous solubility, which limits its use for clinical purposes (Wang et al. 1997; Priyadarsini 2014). The designed Cur QDs had a particles size, a polydispersity index and a zeta potential of 2.5 nm, 0.172 and −26 mV, respectively (Singh et al. 2017). The Cur QDs were also tested for their antibacterial effect against several bacterial strains, including S. aureus and MRSA. The MIC values of Cur QDs were in the range of 3.91 to 15.65 µg mL−1, while the MIC values of the free Cur were significantly higher (175 to 350 µg mL−1). Interestingly, Cur QDs revealed a higher biofilm inhibitory activity on the formation of MRSA biofilms comparing to S. aureus biofilms. Additionally, the Cur QDs exhibited a strong biofilm degrading activity against S. aureus 3-day old biofilms, with disintegration of the biofilm matrix at a concentration of 0.125 µg mL−1. Confocal microscopy was used to validate the antibiofilm effects of Cur QDs, and it was possible to observe that Cur QDs complexes were localized manly at the superficial layers of the biofilm. It was hypothesized by the authors that the strong activity and aqueous solubility of Cur QDs might be related to the small size of these particles, which may lead to a higher penetration and interaction with the biofilm matrix and a higher cellular uptake (Singh et al. 2017).
CONCLUDING REMARKS
The high number of infections, the increasing multiple-antibiotic resistance cases, the health and economic burden and the lack of an effective therapy that does not require surgery for S. aureus biofilms have boosted the research to improve the available therapies. In this context, several nanosystems were developed to eradicate S. aureus biofilms established on the surface of an implant device.
Some systems described above were used to enhance the bioavailability of antibiotics and their controlled release, while reducing their adverse systemic side effects. As an alternative strategy, antibacterial agents such as nitric oxide, enzyme inhibitors and matrix disruptive agents were encapsulated alone or in combination with antibiotics into nanosystems. Additionally, some NPs reported in the literature were functionalized with ligands for a targeted delivery of the antibacterial agents to the biofilm and for increasing chemical stability of the nanosystems.
Research in nanotechnology to fight bacterial biofilms has been also focused on developing systems with intrinsic antibacterial activity such as metallic NPs, to overcome the antibiotic resistance phenomenon. Among these, magnetic NPs are also highlighted for their potential for a targeted delivery to biofilms, when exposed to an external magnetic field.
The studies reported in the literature demonstrated in vitro efficiency against S. aureus either in its planktonic form or within a biofilm structure. Even though with promising results, only a few studies achieved eradication of the bacteria. Moreover, most of these studies lack ex vivo and in vivo assays to evaluate biofilm eradication.
Although in vitro biofilm models highlight the potential of nanosystems for the eradication of these bacterial structures, they are still far from representing the in vivo conditions. The high complexity of biological systems is, usually, not considered in in vitro models. In a biological context, the nanosystems interact with biological fluids and bacterial and host cells. These cells produce extracellular polymeric substances and cellular debris that may hamper the efficiency of nanosystems (Buhmann et al. 2016). Thus, the use of serum in the biofilm culture medium (Rosman et al. 2014) and of the co-culture with host cells (Subbiahdoss et al. 2011) is a step forward in the development of in vitro biofilms with increasing relevance at a clinical level.
Additionally, reference bacterial strains to conduct in vitro studies are currently lacking, which leads to issues of reproducibility and may raise questions regarding the in vivo potential of the developed nanosystems (Roberts et al. 2015; Buhmann et al. 2016).
Perhaps, the major limitation of in vitro efficacy studies concerns the use of 24 or 48 h old biofilms. Considering that in vivo the biofilm maturation occurs during a much longer period, it is expected that the in vivo efficiency of nanosystems will be lower than in in vitro assays. In fact, Lellouche et al. (2012) compared the efficiency of NPs against 1- and 7-day old biofilms of S. aureus and E. coli. For both strains, the eradication efficiency of the treatment decreased 20–40% for 7-day old biofilms. Thus, in vitro assays based on 1-day old biofilms may overestimate the antimicrobial activity of therapeutic agents.
Besides in vitro and in vivo efficiency evaluation of nanosystems, it is also necessary to assess their cytotoxicity. Some of the reported studies lack in vitro assays to assess the safety and the biocompatibility of the designed systems. However, some in vivo mechanisms, such as toxicokinetics, translocation and coordinated response of tissues, are not represented in the current in vitro models (Lopes et al. 2014). Thus, further research is required to evaluate the in vivo toxicity of nanomaterials, regarding biodistribution and interactions with organs and tissues (Arora, Rajwade and Paknikar 2012).
In conclusion, nanotechnology has a tremendous potential to treat biofilm-associated infections caused by pathogens such as S. aureus. Despite this potential, no clinical trials using nanotechnology to fight bacterial biofilms are going on. Therefore, it is urgent to focus on the in vivo efficacy and safety of the nanosystems, essential to initiate further clinical studies.
FUNDING
RMP, DLC and CN are thankful to Fundação para a Ciência e Tecnologia (FCT) for the PhD Grant [SFRH/BD/130319/2017], the PhD Grant [ PD/BD/105957/2014] and the investigator Grant [IF/00293/2015], respectively. This work was supported by FCT through the FCT PhD Programmes and by Programa Operacional Capital Humano (POCH), specifically by the BiotechHealth Programme (Doctoral Programme on Cellular and Molecular Biotechnology Applied to Health Sciences). The work was supported by UID/QUI/50006/2019 with funding from FCT/MCTES through national funds as well as from the Fund for Scientific Research Flanders (FWO grant WO.009.16N) to PVD. This work is under the framework of the project POCI-01–0145-FEDER-31444, financed by Fundo Europeu de Desenvolvimento Regional (FEDER) - through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalisation ( POCI) - and by national funds through Fundação para a Ciência e Tecnologia (FCT).
Conflict of interests. None declared.
 Impact of nanosystems in Staphylococcus aureus biofilms treatment
Impact of nanosystems in Staphylococcus aureus biofilms treatment
