
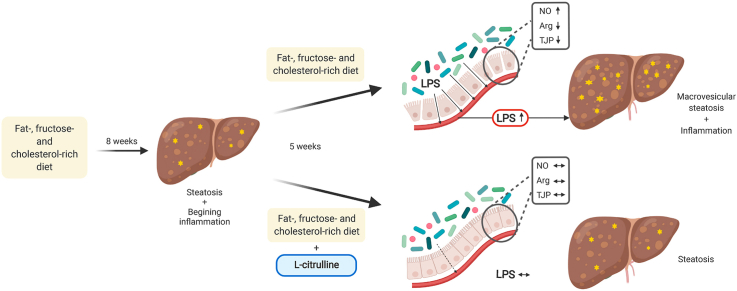
Non-alcoholic fatty liver disease (NAFLD) is by now the most prevalent liver disease worldwide. The non-proteogenic amino acid l-citrulline (L-Cit) has been shown to protect mice from the development of NAFLD. Here, we aimed to further assess if L-Cit also attenuates the progression of a pre-existing diet-induced NAFLD and to determine molecular mechanisms involved. Female C57BL/6J mice were either fed a liquid fat-, fructose- and cholesterol-rich diet (FFC) or control diet (C) for 8 weeks to induce early stages of NASH followed by 5 more weeks with either FFC-feeding +/- 2.5 g L-Cit/kg bw or C-feeding. In addition, female C57BL/6J mice were either pair-fed a FFC +/- 2.5 g L-Cit/kg bw +/- 0.01 g/kg bw i.p. N(ω)-hydroxy-nor-l-arginine (NOHA) or C diet for 8 weeks.
The protective effects of supplementing L-Cit on the progression of a pre-existing NAFLD were associated with an attenuation of 1) the increased translocation of bacterial endotoxin and 2) the loss of tight junction proteins as well as 3) arginase activity in small intestinal tissue, while no marked changes in intestinal microbiota composition were prevalent in small intestine. Treatment of mice with the arginase inhibitor NOHA abolished the protective effects of L-Cit on diet-induced NAFLD. Our results suggest that the protective effects of L-Cit on the development and progression of NAFLD are related to alterations of intestinal arginase activity and intestinal permeability.

•
l-citrulline diminished progression of non-alcoholic fatty liver disease (NAFLD).
•l-citrulline protects from fructose-induced small intestinal barrier dysfunction.
•NASH development is associated with a loss of arginase activity in small intestine.
•l-citrulline improves intestinal arginase activity in diet-induced NAFLD.
•Arginase inhibitor attenuates effects of l-citrulline on NAFLD development.
As the prevalence of non-alcoholic fatty liver disease (NAFLD) is estimated to be ~25 % in the general global population, NAFLD is by now the most prevalent liver disease worldwide [1]. NAFLD comprises a wide range of disease stages ranging from the fully reversible early stages e.g., simple steatosis and steatohepatitis to late non-reversible stages like liver cirrhosis [2,3]. Genetic predisposition and general overnutrition are thought to be key risk factors in the development of NAFLD (for overview [4,5]); however, in recent years, data accumulated that alterations of intestinal microbiota composition and barrier dysfunction associated with an increased translocation of bacterial toxins may also be critical in the onset and progression of the disease [[6], [7], [8], [9]]. Indeed, interventions targeting intestinal microbiota composition and/or barrier function like a supplementation of pre- or probiotics have been suggested to alleviate NAFLD (for overview [10]). However, despite intense efforts to unravel molecular mechanisms involved and some promising results from pre-clinical and clinical studies, life-style modifications, e.g., caloric restriction diets and increased physical exercise, are still the only approved treatments of the disease [11].
Results of recent studies suggest that an oral supplementation of pharmacological doses of the non-proteogenic amino acid l-citrulline (L-Cit) may have beneficial effects on diseases of various etiologies including NAFLD [[12], [13], [14], [15], [16]]. It has been further suggested that these effects may at least partially be related to alterations of nitric oxide (NO) synthesis (for overview [17]). For example, results of our own group but also those of others suggest that an oral supplementation of L-Cit is associated with improved intestinal barrier function in rodents with a diet-induced NAFLD [15,16]. Also, results in rodent models of endotoxemia suggest that a supplementation of L-Cit and l-arginine, both restore intracellular NO production and reduce iNOS levels [18], the latter having been suggested to be critical in intestinal barrier dysfunction [19,20]. However, while showing a protection against the loss of tight junction proteins and the increases in levels of 3-nitrotyrosine protein adducts (3-NT), being indicative of an increased NO synthesis [15,21], molecular mechanisms underlying the beneficial effects of an oral L-Cit supplementation in diet-induced NAFLD have not yet been clarified. Furthermore, if a supplementation of the amino acid L-Cit also possesses therapeutic effects when the disease has already evolved, and dietary pattern is not adapted to a ‘healthy’ dietary pattern, has also not yet been determined. Therefore, the aim of the present study was to assess in a pair-feeding mouse model of dietary-induced early non-alcoholic steatohepatitis (NASH) if an oral supplementation of L-Cit abolishes the progression of a pre-existing early NASH. Furthermore, molecular mechanisms involved were determined.
All animal procedures were approved by the local Institutional Animal Care and Use Committee (IACUC) and were carried out in a specific pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Six weeks old female C57BL/6J mice (n = 8/group), shown in previous studies of our group to be more susceptible to the development of fructose-induced steatosis [22] and to develop early signs of NASH at a similar rate as male mice [23], were obtained from Janvier SAS France, and randomized to the following feeding groups: mice fed a liquid standard control diet (C) or a liquid fat-, fructose- and cholesterol-rich diet (FFC). Composition of diets used in the feeding trial are summarized in Supplementary Table S1, and was detailed previously [24,25]. Mice had free access to plain tap water at all times. The following feeding trials were performed: Feeding trial 1: Study design of the trial 1 is summarized in Supplementary Fig. S1. Animals were fed a C and pair-fed a FFC-diet for 8 weeks. In week 8, tissue and blood were collected from some of the C- and FFC-fed mice as detailed below (n = 8/group). Remaining FFC-fed mice were randomized to either receive FFC or FFC fortified with l-citrulline (L-Cit: Sigma Aldrich Chemie GmbH, Steinheim, Germany) (2.5 g/kg bw, FFC + Cit) for the remaining 5 weeks of the experiment (n = 8/group). FFC groups were pair-fed as detailed previously [24,25]. Body weight was measured weekly and L-Cit content in diet was adapted accordingly. After 5 and 11 weeks of feeding, a glucose-tolerance-test (GTT) was performed with mice as detailed previously [26]. Feeding trial 2: Study design of the trial 2 is summarized in Supplementary Fig. S2. Animals (n = 8/group) were pair-fed either C or FFC +/- L-Cit (2.5 g/kg bw) and +/- N(ω)-hydroxy-nor-l-arginine (NOHA: Bachem, Bubendorf, Switzerland) (0.01 g/kg bw in saline or only saline, i.p. 3 x weekly), respectively, for 8 weeks resulting in the following treatment groups: C, C + NOHA, C + L-Cit, C + NOHA + L-Cit, FFC, FFC + NOHA, FFC + L-Cit, FFC + NOHA + L-Cit. In week 6 of trial 2 a GTT was performed.
Using 100 mg/kg ketamine and 16 mg/kg xylazine (Richter Pharma AG, Wels, Austria) mice were anesthetized after either 8 or 13 weeks of feeding and blood from the portal vein was collected. Animals were killed by cervical dislocation and portions of liver and proximal small intestine were either snap-frozen or fixed in neutral-buffered formalin for further analysis. For the everted sac experiments, naïve female C57BL/6J mice fed standard chow were killed by cervical dislocation.
To assess liver histology, liver embedded in paraffin was cut in 4 μm tissue sections, stained with hematoxylin and eosin (H&E) (Sigma Aldrich Chemie GmbH, Steinheim, Germany) and scored using the NAFLD activity score (NAS) adapted from Kleiner et al. [27]. Using a commercially available naphthol AS-D chloroacetate esterase staining kit (91C-1KT, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) as previously detailed [28], number of neutrophil granulocytes was determined per microscopic field in liver sections using a microscope integrated camera (LeicaDM4000 B LED, Leica, Wetzlar, Germany). For each tissue section mean was determined from 8 fields (200 x). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured in a routine laboratory at the University Hospital Jena, Jena, Germany.
Concentration of nitrite in luminal content and tissue of proximal small intestine was measured using a commercially available Griess assay (G2930, Promega, Madison, Wisconsin, USA). In brief, intestinal tissue and luminal content were homogenized or washed, respectively, in ice-cold PBS, centrifuged and nitrite concentration was measured in the obtained supernatant according to manufacturer's instructions.
For determining arginase activity levels in proximal small intestine, tissue was homogenized in 10 mM Tris-HCl, pH 7.4 containing 0.4 % (w/v) Triton X-100, 1 μM pepstatin and 1 μM leupeptin A (P5318 and L9783, Sigma-Aldrich Chemie GmbH, Steinheim, Germany). After centrifuging samples at 13000×g for 10 min, arginase activity was determined in supernatant using Arginase Activity Assay Kit (MAK112, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) according to manufacturer's instructions or by a modified method of Corraliza et al. [29], as detailed previously [30]. Results were normalised to protein concentration (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, Waltham, Massachusetts, USA). In short, small intestinal tissue was homogenized in 50 mM Tris-HCl pH 7.5, 0.1 % Triton X-100, protease inhibitor cocktail, and 10 mM MnCl2. Supernatant (50 μl) was mixed with 0.5 M arginine pH 9.7 (1:1) and incubated at 37 °C for 1 h. Reaction was stopped by adding 800 μl of H2SO4:H3PO4:H2O (1:3:7) and 50 μl of 9 % α-isonitrosopropiophenone. Samples were incubated at 100 °C for 1 h, followed by a 10 min incubation at 60 °C. Urea concentration was measured using a plate reader (SpectraMax, Molecular Devices, San Jose, CA, USA) at wavelength of 540 nm.
Tumor necrosis factor alpha (TNFα) protein levels in liver tissue were measured using a commercially available ELISA (EMT2010-1, Assaypro, St. Charles, Missouri, USA) according to the instructions. Portal plasma endotoxin levels were measured as previously described [31], using a commercially available limulus amebocyte lysate assay (Charles River, Écully, France).
Total RNA from liver or proximal small intestinal tissue was isolated with Trizol (peq GOLD Trifast; Peqlab, Erlangen, Germany). Following a RNA concentration determination, a DNase digestion step was performed, and RNA was reverse transcribed (cDNA synthesis kit, Promega, Mannheim, Germany). Real-time polymerase chain reaction (RT-PCR) was performed to assess target gene expression respective to 18S as previously described in detail [32]. Primer sequences are shown in Supplementary Table S2.
For staining of 4-hydroxynonenal protein adducts (4-HNE) or F4/80 positive cells in liver tissue the following polyclonal antibodies were used (4-HNE: H-1110-100, AG Scientific, San Diego, California, USA; F4/80: ab6640, Abcam, Cambridge, Massachusetts, USA) as previously described [32,33]. To measure 3-nitrotyrosine protein adducts (3-NT) and occludin as well as zonula occludens 1 (ZO-1) protein in paraffin-embedded proximal small intestinal tissue section the polyclonal antibodies were used (3-NT: sc-32757, Santa Cruz Biotechnology, Santa Cruz, California, USA; occludin: 71–500, Invitrogen, Waltham, Massachusetts, USA; ZO-1: 617300, Thermo Fisher Scientific, Waltham, Massachusetts, USA) as described previously [32]. For antigen retrieval, tissue sections were treated with citrate buffer (3-NT) or protease (occludin and ZO-1). Peroxidase linked secondary antibody and diaminobenzidine were used (Peroxidase Envision Kit; DAKO, Hamburg, Germany) to detect specific primary antibody. Evaluation of 4-HNE, 3-NT, occludin and ZO-1 was performed using a software integrated in the microscope (Leica ApplicationSuite, Leica, Wetzlar, Germany). In brief, the intensity of staining in tissue sections was defined as percent of the tissue field area within the default color range determined by the software. For determining mean staining intensity of each tissue section, data from 8 fields were used. Representative photomicrographs were taken with 200 x and 400 x magnification (Leica DM4000 B LED, Leica, Wetzlar, Germany).
Whole protein extracts were isolated from small intestinal tissue and separated in a SDS-PAGE as detailed previously [28]. After transferring proteins to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, California, USA), and incubating them with primary antibodies (Arginase 1: 93668, Arginase 2: 55003, and β-actin: 4970, Cell Signaling Technology, Massachusetts, USA) overnight, ensued by an incubation with the corresponding HRP-linked secondary antibody. The bands were detected using Super Signal West Dura kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA) [28] and analyzed densitometrically using ChemiDoc XRS System with Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA).
Samples were collected from small intestine of mice, snap frozen in liquid nitrogen and stored at -80 °C until use. Whole genomic bacterial DNA was isolated from the samples using FastDNA™ Spin kit for soil following the manufacturer's instructions (MP Biomedicals, Solon, Ohio). The extracted DNA was used for microbiota profiling with barcoded amplicons of the V1–V2 region of 16S rRNA genes as described previously [34]. Briefly, two PCRs were conducted, in the first PCR a 20 μl reaction containing PrimeSTAR Hot Start DNA polymerase (2.5 U, Clontech Laboratories, Mountain View, California, USA), 2.5 mM dNTP mixture, 0.2 μM primers and 1 μl of template DNA was prepared; an initial denaturation at 95 °C for 3 min was followed by 20 cycles of denaturation at 98 °C for 10 s, subsequent annealing at 59 °C for 10 s, extension step at 72 °C for 45 s and a final extension for 2 min at 72 °C; 1 μl from the resultant product was taken to perform the second PCR with the previous conditions in a 50 μl reaction for 15 cycles. Amplification was confirmed on a 2 % agarose electrophoresis gel. PCR products were normalized and purified with the SequalPrep Normalization Plate Kit (Invitrogen, Waltham, Massachusetts, USA). Amplicon pools were 250 bp pair-end sequenced with Illumina MiSeq platform (Illumina, San Diego, California, USA). A total of 28.393 ± 5.467 sequence reads per sample were processed following the Mothur SOP [35]. The final sequence reads were clustered into operational taxonomic units (OTUs) at > 97 % similarity. Singletons and OTUs with sequence length < 250 bp were removed from the data set. A total of 772 OTUs were taxonomically assigned using the naïve Bayesian RDP classifier and manually compared with RDP database using Seqmatch function. Sequences were submitted to European Nucleotide Archive under the accession number PRJEB39588.
Naïve C57BL/6J female mice (n = 7/treatment) were killed by cervical dislocation, small intestine was rapidly removed and everted with a rod as described by others [36]. Small intestinal tissue was cut into equal length segments. Each sac was filled with 1 x Krebs-Henseleit-bicarbonate-buffer containing 0.2 % (w/v) bovine serum albumin (KRH buffer). After being incubated in gassed KRH buffer (95 % O2/5 % CO2) supplemented with 0.4 mM L-Cit for 10 min at 37 °C, sacs were further incubated for 1 h at 37 °C in gassed KRH buffer +/- 5 mM fructose in the presence or absence of 0.4 mM L-Cit. Tissue permeability was assessed by exposing tissue sacs to 0.1 % (w/v) d-xylose (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) in the incubation solutions for 5 min prior to termination of the incubation. Doses of L-Cit used in these experiments were determined in pilot studies (n = 4 naïve female C57BL/6J mice) (see Supplementary Fig. S3). In these experiments, it was shown that 0.04 and 0.4 mM L-Cit markedly attenuated the fructose-induced permeation of d-xylose and arginase activity, while higher and lower doses of L-Cit had no or even adverse effects. Based on the doses used in the in vivo experiments and the results of these pilot experiments, 0.4 mM L-Cit was selected for all further experiments in the everted sac model. Intestinal tissue from each sac was snap frozen for further analysis. Measurement of xylose concentration was performed using a commercially available kit (d-Xylose Assay Kit, Megazyme, Bray, Wicklow, Ireland).
All values are shown as mean ± SEM. Using Grubb's test outliers were identified. p ≤ 0.05 was determined to be significant and statistical differences between groups were determined using unpaired student t-test, one-way ANOVA, or two-way ANOVA where appropriate (Graph Pad Prism Version 7.0, La Jolla, USA). In case of inhomogeneity of variances data were log-transformed. For the analysis of Illumina amplicon sequencing data PRIMER was used (v.6.1.16, PRIMER-E; Plymouth Marine Laboratory, Plymouth, UK). In brief, samples were standardized by total, square root transformed and a similarity matrix created using the Bray-Curtis coefficient [37] and plotted in a non-metric multidimensional scaling (nMDS) plot. Statistical differences between dietary treatments were evaluated by permutational multivariate analysis of variance (PERMANOVA) and p ≤ 0.05 was considered to be significantly different.
Despite similar caloric intake and body weight gain, FFC-fed mice developed marked signs of NAFLD within the first 8 weeks of feeding (Table 1, Fig. 1). Indeed, NAS, numbers of neutrophil granulocytes and absolute liver weight as well as liver to body weight ratio were all significantly higher in FFC-fed mice when compared to control diet (C) fed animals (all parameters p < 0.05) (Fig. 1, Table 1). Number of F4/80 positive cells in livers of FFC-fed mice were by trend higher, too, than in livers of C-fed mice (p = 0.0551) whereas neither ALT nor AST activity differed between groups. After 13 weeks, these signs of NAFLD had slightly progressed in FFC-fed mice with more hepatocytes showing macrovesicular fat accumulation and inflammatory foci as well as higher numbers of neutrophil granulocytes and F4/80 positive cells in liver tissue than after 8 weeks of feeding. In contrast and despite similar weight gain and caloric intake, NAS, numbers of neutrophil granulocytes and F4/80 positive cells as well as ALT and AST activity in plasma were significantly lower in FFC + L-Cit-fed animals when compared to FFC-fed mice (Fig. 1, Table 1). As steatosis was only by ~34 % lower in livers of FFC + L-Cit-fed mice when compared to FFC-fed animals, absolute liver weight and liver to body weight ratio did not differ between groups (Table 1). In line with these findings, levels of TNFα protein and 4-HNE protein adducts were also significantly lower in livers of FFC + L-Cit-fed mice when compared to FFC-fed animals (Fig. 2, Supplementary Fig. S4). In contrast, as data varied considerably, neither fasting glucose nor area under the curve (AUC) of glucose-tolerance-test differed between groups after 5 or 11 weeks of feeding (Table 1).

| Diet groups | |||||
|---|---|---|---|---|---|
| 8 weeks | 13 weeks | ||||
| C | FFC | C | FFC | FFC + L-Cit | |
| Caloric intake (kcal/g bw/d) | 0.45 ± 0.01 | 0.48 ± 0.01 | 0.46 ± 0.01 | 0.44 ± 0.01 | 0.44 ± 0.01 |
| Absolute body weight gain (g) | 3.4 ± 0.2 | 3.9 ± 0.5 | 4.8 ± 0.5 | 4.4 ± 0.3 | 4.4 ± 0.3 |
| Absolute body weight (g) | 21.9 ± 0.5 | 22.3 ± 0.5 | 23.4 ± 1.0 | 23.4 ± 0.3 | 23.2 ± 0.4 |
| Liver weight (g) | 1.1 ± 0.04 | 1.5 ± 0.03 * | 1.1 ± 0.05 | 1.6 ± 0.04 | 1.5 ± 0.05 |
| Liver/body weight ratio (%) | 5.0 ± 0.1 | 6.7 ± 0.1 * | 4.7 ± 0.1 | 6.8 ± 0.1 | 6.7 ± 0.2 |
| NAS Steatosis | 0.2 ± 0.1 | 2.4 ± 0.3 * | 0.3 ± 0.1 | 2.9 ± 0.1 * | 2.0 ± 0.0 |
| NAS Inflammation | 0.1 ± 0.1 | 0.4 ± 0.1 * | 0.1 ± 0.1 | 0.8 ± 0.1 * | 0.3 ± 0.1 |
| ALT (U/L) | 21.0 ± 2.0 | 46.0 ± 11.9 | 23.1 ± 4.6 | 33.5 ± 2.6 * | 25.5 ± 1.5 |
| AST (U/L) | 41.7 ± 2.4 | 73.7 ± 17.2 | 51.9 ± 6.0 | 58.6 ± 2.5 * | 49.7 ± 3.1 |
| Fasting blood glucose (mg/dL) | 135 ± 9 | 157 ± 8 | 144 ± 7 | 148 ± 5 | 152 ± 6 |
| GTT, AUC (0–120 min) | 31931 ± 2590 | 39996 ± 4312 | 32512 ± 640 | 33352 ± 1503 | 36367 ± 4917 |
a Values are mean ± SEM, n = 7–8. Unpaired t-test was used to compare C and FFC group after 8 or FFC and FFC+L-Cit after 13 weeks of feeding (*, p ≤ 0.05). Liver histology was evaluated using NAFLD activity score (NAS) adapted from Kleiner et al. [27]. AUC, area under the curve; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; GTT, glucose-tolerance-test; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.

![Effect of L-Cit supplementation on indices of liver damage in female mice with FFC-induced NASH. (A) Representative photomicrographs of hematoxylin and eosin (H&E) stained liver sections (200 x, 400 x), (B) evaluation of liver histology using NAFLD activity score (NAS) adapted from Kleiner et al. [27], and number of (C) neutrophil granulocytes, as well as (D) F4/80 positive cells in liver sections. Data are presented as mean ± SEM, n = 7–8. Unpaired t-test was used to compare C and FFC group after 8 or FFC and FFC + L-Cit after 13 weeks of feeding, *p ≤ 0.05. C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.](/dataresources/secured/content-1765862507748-adc5b536-9ead-41f3-b382-0fae7fed3df8/assets/gr1.jpg)
Effect of L-Cit supplementation on indices of liver damage in female mice with FFC-induced NASH. (A) Representative photomicrographs of hematoxylin and eosin (H&E) stained liver sections (200 x, 400 x), (B) evaluation of liver histology using NAFLD activity score (NAS) adapted from Kleiner et al. [27], and number of (C) neutrophil granulocytes, as well as (D) F4/80 positive cells in liver sections. Data are presented as mean ± SEM, n = 7–8. Unpaired t-test was used to compare C and FFC group after 8 or FFC and FFC + L-Cit after 13 weeks of feeding, *p ≤ 0.05. C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.

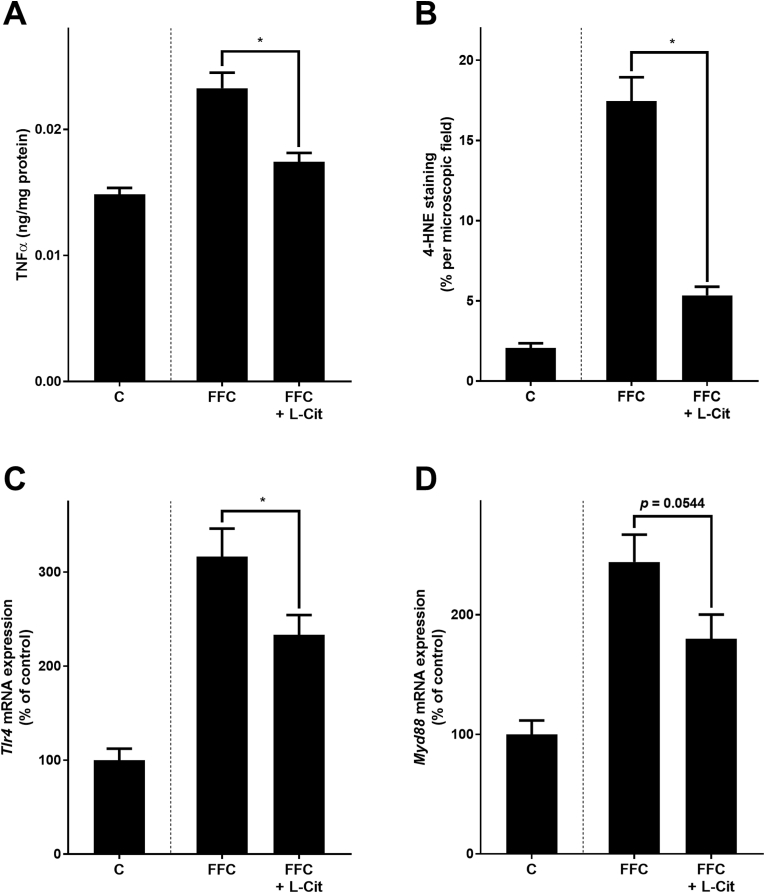
Effect of L-Cit supplementation on markers of inflammation and lipid oxidation, as well as on Tlr4-dependent signaling pathways in livers of female mice with FFC-induced NASH. (A) TNFα levels in hepatic tissue, (B) quantification of 4-HNE protein adducts staining of liver sections, and mRNA expression of (C) Tlr4 and (D) Myd88 in hepatic tissue. Data are presented as mean ± SEM, n = 8. Unpaired t-test was used to compare FFC and FFC + L-Cit after 13 weeks of feeding, *p ≤ 0.05. 4-HNE, 4-hydroxynonenal protein adducts; C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; Myd88, myeloid differentiation primary response 88; NASH, non-alcoholic steatohepatitis; Tlr4, toll-like receptor 4; TNFα, tumor necrosis factor alpha.
In line with previous findings of our group [15], Tlr4 and myeloid differentiation primary response 88 (Myd88) mRNA expressions in liver tissue and bacterial endotoxin levels in portal plasma were lower in FFC + L-Cit-fed mice than in FFC-fed animals (Tlr4: p < 0.05; Myd88: p = 0.0544; endotoxin: p < 0.05) (Fig. 2, Fig. 3). Furthermore, protein level of the tight junction proteins occludin and ZO-1 were significantly higher in proximal small intestine of FFC + L-Cit-fed mice than that of FFC-fed animals (Fig. 3, Supplementary Fig. S5). To determine if protective effects of L-Cit on the progression of NAFLD were associated with changes in intestinal microbiota community structure, 16S rRNA gene sequencing was performed. Despite a statistical difference observed between all groups (p = 0.04), pairwise comparisons did not show differences between community structure of microbiota in proximal small intestine of the C- and either of the FFC-fed groups nor between FFC-fed groups (Fig. 3). In line with these findings, mean abundance of bacterial strains was similar between FFC- and FFC + L-Cit-fed groups (Fig. 3 and Supplementary Table S3). Furthermore, neither mRNA expression of G-protein-coupled receptor 41 and 43 (Gpr41, Gpr43), proposed to be activated by short-chain fatty acids and to mediate their immune-modulating effects [38], nor levels of nitrite in luminal content derived from proximal small intestine differed between FFC-fed groups (Table 2 and Fig. 4A). Still, in line with previous findings of our group [15], nitrite and 3-nitrotyrosine (3-NT) protein adduct concentration in proximal small intestinal tissue were both significantly lower in FFC + L-Cit-fed animals when compared to FFC-fed animals, being almost at the level of controls (Fig. 4, Supplementary Fig. S4). Additionally, arginase activity, shown to be the opponent of inducible nitric oxide synthase (iNOS) but also in recent years discussed to be critical in the development of inflammatory bowel diseases [39,40], was significantly lower in proximal small intestine of FFC-fed mice when compared to FFC + L-Cit-fed animals (Fig. 4). Again, levels determined in FFC + L-Cit-fed mice were close to those of controls (Fig. 4). Somewhat surprisingly, neither mRNA nor protein levels of arginase 2 differed between groups. In line with the findings of others in humans and animals [41,42] arginase 1 protein was not detectable in proximal small intestine (Supplementary Fig. S6).

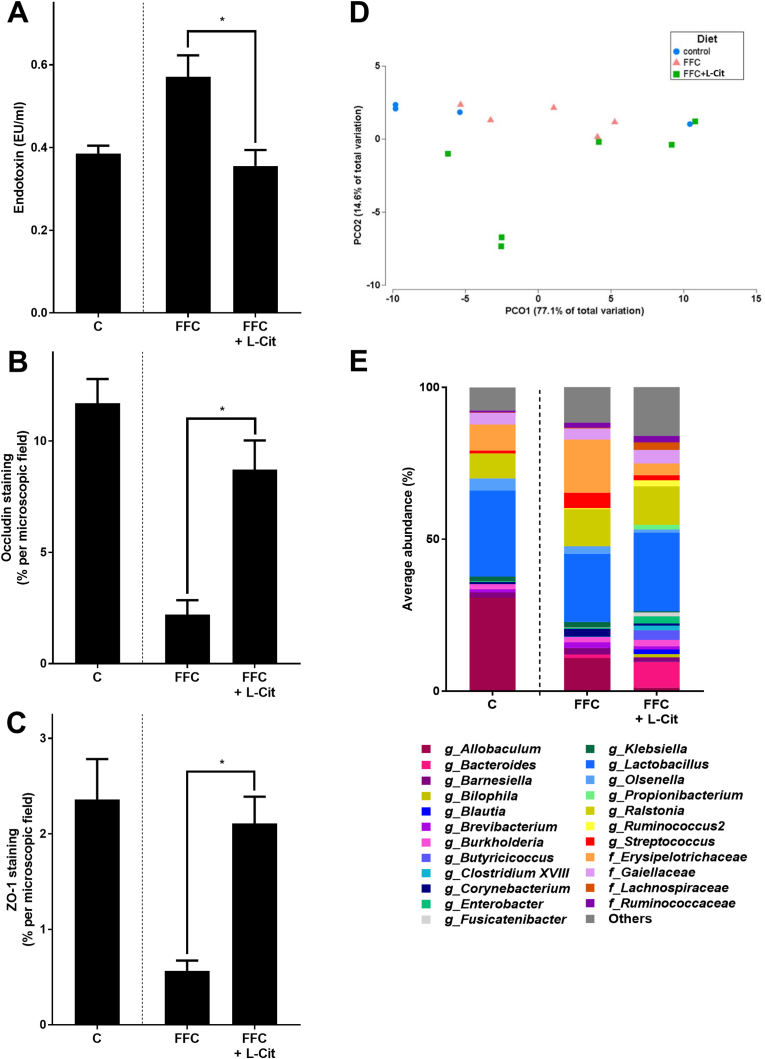
Effect of L-Cit supplementation on intestinal barrier function in female mice with FFC-induced NASH. (A) Bacterial endotoxin levels in portal plasma, densitometric analysis of (B) occludin and (C) ZO-1 staining in proximal small intestine. (D) Non-metric multidimensional scaling (nMDS) showing the bacterial communities in proximal small intestine where each point represents one sample, and (E) average relative abundance of genera in proximal small intestine. Data are presented as mean ± SEM, n = 8, except for microbiota analysis where n = 4–6 were analyzed. Unpaired t-test was used to compare FFC and FFC + L-Cit after 13 weeks of feeding, *p ≤ 0.05. C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NASH, non-alcoholic steatohepatitis; ZO-1, zonula occludens 1.

| Diet groups | |||
|---|---|---|---|
| C | FFC | FFC + L-Cit | |
| Gpr41 mRNA expression (% of control) | 100 ± 14.2 | 88.7 ± 10.8 | 78.6 ± 12.7 |
| Gpr43 mRNA expression (% of control) | 100 ± 20.0 | 89.4 ± 16.1 | 83.7 ± 17.8 |
a Values are mean ± SEM, n = 7–8. Unpaired t-test was used to compare FFC and FFC + L-Cit after 13 weeks of feeding (*, p ≤ 0.05). C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich-diet; Gpr41, G-protein-coupled receptor 41; Gpr43, G-protein-coupled receptor 43; NASH, non-alcoholic steatohepatitis.

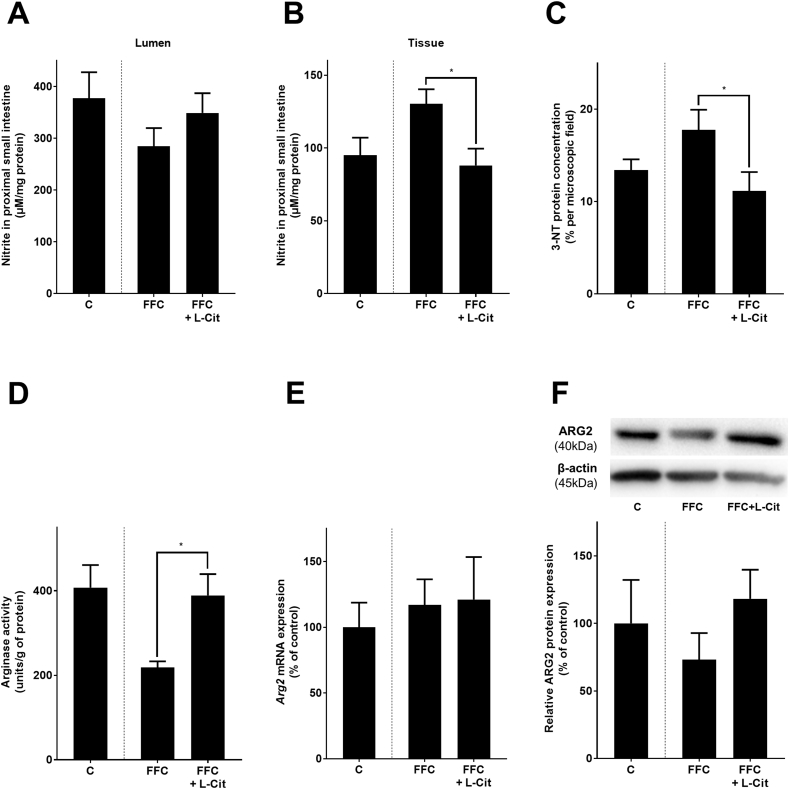
Effect of L-Cit supplementation on NO synthesis and arginase activity in proximal small intestine of female mice with FFC-induced NASH. Levels of nitrite in (A) lumen content, and (B) tissue obtained from proximal small intestine, (C) densitometric analysis of 3-NT staining in proximal small intestine, (D) arginase activity in proximal small intestine, (E) mRNA expression of Arg2 in proximal small intestine, (F) relative expression of ARG2 protein in proximal small intestine with representative western blot. Data are presented as mean ± SEM, n = 8, except for nitrite in lumen content n = 5. Unpaired t-test was used to compare FFC and FFC + L-Cit after 13 weeks of feeding, *p ≤ 0.05. 3-NT, 3-nitrotyrosine; Arg2, arginase 2; C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NASH, non-alcoholic steatohepatitis.
To assess if an alteration of arginase activity is critical in the development of intestinal permeability in settings of diet-induced NAFLD in mice and if L-Cit might exert its effects on intestinal barrier function and subsequently NAFLD through modulating arginase activity, mice were concomitantly treated with the arginase inhibitor NOHA while being fed an FFC- or an FFC-diet supplemented with L-Cit. As no differences regarding markers of liver damage between C-fed and C + NOHA-, C + L-Cit- and C + NOHA + L-Cit-fed mice were found, data from C-fed mice are shown to represent all four control groups. As expected, after 8 weeks of feeding, FFC-fed mice developed marked macrovesicular steatosis with beginning inflammation. In line with the above reported therapeutic effects of an oral L-Cit supplementation and earlier findings of our group [15], L-Cit markedly attenuated the development of NAFLD with NAS being significantly lower than in all other FFC-fed groups. In FFC + L-Cit-fed mice concomitantly treated with NOHA (FFC + NOHA + L-Cit), these protective effects of the oral L-Cit supplementation were almost completely abolished with NAS being similar to those determined in livers of FFC- and FFC + NOHA-fed animals. However, as all FFC-fed mice regardless of additional treatments showed signs of steatosis and very early inflammation, neither ALT nor AST activity in plasma nor liver weight or liver to body weight ratio differed between groups. Also, neither fasting levels nor AUC of GTT differed between FFC-fed groups. Still, bacterial endotoxin levels in portal vein were similarly elevated in FFC- and FFC + NOHA-fed mice, whereas in FFC + L-Cit-fed animals bacterial endotoxin levels in portal plasma were significantly lower than in FFC-fed mice. This effect of L-Cit was attenuated in FFC + NOHA + L-Cit-fed mice (Fig. 5, Table 3).

![Effect of L-Cit supplementation on markers of intestinal permeability and arginase activity in proximal small intestine in female mice with FFC-induced NASH in the presence of arginase inhibitor NOHA and in ex vivo experiments. (A) Representative photomicrographs of hematoxylin and eosin (H&E) stained liver sections (200 x), (B) evaluation of liver histology using NAFLD activity score (NAS) adapted from Kleiner et al. [27], (C) endotoxin in portal plasma, (D) arginase activity, and (E) xylose permeation in everted sacs of small intestine. Data are presented as mean ± SEM, n = 7–8, except for xylose permeation n = 4. Two-way ANOVA was used to compare FFC-fed groups after 8 weeks of feeding ap ≤ 0.05 compared to FFC-fed mice, bp ≤ 0.05 compared to FFC + NOHA-fed mice, dp ≤ 0.05 compared to FFC + NOHA + L-Cit-fed mice. One-way ANOVA was used to compare groups of ex vivo experiments, *p ≤ 0.05. L-CitE, l-citrulline supplementation effect; TE, treatment with NOHA effect; TExL-CitE, interaction between treatment with NOHA and l-citrulline supplementation. C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; NOHA, N(ω)-hydroxy-nor-l-arginine; NS, not significant.](/dataresources/secured/content-1765862507748-adc5b536-9ead-41f3-b382-0fae7fed3df8/assets/gr5.jpg)
Effect of L-Cit supplementation on markers of intestinal permeability and arginase activity in proximal small intestine in female mice with FFC-induced NASH in the presence of arginase inhibitor NOHA and in ex vivo experiments. (A) Representative photomicrographs of hematoxylin and eosin (H&E) stained liver sections (200 x), (B) evaluation of liver histology using NAFLD activity score (NAS) adapted from Kleiner et al. [27], (C) endotoxin in portal plasma, (D) arginase activity, and (E) xylose permeation in everted sacs of small intestine. Data are presented as mean ± SEM, n = 7–8, except for xylose permeation n = 4. Two-way ANOVA was used to compare FFC-fed groups after 8 weeks of feeding ap ≤ 0.05 compared to FFC-fed mice, bp ≤ 0.05 compared to FFC + NOHA-fed mice, dp ≤ 0.05 compared to FFC + NOHA + L-Cit-fed mice. One-way ANOVA was used to compare groups of ex vivo experiments, *p ≤ 0.05. L-CitE, l-citrulline supplementation effect; TE, treatment with NOHA effect; TExL-CitE, interaction between treatment with NOHA and l-citrulline supplementation. C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; NOHA, N(ω)-hydroxy-nor-l-arginine; NS, not significant.

| Diet groups | Two-way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|
| C | FFC | FFC +NOHA | FFC +L-Cit | FFC +NOHA + L-Cit | TE | L-CitE | TEx L-CitE | |
| Caloric intake (kcal/g bw/d) | 0.40 ± 0.01 | 0.45 ± 0.01 | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.44 ± 0.01 | NS | <0.05 | NS |
| Absolute body weight gain (g) | 3.8 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.7 | 3.3 ± 0.4 | 3.2 ± 0.5 | NS | NS | NS |
| Absolute body weight (g) | 21.5 ± 0.5 | 21.6 ± 0.3 | 21.4 ± 0.3 | 21.5 ± 0.2 | 22.2 ± 0.7 | NS | NS | NS |
| Liver weight (g) | 1.04 ± 0.03 | 1.48 ± 0.03 | 1.37 ± 0.07 | 1.34 ± 0.03 | 1.25 ± 0.07a | NS | <0.05 | NS |
| Liver/body weight ratio (%) | 4.8 ± 0.1 | 6.8 ± 0.1 | 6.4 ± 0.3 | 6.1 ± 0.1 | 5.7 ± 0.2a | NS | <0.05 | NS |
| NAS Steatosis | 0.4 ± 0.1 | 2.5 ± 0.2 | 2.7 ± 0.1 | 2.1 ± 0.3b | 2.5 ± 0.1 | NS | NS | NS |
| NAS Inflammation | 0.02 ± 0.02 | 0.94 ± 0.15 | 1.37 ± 0.18 | 0.12 ± 0.08a,b,d | 1.17 ± 0.10 | <0.05 | <0.05 | NS |
| ALT (U/L) | 15.4 ± 0.7 | 32.1 ± 4.0 | 40.0 ± 3.5 | 28.5 ± 3.7 | 27.0 ± 4.6 | NS | <0.05 | NS |
| AST (U/L) | 36.0 ± 2.0 | 60.9 ± 5.0 | 74.5 ± 5.2 | 53.4 ± 6.2 | 56.4 ± 8.3 | NS | NS | NS |
| Fasting blood glucose (mg/dL) | 128 ± 6 | 123 ± 4 | 116 ± 10 | 106 ± 4 | 119 ± 5 | NS | NS | NS |
| GTT, AUC (0–120 min) | 25107 ± 1958 | 28415 ± 1478 | 26952 ± 2803 | 25779 ± 1521 | 27305 ± 1101 | NS | NS | NS |
a Values are mean ± SEM, n = 8. Two Way ANOVA test was used to compare FFC-fed groups after 8 weeks of feeding (a p ≤ 0.05 compared to FFC-fed mice, b p ≤ 0.05 compared to FFC + NOHA-fed mice, d p ≤ 0.05 compared to FFC + NOHA + L-Cit-fed mice). Liver histology was evaluated using NAFLD activity score (NAS) adapted from Kleiner et al. [27]. L-CitE, l-citrulline supplementation effect; TE, treatment with NOHA effect; TExL-CitE, interaction between treatment with NOHA and l-citrulline supplementation. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve; C, control diet; L-Cit, l-citrulline; FFC, fat-, fructose- and cholesterol-rich diet; GTT, glucose-tolerance-test; NAS – NAFLD activity score; NASH, non-alcoholic steatohepatitis; NOHA, N(ω)-hydroxy-nor-l-arginine; NS, not significant.
To further determine if fructose present in the diet was critical in mediating the effects on arginase activity and subsequently on intestinal permeability and if L-Cit alters arginase activity and permeability in small intestinal tissue, studies using an ex vivo everted sac model of small intestinal tissue were used. Already an incubation of everted sacs prepared from small intestinal tissue of naïve mice with 5 mM fructose for 1 h resulted in a significant reduction of arginase activity of ~30 % and a significant increase of tissue permeability of ~100 %, the latter being assessed using xylose permeation assay (arginase activity: C vs. F p < 0.05, permeability, C vs. F p < 0.05). This drop in arginase activity and increase in permeability was almost completely attenuated when fructose-challenged everted sacs of small intestinal tissue were concomitantly incubated with 0.4 mM L-Cit (arginase activity: p < 0.05 for F vs. F + L-Cit; xylose concentration: p = 0.0533 for F vs. F + L-Cit) (Fig. 5).
While NAFLD is by now the most prevalent liver disease worldwide, therapeutic options are still rather limited and mainly focusing on life-style interventions [11] shown to be frequently afflicted with low adherence and high relapse rates [43,44]. In the present study, we furthered previous studies of us and others in which it was shown that the concomitant supplementation of L-Cit while inducing NAFLD diminished the disease development [15,16]. Indeed, here it was shown that L-Cit attenuated the progression of a diet-induced pre-existing NAFLD, even when the intake of the NAFLD-inducing diet was continued. And while steatosis was still present, FFC-fed mice receiving pharmacological doses of L-Cit for the last 5 weeks of the trial had less macrovesicular fat accumulation in the liver and almost no inflammatory alterations. Furthermore, number of neutrophil granulocytes and F4/80 positive cells as well as protein levels of TNFα and concentration of 4-HNE protein adducts were almost at the level of controls. However, while we found clear beneficial effects on liver, glucose tolerance was not improved in FFC + L-Cit-fed mice. These findings are also in line with those of others showing that L-Cit alone had only limited effects on glucose tolerance and fasting glucose levels in rats with diet-induced obesity. Still, in the same study it was also shown that when combined with atorvastatin, L-Cit may even have additive effects on glucose homeostasis in overweight rats [45].
Taken together, results of the present study further bolster the hypothesis, that supplementing L-Cit orally at pharmacological doses may attenuate the development and progression of NAFLD in rodent models of the disease while having no or only very limited effect on insulin resistance. However, if similar effects are also found when NAFLD has progressed to even later stage of the disease, e.g., later stages of NASH and NASH with beginning fibrosis, respectively, remains to be determined. Also, it needs to be determined if lower doses of the amino acids would also be sufficient to attenuate the development and progression of the disease. Indeed, doses of L-Cit used in the present study (2.5 g/kg bw) cannot be achieved by ‘normal’ nutrition and it cannot be ruled out that systemic effects may occur, especially when intake is prolonged. Furthermore, if humans with NAFLD would also benefit from a L-Cit treatment remains to be determined as well as doses needed to achieve beneficial effects.
Results of several studies suggest that changes of intestinal microbiota composition and intestinal barrier dysfunction associated with an increased translocation of bacterial endotoxin but also other bacterial toxins and viruses are critical contributors in the onset and progression of NAFLD [46]. Indeed, it has been shown in human and animal studies that not only expression of TLR4 but also other TLRs are induced in liver in patients and animals with NAFLD [9,47] and that a genetic deletion of TLR4 as well as TLR2 and TLR9 may attenuate the development of NAFLD in mice [[48], [49], [50]]. However, molecular mechanisms involved in intestinal barrier dysfunction in settings of NAFLD are yet not fully understood. In line with previous findings of our group and others [15,16], in the present study, we show that an oral supplementation of pharmacological doses of L-Cit restores intestinal barrier function, thereby also leading to a normalization of bacterial endotoxin levels in portal plasma and of Tlr4 and Myd88 expression in liver tissue. As it has been shown by others that bacteria also possess enzymes involved in the citrulline NO cycle, e.g. argininosuccinate synthase [[51], [52], [53]] and may add to the formation and bioavailability of NO in the gut [54,55], we determined intestinal microbiota community composition and levels of nitrite in lumen of proximal small intestine. Contrasting the findings of others in fecal samples of rodents with diet-induced NAFLD and NAFLD patients [56,57], composition of intestinal microbiota in small intestine was not markedly different between C- and both FFC-fed groups. Also, even after 5 weeks of receiving an oral L-Cit supplementation with their diet, neither intestinal microbial community structure nor nitrite in luminal content obtained from small intestine differed significantly between FFC- and FFC + L-Cit-fed mice. Furthermore, expressions of Gpr41 and Gpr43, suggested to be activated through short chain fatty acids and to thereby modulate intestinal immune response and barrier function [38,58], were also similar between FFC-fed groups further suggesting that other factors might have been involved in the beneficial effect of L-Cit (see below). Still, with the methods used in the present study to assess microbiota composition in small intestine e.g., 16S rRNA sequencing as well as the experimental setup, it cannot be ruled out that intestinal microbiota and/or metabolites derived from microbial metabolism may have contributed to the beneficial effects found for the supplementation of L-Cit on the development NAFLD, too.
An increased production of nitrite in intestinal tissue and formation of 3-NT protein adducts have been suggested before to be critical in intestinal barrier dysfunction [59]. In the present study, the elevation of nitrite and 3-NT protein adduct levels found in small intestine of FFC-fed mice were almost completely attenuated in small intestine of FFC + L-Cit-fed mice. In line with these findings, a supplementation of L-Cit has been shown to normalize iNOS-dependent NO synthesis in other organs, too [60]. The decrease in nitrite and 3-NT protein adduct levels found in FFC + L-Cit-fed mice was associated with an attenuation of the loss of arginase activity in small intestinal tissue. Arginase has been shown before to be the counter regulator of nitric oxide synthases, including also iNOS, thereby also regulating the bioavailability of NO [61]. Also, in recent years, results obtained in humans with inflammatory bowel disease and of rodent models of inflammatory bowel diseases suggest that alterations of intestinal arginase and herein especially arginase 1 found in macrophages and endothelial cells [62], may be critical in the development of inflammatory alterations in these diseases [40,63]. In the present study, contrasting these findings of others, protein levels of arginase 1 were below the level of detection. Differences between our findings and that of others might have resulted from differences in models e.g., NAFLD vs. inflammatory bowel disease and the lack of any overt inflammatory alterations in small intestine in the present study. Also, neither mRNA nor protein expression of arginase 2, found to be an arginase isoenzyme expressed in enterocytes [41,42], differed between FFC groups. In support that the beneficial effects of L-Cit on intestinal permeability may depend upon its regulatory effects on arginase activity, the loss of activity of the enzyme and increases in permeability induced by the presence of fructose in everted sacs of small intestinal tissue was almost completely abolished when L-Cit was present at doses as low as 0.4 mM. Indeed, in the present study we also found that these effects were dose-dependent with lower doses of the amino acid showing no or limited effects on both arginase activity and permeability and higher doses showing adverse effects on permeability while further increasing arginase activity (see Supplementary Fig. S3). Also, in vivo, the beneficial effects of L-Cit on liver histology and portal endotoxin levels were almost completely abolished when FFC + L-Cit-fed mice were concomitantly treated with the arginase inhibitor NOHA. Interestingly, neither mRNA nor protein expression of arginase 2 differed between FFC-groups; however, it has been shown before by others that L-Cit can act as a regulator of arginase activity [64].
Results of others also support the hypothesis that arginase activity and the related changes in NO bioavailability may be critical in the regulation of intestinal barrier function. For instance, Horowitz et al. showed that in patients with ulcerative colitis or Crohn's disease, urea production was increased while NO levels were lower suggesting that arginase activity was altered in these patients [63]. In this study but also other studies the effects of the changes in arginase activity - be this achieved through injection of arginase or the supplementation of L-Cit - were linked to changes in microcirculation [65]. Indeed, changes in microcirculation in small intestinal tissue have repeatedly been linked to the development of impairments of intestinal barrier function [66,67]. Also, it has been shown in other tissues, that arginase activity may be involved in the regulation of permeability or tissue leakage, too [68]. Indeed, studies have shown that arginase may modulate endothelial function [69,70]. Somewhat contrasting the findings of the present study, Akazawa et al. showed that inhibition of arginase with NOHA improved dextran sulfate sodium-induced colitis in mice [71]; however, as already discussed above, differences might have resulted from differences in disease etiology e.g., overt inflammation vs. no inflammatory alterations. This suggests that there might be a threshold of an ‘optimal’ arginase activity in intestinal tissue. However, this will need to be determined in future studies.
Taken together, results of our study suggest that a loss of arginase activity in small intestinal tissue, probably induced by the presence of fructose in the diet, may be critical in the development of intestinal barrier dysfunction. Our data also suggest that an oral supplementation of L-Cit can modulate intestinal arginase activity and that this is associated with an improvement of intestinal barrier function in setting of diet-induced NAFLD in mice. If similar effects of L-Cit are also found in humans as well as molecular mechanisms involved need to be determined in future studies. Also, due to the experimental setup and methods used, it cannot be ruled out that other factors such as microbial metabolites or other organs might have added to the effects found in arginase activity. Furthermore, if L-Cit affects arginase activity through direct effects or through its conversion to arginine or metabolites derived through the metabolism of arginine/citrulline remains to be determined, too.
In summary, our data suggest that an oral supplementation of L-Cit at pharmacological doses prevents the progression of NAFLD in mice with a pre-existing NASH through mechanisms involving a protection against intestinal barrier dysfunction in small intestine. Our results further suggest that these effects of supplementing L-Cit on intestinal barrier function are related to a protection against the increased NO synthesis and loss of arginase activity induced by dietary fructose. However, exact molecular mechanisms underlying the L-Cit-dependent regulation of arginase activity e.g., if the effects found are related to a conversion of L-Cit to l-arginine or to metabolites derived from a metabolism of the amino acid by intestinal microbiota or other organs remain to be determined in further studies. Also, further studies are needed to clarify how arginase is involved in the regulation of intestinal barrier function. Future studies will also have to assess if similar beneficial effects of an oral L-Cit supplementation are also found in patients with NAFLD as well as doses necessary. Indeed, it needs to be determined if lower doses of L-Cit may have similarly beneficial effects and/or if doses used in the present study may also exert systemic effects especially when applied over an extended period of time.
Funded by grants from the
IB designed research; DR, ABa, AHA, ABr, AN, CJJ, VS, and FJ conducted research; DR, ABa, AHA, ABr, AN, VS, and ACS analyzed data; DR and IB wrote the paper; and IB had primary responsibility for final content. All authors have read and approved the final manuscript.
Rajcic, Baumann, Hernández-Arriaga, Brandt, Nier, Jin, Sánchez, Jung, and Camarinha-Silva, declare to have no conflicts of interest. Bergheim received funding from Yakult Ltd. for an unrelated research project. The authors have no additional financial interests.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
The authors would like to thank Cathrin Sellmann for her support with carrying out the animal experiments and some of the analysis. Graphical abstract was created with BioRender.com. Open access funding provided by the University of Vienna.