
NMT and RZA contributed equally to this study.
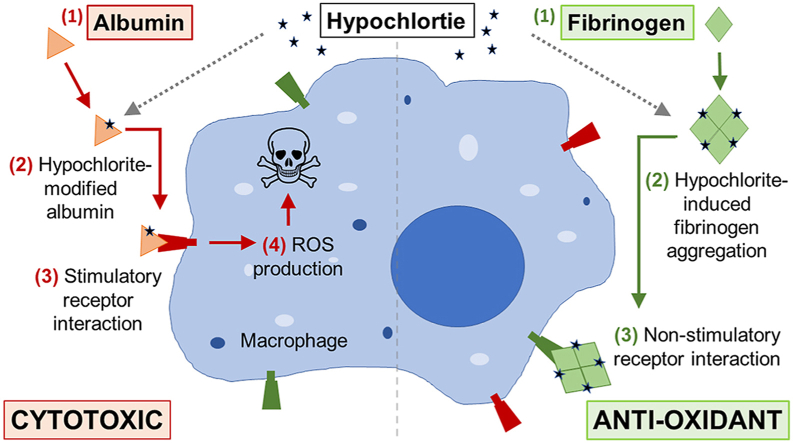
Fibrinogen, a major constituent of blood plasma, is highly susceptible to reaction with biological oxidants. It has been proposed that fibrinogen plays a role in antioxidant defence, but oxidation of fibrinogen is also known to disrupt normal blood clotting and is implicated in the pathology of atherosclerosis. In the present study, we show that the biological oxidant hypochlorite promotes the formation of soluble high molecular weight fibrinogen assemblies ≥40 × 106 Da, that do not accumulate when fibrinogen is induced to aggregate by other stresses such as heating or hydroxyl-mediated damage in vitro. Hypochlorite-modified fibrinogen is stable at 37 °C as assessed by precipitation assays, and has reduced susceptibility to iron-induced (hydroxyl-mediated) precipitation compared to native fibrinogen. In contrast to hypochlorite-modified albumin, which is known to be immunostimulatory, hypochlorite-modified fibrinogen does not induce RAW 264.7 (macrophage-like) cells or EOC 13.31 (microglia-like) cells to produce reactive oxygen species or induce cell death. Furthermore, depletion of fibrinogen from human blood plasma increases the immunostimulatory property of blood plasma after it is supplemented with hypochlorite in situ. We propose that reaction of hypochlorite with fibrinogen in blood plasma potentially reduces the accumulation of other hypochlorite-modified species such as immunostimulatory hypochlorite-modified albumin. The latter represent a novel role for fibrinogen in blood plasma antioxidant defence.

•
Plasma proteins have distinct responses to reaction with hypochlorite.
•Hypochlorite-modified fibrinogen is prone to aggregation.
•Hypochlorite-modified albumin is resistant to aggregation.
•Hypochlorite-modified albumin induces ROS production and is cytotoxic.
•Hypochlorite-modified fibrinogen does not induce ROS production or cell death.
Fibrinogen is an abundant secreted protein that forms insoluble fibrin clots following cleavage by thrombin. Clot formation is a highly regulated process that is resolved by dissolution of the insoluble fibrin clot via plasmin-mediated degradation. Compared to other abundant plasma proteins, fibrinogen is particularly vulnerable to damage (i.e. misfolding) which promotes its aggregation and aberrant deposition. For example, fibrinogen has been identified as a major endogenous client for the activity of extracellular chaperones following the incubation of human plasma with mild, physiologically-relevant stress in vitro [[1], [86]]. Additionally, it has been demonstrated that compared to other abundant plasma proteins including serum albumin, fibrinogen is remarkably susceptible to oxidative modification in situ [2]. It has been proposed that the susceptibility of fibrinogen to oxidation relates to an important role in antioxidant defence [[3], [4], [5]]. On the other hand, the oxidation of fibrinogen is linked to aberrant clot formation [6] and iron(III)-induced parafibrin deposition [[7], [8], [9]], which promote endothelial cell apoptosis [10] and atherosclerosis [11]. Therefore, there is a need to more fully elucidate the biological consequences of fibrinogen oxidation in order better understand the role of oxidised fibrinogen in health and disease.
Hypochlorite (OCl−) is a potent biological protein misfolding agent [12] that is produced by the enzyme myeloperoxidase during inflammation. During severe inflammation it is estimated that in vivo levels of hypochlorite can reach the millimolar range (reviewed in Ref. [13]). Furthermore, the accumulation of hypochlorite-modified proteins is implicated in the pathology of many disease states including atherosclerosis [14], Alzheimer's disease [15], kidney disease [16], rheumatoid arthritis [17], osteoarthritis [18], and chronic lung disease [19]. Consistent with the susceptibility of fibrinogen to oxidation in vivo, hypochlorite-modified fibrinogen is proposed to be a major constituent of high molecular weight advanced oxidation protein products (HMW-AOPP) [[20], [21], [22]], which are elevated in blood plasma in uremia [23,24].
AOPP are typically generated in vitro by treating purified serum albumin with hypochlorite. These albumin-derived AOPP have been demonstrated to influence a broad range of biological activities including, but not limited to: (i) CD36-mediated platelet activation [25], (ii) macrophage differentiation [26], (iii) uptake of high-density lipoprotein particles [27], and (iv) stimulation of neutrophil and monocyte immune responses [28,29]. With the exception of previous studies reporting that hypochlorite-induced modification of fibrinogen impairs fibrin clot formation [6,[30], [31], [32]], overwhelmingly, prior studies have used metal-catalysed systems that generate hydroxyl radicals or ultraviolet light generate oxidised fibrinogen in vitro [[7], [8], [9], [10],[33], [34], [35]]. As a result, it is not known if hypochlorite-modified fibrinogen induces the same deleterious cellular effects that have been reported for hypochlorite-modified albumin.
Given the high susceptibility of fibrinogen to oxidation and the potency with which hypochlorite-induces protein modification, in the present study we examined the effect of hypochlorite on the aggregation and precipitation of fibrinogen in vitro. In order to gain insight regarding the biological importance of fibrinogen -derived AOPP, we also analysed the ability of hypochlorite-modified fibrinogen to stimulate ROS production using the macrophage-like cell line RAW 264.7 and the microglia-like cell line EOC 13.31 and examined the binding of hypochlorite-modified fibrinogen to macrophage receptors.
It has been reported that fibrinogen in HMW-AOPP is aberrantly cross-linked via oxidative modifications [[20], [21], [22]]. In the present study, we treated purified fibrinogen with 0–320 μM sodium hypochlorite (NaOCl) and analysed the samples for evidence of cross-linking using denaturing gel electrophoresis (Fig. 1). Under the conditions used, the majority of the fibrinogen migrated as a single band >250 kDa under non-reducing conditions (Fig. 1A) and as three bands at ~64, 56, and 47 kDa under reducing conditions (Fig. 1B). This result is consistent with the expected migration of the non-reduced 340 kDa fibrinogen dimer and the reduced fibrinogen α, β, γ subunits, respectively. Under non-reducing conditions hypochlorite-treatment induced a dose-dependent formation of SDS-resistant HMW species up to ~5% of the total protein at 320 μM NaOCl (Fig. 1A). Some of these HMW species were able to enter the gel, but the majority were retained in the base of the sample wells indicative of having very large mass (Fig. 1A). Furthermore, after treating fibrinogen with 320 μM NaOCl, even under reducing conditions, HMW species were retained in the sample well of the gel (Fig. 1B). When analysing fibrinogen treated with ≥80 μM NaOCl, under reducing conditions, several faint bands and one more prominent band at ~140 kDa were detected; the latter possibly represents the formation of a cross-linked α-chain dimer (Fig. 1B) [36]. Consistent with the results obtained using purified fibrinogen, supplementation of pooled human blood plasma with 320 μM NaOCl induced the formation of HMW fibrinogen species in situ (Fig. 1C).

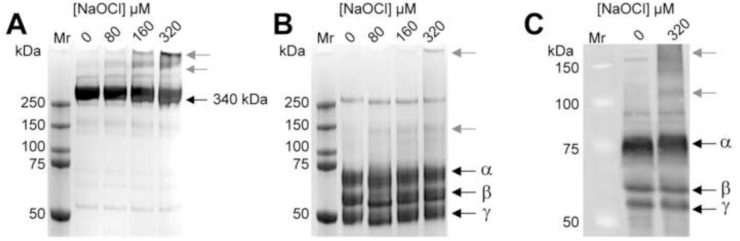
Images of SDS-PAGE gels showing the effect of NaOCl on purified human fibrinogen and Western blot analysis of fibrinogen in NaOCl-treated human plasma. Purified human fibrinogen (2 mg/ml) was pre-treated with NaOCl at the concentrations shown and subsequently subjected to SDS-PAGE analysis under (A) non-reducing or (B) reducing conditions. (C) Pooled human plasma from two healthy individuals was diluted to 2 mg/mL in PBS/Az and supplemented with 320 μM NaOCl prior to Western blot analysis using an anti-fibrinogen antibody. Bands corresponding to the expected mass of native fibrinogen or its subunits are indicated using black arrows. Bands corresponding to NaOCl-induced cross-linked species are indicated using grey arrows.
By native gel electrophoresis analysis, aggregation of fibrinogen was visible after treatment with 40 μM NaOCl (i.e. at a lower NaOCl concentration than causes visible cross-linking; Figs. 1B & 2A). Furthermore the relative abundance of protein migrating more slowly than native fibrinogen increased in a dose-dependent manner over the range 0–320 μM NaOCl (Fig. 2A). The results of corresponding experiments performed using hydrogen peroxide in the presence or absence of copper (which catalyses the generation of hydroxyl radicals) indicate that soluble aggregated fibrinogen does not accumulate in the presence of comparable molar concentrations of hydrogen peroxide (Fig. S1A). It has previously been shown that α2-macroglobulin (α2M) is functionally regulated by reaction with hypochlorite, which induces the dissociation of native tetrameric α2M into stable dimers [[37], [38], [39], [40]]. Consistent with the higher susceptibility of fibrinogen to oxidative modification, hypochlorite-induced aggregation of fibrinogen was observed at approximately 4-fold lower NaOCl concentrations than that required to induce the dissociation of α2M (Fig. 2A and B, respectively). Corresponding treatment of serum albumin with hypochlorite did not influence its migration by native PAGE (Fig. 2C), but aggregation of creatine phosphokinase (CPK; a cytosolic enzyme) to form HMW aggregates was detected after comparable treatment with NaOCl (Fig. 2D). Although aggregation of albumin was not observed under the conditions used, treatment with hypochlorite reduced the absorbance of the protein solution at 280 nm indicating that protein modification did occur (Fig. S2).

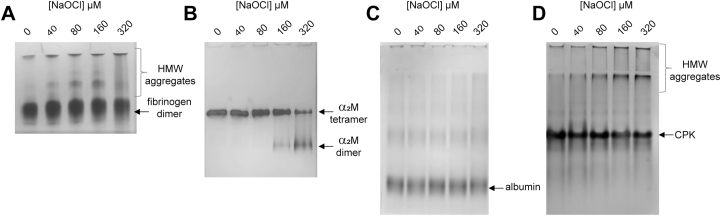
Images of native PAGE gels showing the effect of NaOCl on the migration of fibrinogen,α2M, albumin and CPK. Purified protein (2 mg/ml in PBS) was pre-treated with NaOCl overnight at ambient room temperature and subjected to native PAGE analysis. The positions of (A) the native fibrinogen dimer and NaOCl-induced HMW fibrinogen aggregates; (B) the native α2M tetramer and NaOCl-induced α2M dimer; (C) serum albumin; (D) CPK and NaOCl-induced HMW CPK aggregates are indicated on the images.
Hypochlorite-induced misfolding of fibrinogen promotes its non-covalent association Previous estimates of the mass of HMW-AOPP indicate that they can reach ≥2 × 106 Da [22]. Given that the results of native PAGE analysis indicated that some very large aggregated fibrinogen species were unable to enter the gel (Fig. 2A), hypochlorite-treated fibrinogen was subsequently analysed using size exclusion chromatography. Consistent with the results of native PAGE analysis, treatment of fibrinogen with NaOCl induced the aggregation of fibrinogen in a dose-dependent manner (Fig. 3A). Strikingly, some of the hypochlorite-treated fibrinogen eluted at the void volume of the column, which corresponds to a molecular weight ≥40 × 106 Da. When treated with 2 mM NaOCl (i.e. a 700-fold molar excess), the majority of fibrinogen formed HMW species in solution. Soluble HMW species of this magnitude are observed in vitro when proteins are induced to misfold in the presence of a holdase chaperone such as clusterin [41], but are not widely known to form otherwise. For example, incubation of fibrinogen in the presence of high levels of hydroxyl radicals promotes its precipitation and only a small fraction of the protein is retained in solution as HMW species (Figs. S1B and C).

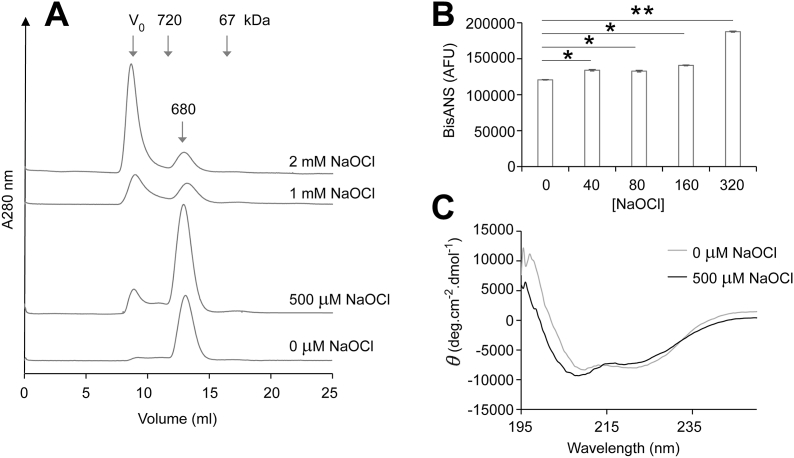
Size exclusion chromatography, bis-ANS assay and circular dichroism analysis of NaOCl-treated fibrinogen. (A) Fibrinogen (2 mg/ml) was pre-treated with NaOCl and analysed by size exclusion chromatography using a Superose™ 6 column. The data shown are the absorbance at 280 nm (A280 nm) of individual samples and are representative of several different experiments. The positions of molecular mass standards (kDa) and the native fibrinogen dimer (680 kDa) are shown. The exclusion volume (Vo) corresponds to molecules of ≥4 × 107 Da. (B) Corresponding bisANS assay of fibrinogen pre-treated with NaOCl. The results are the mean bisANS fluorescence ± SD (n = 4) and are adjusted for the background fluorescence of bisANS alone. * Denotes significantly elevated bisANS fluorescence of NaOCl-treated samples compared to native fibrinogen (p < 0.01; Tukey HSD). ** Denotes significantly elevated bisANS fluorescence compared to all other samples in the assay (p < 0.01; Tukey HSD). (C) Far-UV circular dichroism spectra of fibrinogen (2 mg/ml) after treatment ± 500 μM NaOCl. Each dataset shown is the mean of six scans.
Consistent with the idea that non-covalent association precedes crosslinking, treatment of fibrinogen with NaOCl was associated with an increase in bisANS fluorescence, which is indicative of surface exposed hydrophobicity (Fig. 3B). When fibrinogen was treated with 40–160 μM NaOCl the increase in bisANS fluorescence compared to native fibrinogen was relatively small (~10%), however, treatment of fibrinogen with 320 μM NaOCl substantially increased the resulting bisANS fluorescence (Fig. 3B). The results of circular dichroism analyses indicate that soluble hypochlorite-modified fibrinogen has perturbed secondary structure compared to native fibrinogen (Fig. 3C). Following hypochlorite treatment of fibrinogen the minima at 222 nm was significantly increased (P < 0.0001; Student's t-test) indicating that there was a reduction in alpha-helical content. This data support the conclusion that hypochlorite-induced modification induces misfolding and aggregation of fibrinogen to generate soluble HMW species.
Fibrinogen is readily induced to precipitate at the supra-physiological temperature of 45 °C in vitro [41]. To examine whether or not hypochlorite-induced modification of fibrinogen affects its heat-induced precipitation a series of turbidity assays were performed (Fig. 4). The data show that when incubated at 45 °C, treatment with hypochlorite enhances the heat-induced precipitation of fibrinogen in a dose-dependent manner (Fig. 4A). At the endpoint of the assay the turbidity of all fibrinogen solutions incubated with ≥80 μM NaOCl exceeded that of native fibrinogen (Fig. 4A). Consistent with the results of the turbidity assays, the results of native PAGE analysis indicate that hypochlorite-treated fibrinogen is preferentially lost from solution at 45 °C compared to native fibrinogen (Fig. 4B). In contrast to hypochlorite-induced aggregation, heating alone does not promote the accumulation of soluble HMW forms of aggregated fibrinogen (Fig. 4B and Fig. S3). When the turbidity assay was repeated for 64 h at 41 °C (a temperature attained in humans with high fever [42]), there was a modest increase in turbidity in the fibrinogen sample that had been treated with 320 μM NaOCl. However, precipitation of fibrinogen was not detected after treatment with lower concentrations of NaOCl (Fig. 4C). We next generated a solution containing predominately HMW fibrinogen by treating fibrinogen at 37 °C for 24 h with 2 mM NaOCl. There was no detectable precipitation of fibrinogen under these conditions (Fig. 4D). By comparison, treatment of CPK with hypochlorite, which promotes its aggregation (Fig. 2D), also substantially reduced its solubility at 37 °C as assessed by turbidity assay and densitometry analysis of the fraction of the protein remaining soluble (Fig. S4).

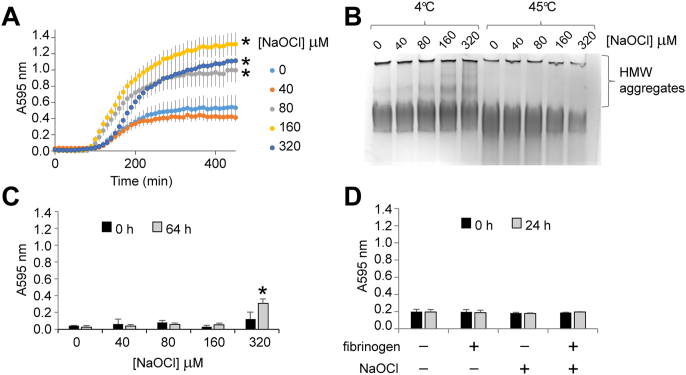
Turbidity assays showing the effect of NaOCl on heat-induced fibrinogen precipitation. (A) Purified human fibrinogen (2 mg/ml) was pre-treated with NaOCl and then incubated at 45 °C while the A595 nm was continuously monitored. The results shown are the mean absorbance of triplicate samples ± SEM. * Denotes significantly increased endpoint turbidity of NaOCl-treated fibrinogen compared to fibrinogen in PBS/Az alone (p < 0.05 Tukey HSD). (B) Image of a native gel showing NaOCl-treated fibrinogen after incubation at 45 °C for 8 h (as in panel A) or after being stored at 4 °C for the same period. (C) Fibrinogen (as described in panel A) was incubated at 41 °C and the A595 nm continuously monitored. For clarity only endpoint measurements are shown. The results are the mean absorbance of triplicate samples ± SD. * Denotes significantly increased turbidity of fibrinogen treated with 320 μM after 64 h of incubation compared to fibrinogen in PBS/Az alone (p < 0.05 Tukey HSD). (D) Fibrinogen was incubated in the presence or absence of 2 mM NaOCl in PBS/Az at 37 °C and the A595 nm continuously monitored. For clarity only endpoint measurements are shown. The results are the mean absorbance of triplicate samples ± SD. The data shown are representative of several independent experiments.
Incubation of fibrinogen with trivalent iron results in the generation of hydroxyl radicals that convert fibrinogen to insoluble fibrin-like material in vitro [7]. This process has been proposed to contribute to aberrant fibrinogen deposition in cardiovascular disease, cancer, Alzheimer's disease and diabetes [[7], [8], [9],[43], [84]]. In the current study we examined whether pre-treatment of fibrinogen with hypochlorite influences its susceptibility to iron-induced precipitation. Under the conditions used, treatment of fibrinogen with 1 mM FeCl3 rapidly increased the turbidity of the solution 10-fold above the absorbance of the FeCl3 solution alone (Fig. 5A). Pre-treatment of fibrinogen with 40–160 μM NaOCl (followed by extensive dialysis to remove unreacted hypochlorite) did not have a significant effect on the turbidity of the solutions following treatment with FeCl3, however, pre-treatment with 320 μM NaOCl reduced the iron-induced precipitation of fibrinogen by approximately 30% (Fig. 5A). Compared to the results of native PAGE analysis following heat-induced precipitation of fibrinogen (which preferentially removes HMW hypochlorite-modified fibrinogen from solution; Fig. 4B), HMW hypochlorite-modified fibrinogen was still visible in solution following treatment with FeCl3 (Fig. 5B). Interestingly, although FeCl3 induces the precipitation of native fibrinogen, native PAGE analysis indicates that it does not induce the formation of soluble HMW fibrinogen (Fig. 5B).

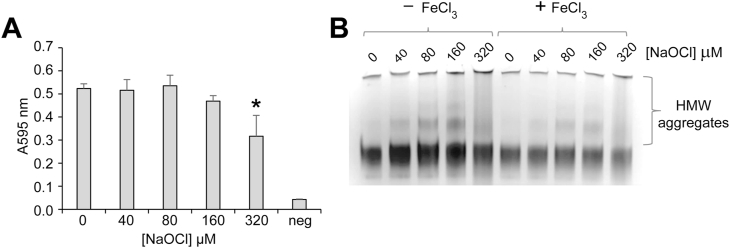
Turbidity assay and image of native PAGE gel showing the effect of NaOCl-induced modification on Fe3Cl-induced fibrinogen precipitation. (A) Purified human fibrinogen (2 mg/ml) was pre-treated with NaOCl in PBS/Az. Following the addition of 1 mM FeCl3 the A595 nm was immediately measured. The results shown are the mean absorbance of triplicate samples ± SD. * Denotes significantly reduced A595 nm of fibrinogen pre-treated with 320 μM NaOCl compared to native fibrinogen (p < 0.01; Tukey HSD). The background absorbance of 1 mM FeCl3 in the reaction buffer is also shown (neg). (B) Corresponding image of a native PAGE gel showing samples described in (A) in the presence or absence of FeCl3. The data shown are representative of several independent experiments. The positions of HMW aggregates of fibrinogen are indicated.
Hypochlorite-induced modification can directly induce proteins to acquire immunostimulatory and cytotoxic properties. For example, hypochlorite-modified albumin has been shown to stimulate monocyte respiratory burst in vitro [44] and induce apoptosis [[45], [46], [47]]. In the present study we used flow cytometry to assess the ability of hypochlorite-modified fibrinogen to stimulate the production of ROS by the macrophage-like cell line RAW 264.7. The results show that compared to treatment with hypochlorite-modified albumin, which robustly increases aminophenyl fluorescein (APF) fluorescence (indicating the production of ROS), comparable treatment with hypochlorite-modified fibrinogen does not increase APF fluorescence above the level of control cells that were not treated with any protein (Fig. 6Ai). Furthermore, in the absence of pre-treatment with NaOCl neither albumin nor fibrinogen stimulated cellular ROS production. In corresponding experiments involving the toll-like receptor 4-deficient microglia-like cell line EOC 13.31, similar results were obtained (Fig. 6Aii). The effect of fibrinogen-depletion on the immunostimulatory property of hypochlorite-treated human plasma was also examined. Fractionation using ammonium sulphate removed 55 ± 16% of plasma fibrinogen with 84 ± 7.5% purity as assessed by densitometry analysis (mean ± SD; n = 4; Fig. S5). The results show that APF fluorescence is not induced following incubation of RAW 264.7 cells with normal human plasma or batch-matched fibrinogen-depleted human plasma, however, supplementation of the plasma with NaOCl prior to incubation with RAW 264.7 cells results in increased cellular AFP fluorescence (Fig. 6B). Furthermore, NaOCl-treated fibrinogen-depleted plasma induced greater AFP fluorescence than NaOCl-treated normal human plasma (Fig. 6B). To determine whether or not the binding of hypochlorite-modified fibrinogen influences cell viability MTS assay was performed. The data show that RAW 264.7 cells had reduced viability following 48 h incubation with hypochlorite-modified albumin but treatment with an equivalent amount of hypochlorite-modified fibrinogen did not reduce cell viability compared to untreated control cells or cells treated with native control proteins (Fig. 6C).

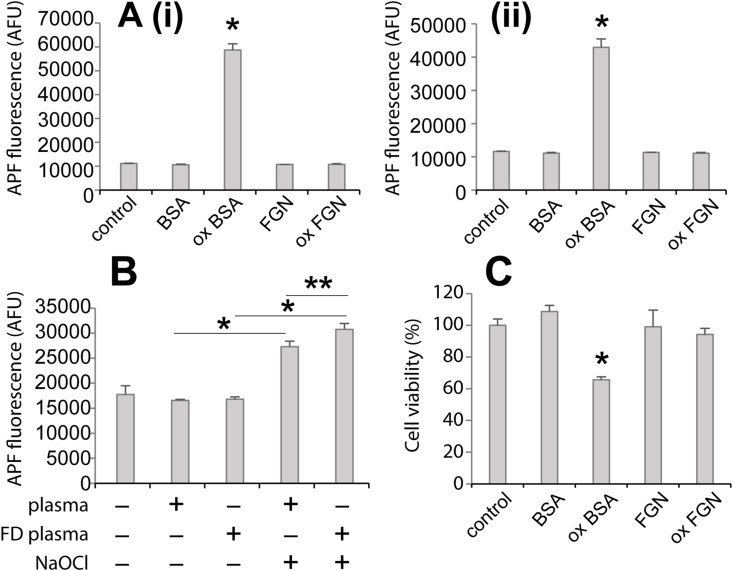
Flow cytometry analysis showing the effect of NaOCl on cellular ROS production induced by albumin, fibrinogen, human plasma or fibrinogen-depleted (FD) human plasma. Panel (A) RAW 264.7 cells (i) or EOC 13.31 cells (ii) were incubated with 5 μM APF in HBB for 45 min at 37 °C prior to incubation with 200 μg/mL native bovine serum albumin (BSA) or fibrinogen (FGN) or protein that had been pre-treated with 500 μM NaOCl (designated ox BSA and ox FGN) for 45 min at 37 °C in HBSS. Control cells not supplemented with protein were incubated in 5 μM APF in HBSS and then HBSS alone. The APF fluorescence, indicative of ROS production was measured by flow cytometry. The results shown are the composite mean APF fluorescence of 5000 viable cells (n = 3 ± SD) in arbitrary fluorescence units (AFU) and are representative of several independent experiments. * Denotes increased APF fluorescence induced by ox BSA compared to all other samples (p < 0.01; Tukey HSD). Panel (B) RAW 264.7 cells were incubated with APF as described in (A) prior to incubation with human plasma orFD human plasma that had been pre-treated ± 500 μM NaOCl. All plasma samples were diluted so that the final protein concentration was 200 μg/mL in HBB. The APF fluorescence was measured as described in (A). The results shown are the composite mean APF fluorescence of 5000 viable cells (n = 3 ± SD) in arbitrary fluorescence units (AFU) and are representative of several independent experiments. * Denotes increased APF fluorescence induced by pre-treatment of plasma or FD plasma with NaOCl (p < 0.01; Tukey HSD) and ** denotes increased APF fluorescence induced by ox FD plasma versus ox plasma (p < 0.05; Tukey HSD). Panel (C) RAW 264.7 cells were cultured in the presence of native BSA or fibrinogen or protein that had been pre-treated with NaOCl as in (A) at 100 μg/mL in serum-free DMEM for 48 h before measuring the relative numbers of viable cells using an MTS assay. The results shown are expressed as a percentage of the absorbance measured in control samples that were cultured in serum-free media alone (n = 4 ± SD).
Macrophage receptors scavenger receptor A (SRA) and macrophage-1 antigen (MAC-1) are reported to preferentially bind to misfolded proteins compared to their native counterparts [48,49]. RAW 264.7 cells were verified as expressing cell surface SRA and MAC-1 (Fig. S6). The binding of biotinylated hypochlorite-modified fibrinogen to these cells was approximately 8-fold greater than that of biotinylated native fibrinogen as assessed by flow cytometry (Fig. 7A). The low levels of binding of native fibrinogen to the cell surface were not significantly reduced by pre-incubation of the cells with either anti-MAC-1 antibody or anti-SRA antibody (Fig. 7A). In contrast, the binding of hypochlorite-modified fibrinogen was reduced by around 25% when cells were pre-incubated with an inhibitory anti-SRA antibody (Fig. 7).

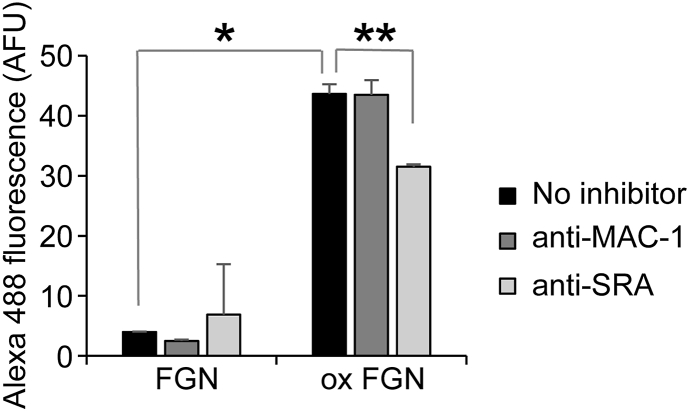
Flow cytometry analysis showing the effect of NaOCl on the binding of fibrinogen to the surface of RAW 264.7 cells. RAW 264.7 cells were pre-incubated with either anti-MAC-1 antibody (5C6), anti-SRA (2F8) or without inhibitor before incubation with biontinylated fibrinogen or fibrinogen that had been pre-treated with NaOCl (ox FGN) at 25 μg/ml in HBB at 4 °C. The cell surface binding of biontinylated fibrinogen was detected using streptavidin-Alexa Fluor 488. The results shown are the composite geometric mean Alexa Fluor 488 fluorescence of 5000 viable cells (n = 3 ± SEM) and is adjusted for background fluorescence. * Denotes increased binding of ox FGN compared to FGN (p < 0.01; Tukey HSD). ** Denotes reduced binding of ox FGN following pre-treatment of cells with anti-SRA antibody (p < 0.01; Tukey HSD).
The results of the present study demonstrate that plasma proteins have distinct responses to hypochlorite-induced stress in vitro - fibrinogen is more susceptible to hypochlorite-induced aggregation compared to other abundant plasma proteins including albumin and α2M. Furthermore, our results support the conclusion that hypochlorite-modified fibrinogen lacks the immunostimulatory properties of hypochlorite-modified albumin. The precise importance of the remarkably high susceptibility of fibrinogen to oxidative modification is currently unclear [50], however, it is plausible that in terms of hypochlorite-induced damage, fibrinogen is a sacrificial protein that reduces the generation of other deleterious hypochlorite-modified species in vivo. Considering that hypochlorite is predicted to reach millimolar concentrations in vivo (reviewed in Ref. [13]), it is reasonable to suggest that the conditions used in this study recapitulate the hypochlorite-induced modification that generates fibrinogen-derived HMW-AOPP in vivo [[20], [21], [22]].
It is thermodynamically favourable for misfolded proteins to aggregate when normally buried hydrophobic regions become surface exposed. Eventually, misfolded protein aggregates can become so large that they exceed the solubility limit and precipitate from solution. Interestingly, this phenomenon occurs when fibrinogen is induced to misfold by heating or hydroxyl-mediated damage, but not in response to treatment with hypochlorite at up to a 300-fold molar excess. Instead, treatment with hypochlorite induces the formation of relatively stable HMW misfolded fibrinogen assemblies. One likely explanation for this difference is that hypochlorite potently induces amino acid side-chain modification, but a very large molar excess is required in order to induce backbone fragmentation [12]. On the other hand, hydroxyl radicals potently modify both amino acid side chains and the protein backbone [51]. These differences highlight the importance of studying the effect of individual biological oxidants on protein structure and function, rather than making assumptions about biological protein oxidation based on the results obtained from a single model system in vitro.
Aberrant deposition of fibrinogen is implicated in a large number of pathologies including cardiovascular disease, cancer, Alzheimer's disease and diabetes [[7], [8], [9],43,[52], [53], [54], [55], [84], [85]]. Although we observed a modest increase in the propensity to precipitate when fibrinogen was treated with ≥320 μM NaOCl and heated for a prolonged period at 41 °C, at normal physiological temperature (37 °C) we did not observe precipitation of fibrinogen, even after treatment with 2 mM NaOCl. Furthermore, hypochlorite-induced modification of fibrinogen was protective against precipitation induced by iron(III)-induced generation of hydroxyl radicals. The precise reasons for this are enigmatic considering that hypochlorite and hydroxyl radicals readily react with the same amino acid targets, however, the packaging of fibrinogen into larger assemblies potentially shields the protein backbone from hydroxyl-mediated damage or disrupts the binding of iron(III) to fibrinogen. This could have relevance when the accumulation of metals and immune system activation occur concomitantly in tissues [56,57].
It is well known that proteins can form very large soluble assemblies when they are induced to misfold in the presence of chaperones including the intracellular small heat shock proteins and the extracellular chaperone clusterin [41,[58], [59], [60], [61]]. To our knowledge chaperone-independent formation of soluble misfolded protein assemblies ≥40 × 106 Da has not previously been reported. It is possible that the autonomous packaging of hypochlorite-modified fibrinogen into soluble assemblies is a programmed process that facilitates the efficiency with which it is cleared from circulation. Supporting this idea, we observed the binding of hypochlorite-modified fibrinogen to SRA, which has previously been implicated in the clearance of other hypochlorite-modified proteins [49]. Furthermore, it has been proposed that the toxicity of protein oligomers is a function of their size and surface exposed hydrophobicity [62], which suggests that efficient packaging of hypochlorite-modified fibrinogen into HMW assemblies underlies its non-toxic characteristic.
Hypochlorite-induced modification can induce proteins to simulate immune cells and induce cell death [25,[63], [64], [65], [66]]. Furthermore, AOPP are considered important mediators of systemic oxidative stress and inflammatory processes in several clinical conditions including diabetes, systemic sclerosis, coronary artery disease and preeclampsia [[67], [68], [69], [70]]. For the first time we demonstrate that hypochlorite-modified fibrinogen does not stimulate cellular ROS production. This is a somewhat surprising result considering that (i) monocytes/macrophages preferentially bind to misfolded fibrinogen [71], (ii) oxidation of fibrinogen via exposure to ultraviolet light reportedly increases its ability to stimulate ROS production by leukocytes [33], and (iii) misfolded fibrinogen bound to negatively charged nanoparticles has been shown to stimulate THP-1 monocyte cells inducing their activation and the release of inflammatory cytokines [48]. Intuitively, the cellular effects of hypochlorite-modified fibrinogen versus misfolded fibrinogen generated using other systems are likely to be a consequence of the different types of modification induced or the extent of modification. Of potential importance, in the current study we demonstrate that hypochlorite-modified fibrinogen does not bind to MAC-1, a receptor for other forms of misfolded fibrinogen that is implicated in the stimulation of leukocytes [48,[72], [73], [74]]. It is a limitation of our study that the method used to generate fibrinogen-depleted plasma also removes a small fraction of contaminating proteins including plasminogen, fibronectin, A2M and immunoglobulin from plasma [21]. Nevertheless, fibrinogen comprises the bulk of protein removed from plasma using this method. As such, our results are consistent with a model in which reaction of hypochlorite with fibrinogen prevents the formation of immunostimulatory species such as hypochlorite-modified albumin in blood plasma.
Considering that the elevation of fibrinogen levels in response to inflammation is an evolutionarily conserved response, it is plausible that fibrinogen is designed to function in the presence of oxidative stress [75]. Nevertheless, vehement debate continues in the field regarding whether the oxidation of fibrinogen is beneficial or harmful with evidence for both pro-thrombotic and anti-thrombotic effects reported [6,33,34,50,[76], [77], [78], [79], [80], [81]]. This landmark study provides an important step towards elucidating the biological importance hypochlorite-modified fibrinogen. Consistent with an important role for fibrinogen as an antioxidant, the results of our study support the conclusion that fibrinogen is uniquely susceptible to hypochlorite-induced aggregation, which does not promote its deposition at normal physiologically-relevant temperatures or confer the ability to stimulate immune cell ROS production. It is plausible, however, that hypochlorite-modified fibrinogen has important cellular consequences that are not yet known. Collectively, the results of his study highlight that in order to understand the diverse biological roles of fibrinogen in health and disease, it is important to consider the specific effects of individual biological oxidants.
All chemicals, proteins and buffer salts were obtained from Sigma-Aldrich, unless otherwise stated. Purified human fibrinogen was from Hyphen Biomed (plasminogen-free) or from Sigma-Aldrich (premium quality essentially plasminogen-free). Native α2M was purified from normal blood plasma as described in Ref. [82]. The collection of human plasma was approved by the joint UOW & ISLHD Health and Medical Human Research Ethics Committee (HE02/080) or the Central Adelaide Local Health Network Research Ethics Committee (HREC/18/CALHN/421).
Fibrinogen (2 mg/ml in phosphate buffered saline, pH 7.4 supplemented with 0.01% (w/v) sodium azide; PBS/Az) was incubated with 0–2 mM sodium hypochlorite (NaOCl) overnight at ambient room temperature. Unless otherwise stated, prior to analysis samples were either extensively dialysed against PBS/Az or desalted using Zeba desalting columns (Life Technologies). The concentration of NaOCl stock solutions were determined by absorbance spectroscopy [83].
Proteins were subjected to native gel electrophoresis using NuPAGE Novex 3–8% Tris-acetate gels and Novex Tris-glycine native buffer (Life Technologies). Denaturing gel electrophoresis was performed using NuPAGE Novex 4–12% Bis-Tris gels and NuPAGE Mes SDS running buffer (Life Technologies). Where specified, samples were reduced by treatment with 5% (v/v) β-mercaptoethanol. Gels were stained using Instant Blue stain (Sigma-Aldrich).
For Western blot analysis heparinised human plasma was supplemented with cOmplete protease inhibitor cocktail according to the manufacturer's instructions and diluted to 2 mg/ml in PBS/Az. The plasma was supplemented with 0 or 320 μM NaOCl and left to incubate at ambient room temperature overnight. Following reduction using 5% (v/v) β-mercaptoethanol, the proteins were separated using a NuPAGE Novex 4–12% Bis-Tris gel and transferred to PVDF membrane using an iblot 2 (Life Technologies) according to the manufacturer's instructions. After blocking overnight at 4 °C using 5% (w/v) skim milk powder in PBS, the membrane was incubated with polyclonal goat anti-fibrinogen antibody (Life Technologies; diluted 1:2000 in blocking solution) and then incubated with a superclonal anti-goat IgG-HRP conjugate (Life technologies; diluted 1:5000 in blocking solution). Blots were imaged by enhanced chemiluminescence using a Chemidoc touch imaging system (Bio-Rad).
Following treatment with NaOCl as described above, fibrinogen (20 μg/ml) was incubated with 10 μM bisANS in PBS and incubated at ambient room temperature for 10 min. The bisANS fluorescence of the samples was measured using a FLUOstar Optima fluorescence plate reader using excitation and emission band pass filters of 360 ± 10 and 490 ± 10 nm, respectively.
For far-UV CD spectroscopy, native or hypochlorite-modified fibrinogen (pre-treated with 500 μM NaOCl) were dialysed against 10 mM sodium phosphate buffer (pH 7.4) and then diluted to 20 μg/ml in the same buffer before the CD spectra were obtained using a Jasco J-810 spectrapolarimeter at 25 °C using a 1-mm path-length cuvette. Samples were filtered (0.45 μm) before analysis.
SEC was carried out using a Superose™ 6 10/300 column (GE Healthcare) at the recommended flow rate of 0.5 ml/min and the absorbance at 280 nm continuously monitored using an ÄKTA FPLC system (GE Healthcare). Mass standards were purified human α2M (720 kDa), bovine serum albumin (67 kDa) or dextran blue (void voluble marker; GE Healthcare). All buffers and samples were filtered (0.45 μm) before use.
Fibrinogen (2 mg/ml) was incubated with 0–2 mM NaOCl in PBS/Az at 45 °C, 41 °C or 37 °C in a CLARIOstar plate reader (BMG Labtech Ltd.) while the absorbance at 595 nm was continuously monitored. For analysis of FeCl3-induced fibrinogen precipitation, fibrinogen was pre-treated with 0–320 μM NaOCl (as described above) and extensively dialysed against PBS/Az. Equal volumes of FeCl3 (2 mM in H2O) and fibrinogen (2 mg/ml in PBS/Az) were combined and the absorbance at 595 nm was read immediately in a CLARIOstar plate reader. Soluble fibrinogen remaining at the conclusion of the assay was recovered after high speed centrifugation to pellet precipitated protein and analysed by native gel electrophoresis.
RAW 264.7, a murine leukemic monocyte/macrophage cell line, was routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma) with low glucose supplemented with 10% (v/v) fetal bovine serum (FBS). EOC13.31 mouse brain microglial cells (kindly donated by J. Yerbury, University of Wollongong, Australia) were maintained in DMEM with high glucose supplemented with 10% (v/v) FBS and 20% (v/v) conditioned medium from LADMAC cells. Both cell lines were cultured at 37 °C in a humidified atmosphere of 5% (v/v) CO2.
Purified albumin and fibrinogen were diluted to 2 mg/ml in PBS/Az or PBS/Az supplemented with 500 μM NaOCl. Following incubation overnight at ambient room temperature the proteins were extensively dialysed against PBS.
Human plasma was depleted of fibrinogen by precipitation with ammonium sulphate according to the method reported in Refs. [22,80]. Fibrinogen-depleted (FD) plasma and batch-matched normal plasma was determined using a Bincinchoninic Acid Kit (Sigma-Aldrich). Normal plasma and FD plasma were then diluted to 2 mg/ml in PBS/Az and incubated overnight in the presence or absence of 500 μM NaOCl at ambient room temperature. The four different plasma samples (normal plasma, FD-depleted plasma, NaOCl-treated plasma and NaOCl-treated FD plasma) were stored at −80 °C before use.
Cellular ROS production was measured using 2-[6-(4′-amino)phenoxy-3H-xanthen-3-on-9-yl] benzoic acid (APF; Life Technologies), which forms a stable and highly fluorescent product in the presence of ROS including hydroxyl radicals, hypochlorite and peroxynitrite (excitation/emission maxima ~490/515 nm). RAW 264.7 macrophage cells or EOC13.31 microglial cells were detached by scraping and washed in Hank's Balanced Salt Solution, pH 7.4 supplemented with 5 mM l-glucose (HBSS). The cells were then incubated with 5 μM APF in HBSS at 37 °C for 30 min with gentle agitation. Excess APF was removed by washing the cells in HBSS by centrifugation. Cells were next incubated with bovine serum albumin (BSA), BSA that had been pre-treated with NaOCl (ox BSA; 2 mg/mL BSA pre-treated with 500 μM NaOCl in PBS/Az by incubation overnight at ambient room temperature and then extensively dialysed against PBS), fibrinogen (FGN), oxidised FGN (ox FGN; 2 mg/mL FGN pre-treated with 500 μM NaOCl in PBS/Az by incubation overnight at ambient room temperature and then extensively dialysed against PBS) or HBSS alone at 37 °C for 30 min with gentle agitation. Following this final incubation step the cells were kept on ice until the APF fluorescence was measured using a CytoFlex S Flow Cytometer (Beckman Coulter) at the Flow Cytometry Facility (College of Medicine and Public Health, Flinders University, South Australia). Dead cells were excluded from the analysis by staining with propidium iodide.
RAW 264.7 cells were cultured in DMEM supplemented with 10% fetal bovine serum for 48 h until approximately 70% confluency and then cultured in the presence or absence of native or hypochlorite-modified fibrinogen or BSA at 100 μg/mL in serum-free DMEM for a further 48 h. Differences in the final numbers of viable cells were measured using CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega), according to the manufacturer's instructions.
Native and hypochlorite-modified fibrinogen were biotinylated using EZ-Link Sulfo–NHS–Biotin (Life Technologies). RAW 264.7 were grown for 48 h without passage and then detached using gentle scraping. The cells were then washed in HBSS (without glucose) by centrifugation and incubated with 1 mg/ml mouse IgG in HBB (30 min at 4 °C) to block Fc receptors. The cells were washed again and incubated with 10 μg/ml rat anti-mouse SRA antibody (clone 2F8; AbD Serotec) in HBSS; 10 μg/ml rat anti-mouse MAC-1 antibody (clone 5C6; AbD Serotec) in HBSS or HBSS alone. Following washing the cells were then incubated with 25 μg/ml biotinylated fibrinogen or hypochlorite-modified fibrinogen for 30 min at 4 °C. The binding of the biotinylated proteins was then detected using a streptavidin-Alexa Fluor 488 conjugate (Life Technologies). Cell surface binding was measured using a BD FACScan (Becton and Dickinson) at the Flow Cytometry Facility (Department of Pathology, University of Cambridge, United Kingdom). The data were analysed using FlowJo7 software (Tree Star Inc.). The data shown are the composite geometric mean fluorescence of three independent samples containing 5000 viable cells as determined by propidium iodide staining.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
This work was supported by the
The authors declare that there are no competing interests.