
We show the synthesis of a redox‐active quinone, 2‐methoxy‐1,4‐hydroquinone (MHQ), from a bio‐based feedstock and its suitability as electrolyte in aqueous redox flow batteries. We identified semiquinone intermediates at insufficiently low pH and quinoid radicals as responsible for decomposition of MHQ under electrochemical conditions. Both can be avoided and/or stabilized, respectively, using H3PO4 electrolyte, allowing for reversible cycling in a redox flow battery for hundreds of cycles.
A vanillin‐based 2‐methoxyhydroquinone is proposed as the catholyte for redox‐flow batteries. We overcame the tendency of such quinones to form reactive radicals that trigger side‐reactions by carefully choosing the medium. This enabled 87.4 % capacity retention after 250 cycles.
The past years have shown a tremendous increase in the generation of green energy, with the ultimate goal to reduce CO2 emissions and dependency on fossil resources.[ 1 , 2 ] While generation has been substantially addressed in many countries by establishing, for example, wind and solar parks, sustainable storage of green energy has not been a major focus of stake holders. However, large‐scale energy storage capacity is essential to compensate mismatch between green energy production and demand. [3] In line with generation, the ideal storage must combine excellent efficiency and ecological footprint to cause a minimum or no additional CO2 emissions. For instance, pumped hydroelectricity, and compressed air storage, are capable of sustainably storing large amounts of energy (up to GWh) at low cost. [4] However, sites to build new storage systems with these technologies are limited. Currently, non‐sustainable large scale storage technologies involve redox flow batteries (RFB). [5] Their core is the electrolyte, which nowadays is mainly based on mined sources (such as vanadium, bromine),[ 6 , 7 , 8 ] with significant environmental impact. Storage capacity of RFB is directly linked to the size of the tanks filled with electrolytes. Organic redox molecules have been reported widely but are only viable at large scale if they can be sustainably produced from renewable sources. [9] Quinones are excellent candidates as they offer rich structural diversity and well‐explored chemistry. [10] However, the majority of the reported quinones stem from non‐renewable feedstock. [9] Further, their synthesis requires harsh conditions and/or air exclusion with a protective environment to prevent a plethora of side reactions. Most quinones used in RFB have been tested in organic solvents such as acetonitrile, showing satisfying results in terms of cyclability and stability. [9] Although side reactions are suppressed, the use of organic solvents makes such systems hardly applicable for large‐volume applications.[ 11 , 12 , 13 ] A general problem, hampering the wide‐spread use of quinones in RFBs, is their inherent tendency to decompose via nucleophilic substitution and addition reactions in alkaline, aqueous solutions. [14] In the acidic pH regime, however, quinones are poorly soluble, decreasing volumetric capacity of RFB.[ 15 , 16 ] Although recent works tackle these challenges,[ 17 , 18 , 19 , 20 , 21 ] they have limited applicability in larger scale.[ 22 , 23 ] In particular, there is an apparent lack of bio‐based quinones as active materials for bio‐based RFB and scalable examples have not been demonstrated yet. The problem is that there are limited raw materials on place for large‐scale quinone production from renewable feedstock.
In nature, a main potential source of quinones is lignin. Lignin is a complex polyaromatic biopolymer which is produced at large scale (50–100 megatons per year) in pulp and paper industry, with the main fraction being incinerated (98 %). However, vanillin (4‐hydroxy‐3‐methoxybenzaldehyde) is one of the few commercial fine chemicals currently produced from lignin with yields of up to 12 % in industrial scale. [24] Therefore, we became interested whether we can use vanillin as a widely available, cheap and sustainable raw material for a redox‐active quinone, that is, 2‐methoxy‐1,4‐hydroquinone (MHQ, Figure 1). The challenges to overcome during conversion include i) to avoid side reactions, ii) and increase solubility in the chosen pH range. For the vanillin‐to‐MHQ conversion, we elaborated different aqueous‐to‐THF solvent fractions and neutral, 0.01, and 0.1 M NaOH in the aqueous phase. The reaction of vanillin with hydrogen peroxide under alkaline conditions turned out to be fast and gave MHQ in high yields of 75.8 % at ambient atmosphere (Supporting Information, Figures S1–S4 and Table S1). [24] After 5 seconds, 96 % of the vanillin was converted in solvent mixtures with low THF content under the chosen reaction conditions as proven by GC‐MS analysis. However, with air present, oxidation and side reactions are observed. The highest yield of MHQ was reached after 30 s reaction time as a compromise between reaction time and lability of MHQ under alkaline conditions. When the reaction time was increased to 10 minutes, significant amounts of the oxidation product of MHQ, 2‐methoxy‐1,4‐quinone (MQ), were observed. MHQ has not been considered so far for any electrochemical applications due to its instability under UV light and its sensitivity to acidic and alkaline conditions. MQ degraded in alkaline media within seconds, and photoproducts were already observed after a few milliseconds.[ 25 , 26 ]

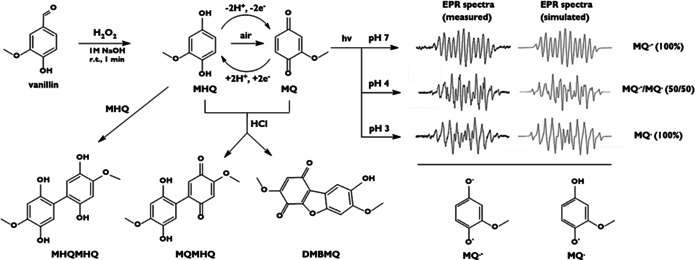
Left: Oxidation of vanillin to MHQ, redox reaction to MQ and potential insoluble dimeric side‐products (MHQMHQ, MQMHQ, DMBMQ. Right: experimentally derived and simulated cw‐ESR spectra of the photoproducts of MQ at pH values of 7.4, 4.0 and 3.0. The hyperfine coupling constants are given in the Supporting Information.
In solution, MHQ readily dimerizes to give the insoluble dimer 4,4′‐dimethoxy‐2,5,2′,5′‐tetrahydroxy‐biphenyl (MHQMHQ), which was characterized by X‐ray diffraction analysis (Supporting Information, Figure S6, Tables S2–S8). In the mother liquor, we identified another side product (2′,5′‐dihydroxy‐4,4′‐dimethoxy‐[1,1′‐biphenyl]‐2,5‐dione, MQMHQ) by 1H‐NMR spectroscopy (Figure S7, †ESI). This dimer was formed either by reaction of MQ with MHQ or by partial oxidation of the MHQMHQ. Although the formation of 3,7‐dimethoxydibenzo[b,d]furan‐1,4,8‐triol (DMBMQ) has been described,[ 25 , 27 ] we could not detect it in our experiments. As the persistence of the MHQ/MQ system is of utmost importance for its use in redox flow batteries, we explored the decomposition mechanism in more detail. One decomposition pathway of the MHQ/MQ system involves radicals, which can be formed by light induced excitation. In analogy to previous findings from investigations on parent 1,4‐benzoquinone,[ 28 , 29 , 30 , 31 , 32 , 33 ] distinct ESR spectra allowed for the analysis of the decomposition intermediates. Figure 1 compares the experimental and simulated ESR spectra after UV excitation of MQ at three different pH values (3.0, 4.0, and 7.4). At pH 7.4, we detected the characteristic ESR spectrum of the radical anion MQ.−. Simulations (Supporting Information, Table S9) revealed an excellent agreement with previously published data on similar systems. [27] At pH 4, the cw‐ESR spectra showed the presence of two different radicals, which were assigned to MQ.− and its protonated form MQ.. This agrees with the pK a‐value of about 4 for MQ. where MQ.− and MQ. occur at a 1:1 ratio. [27] Hence, at pH 3 solely MQ. is observed. Thus, to obtain reversible redox chemistry, it is crucial to operate below pH 4 to suppress formation of semi‐quinone intermediates upon cycling.[ 5 , 34 , 35 ]
However, the pH value is not the only criterion, as radicals are still present upon irradiation, inducing further decomposition reactions. As phosphoric acid is known to stabilize a variety of organic compounds in aqueous solutions, we explored whether it increases stability of MHQ solutions as well. Although the stabilization mechanisms are still under debate, it is likely that side‐reactions are prevented and reactive intermediates are trapped via strong hydrogen‐bridging and chelation effects. [36] Furthermore, phosphates can act as radical scavengers via condensation to, for example, the di‐, tri‐, or metaphosphate, consequently making MQ/MHQ less prone to decompose in aqueous solutions.[ 37 , 38 ] To demonstrate the influence of these stabilizers, we stored solutions of MQ under light exclusion for eight weeks in 0.5 M H3PO4, after which no decomposition products were observed. At this H3PO4 concentration, the MHQ/MQ couple is also stable under electrochemical conditions. The half wave potential of MHQ was centered at 320 mV (Figure 2 a) vs. Ag/AgCl, (515 mV vs. SHE), which did not change during cycling. Varying the scan rate revealed electrochemical reversibility of the MQ/MHQ system in the tested range (2–1000 mV s−1) as seen by the linear dependency of the peak current (ip) versus the square root of the scan rate (ν1/2, Figure 2 b). Furthermore, the redox potential shifts with a slope of 49 mV pH−1 as is typical for 2 e− processes of quinones (Supporting Information, Figure S8). [15] This is in agreement with previous results, showing that at pH values below the pK a of the semiquinone radicals, the oxidation/reduction occurs via an EE mechanism.[ 34 , 39 ]

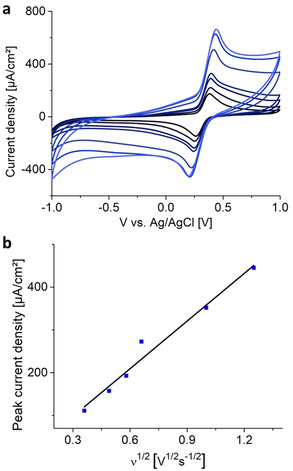
a) Cyclic voltammograms of MHQ in 0.5 M H3PO4 at scan speeds of 50, 100, 150, 200, 500, 800, and 1000 mV s−1 (dark blue to light blue), respectively. b) Plot of I p vs. ν 1/2 of MHQ/MQ (1 mM in 0.5 M H3PO4).
We tested the feasibility of MHQ/MQ for aqueous electrolytes, in an aqueous organic redox flow battery. First, we tested the reversibility of the system in a proof‐of‐concept in symmetric and very diluted state using symmetric sandwich cells. (Supporting Information, Figure S9). At phosphoric acid concentrations below 0.5 M, the active materials degraded rapidly in these cells. After an initial decrease of the capacity, mainly due the influence of residual air in the cell, 0.5 M H3PO4 yielded high coulombic efficiencies over 50 cycles. In contrast, 0.75 M H3PO4 induced partial irreversibility of the electrochemical trials, concomitant with a significant capacity fading in full cells (60 % of the initial capacity; Supporting Information, Figure S9). In 1 M H3PO4, this behavior was even more pronounced and after 25 cycles capacity dropped to less than 10 % of its initial value.
Thus, we continued with the 0.5 M H3PO4 as medium for the pumped RFB trials using para‐benzoquinone (pBQ) as the other electrolyte system (Supporting Information, Figures S10, S11). We cycled the cell for more than 250 cycles with full charge and discharge (Figure 3 a).

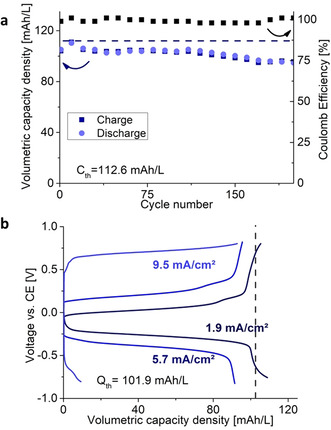
a) Potentiostatic cycling of a pumped RFB with 18.65 mg MQ and 25 mg pBQ as active materials in 0.5 M H3PO4 at ±0.75 V for 5400 s cycle−1. b) Galvanostatic cycling of a pumped RFB containing 20.6 mg MHQ and 23.4 mg pBQ as active materials in 0.5 M H3PO4 at a current density of 1.9, 5.7 and 9.5 mA cm−2. The theoretical capacity of the cells (a: 112.6, b. 101.9 mAh L−1) is indicated by the dashed line.
The initial capacity was close to the theoretical one and retained 87.4 % of its initial capacity while maintaining high coulombic efficiency between 97–99 % and current efficiencies of 91–94 % during 250 cycles. Galvanostatic cycling gave values close to theoretical capacities at current densities of 1.9 mA cm−2 (Figure 3 b). Remarkably, these results were obtained under ambient condition without the exclusion of air. The solubility of MHQ in 0.5 M H3PO4 is ≈140 g L−1. This translates to an energy density of 27.1 Wh L−1 at a voltage difference of 1 V when choosing a suitable counter‐side (such as viologen, S/S2+).
In conclusion, we synthesized MHQ from vanillin and elaborated the foundations to employ the MQ/MHQ redox couple for RFBs. These quinones decompose in presence of a variety of ions as well as light exposure. Using 0.5 M H3PO4 as solvent, we stabilized the MQ/MHQ couple giving 97–99 % coulombic efficiency over 250 cycles in a full RFB. The cycle number corresponds to more than 8 months continuous battery operation in stationary storage with about one charge/discharge cycle per day. At the counter electrode, a well‐known reference compound, pBQ was used, which can also be produced from natural resources (such as oxidation of quinic acid) using simple procedures. Further studies are required to investigate the applicability in large scale and counter‐sides with suitably high voltage difference in the chosen medium. The MQ/MHQ redox couple could be one of the first bio‐based quinones, which is available in large scale (from lignin) and could hit the price of 4 $ kg−1, which is considered the required threshold for industrial applicability. [40]
The authors declare no conflict of interest.
The Austrian Research Promotion Agency (FFG) is gratefully acknowledged for financial support of the project LignoBatt (860429).
1
2
4
6
7
8
9
9
10
11
12
13
14
17
18
19
20
21
23
24
26
27
28
29
30
31
35
36
38
39
40
41